

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PeQuiM- Laboratory of Research in Medicinal Chemistry, Institute of Chemistry, Federal University of Alfenas, 37130-000, Alfenas, MG, Brazil. Curated by ChEMBL | Assay Description Non-competitive inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate ... | Eur J Med Chem 130: 440-457 (2017) Article DOI: 10.1016/j.ejmech.2017.02.043 BindingDB Entry DOI: 10.7270/Q2VX0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50256407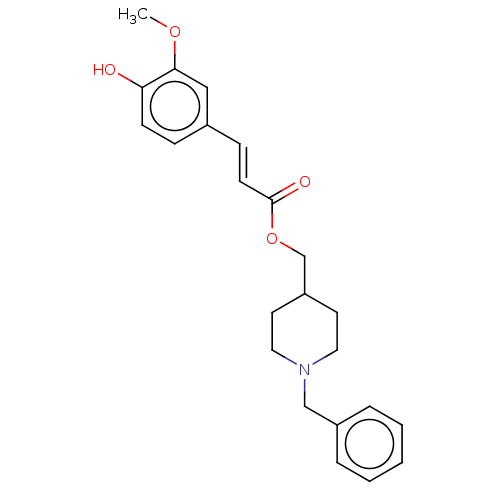 (CHEMBL4088197) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | 1.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
PeQuiM- Laboratory of Research in Medicinal Chemistry, Institute of Chemistry, Federal University of Alfenas, 37130-000, Alfenas, MG, Brazil. Curated by ChEMBL | Assay Description Non-competitive inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate ... | Eur J Med Chem 130: 440-457 (2017) Article DOI: 10.1016/j.ejmech.2017.02.043 BindingDB Entry DOI: 10.7270/Q2VX0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292422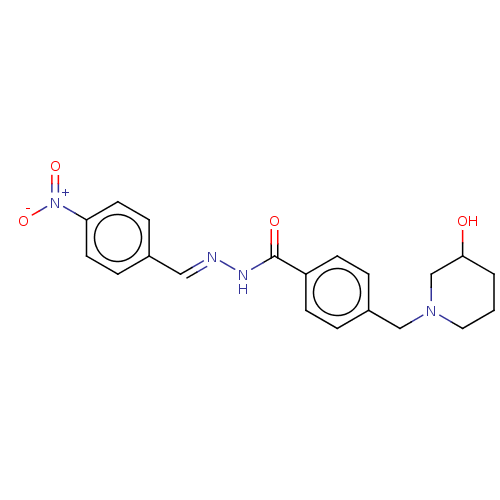 (CHEMBL4175724) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292419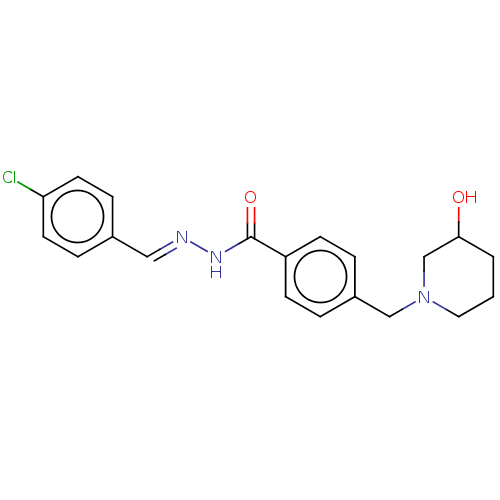 (CHEMBL4163724) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292536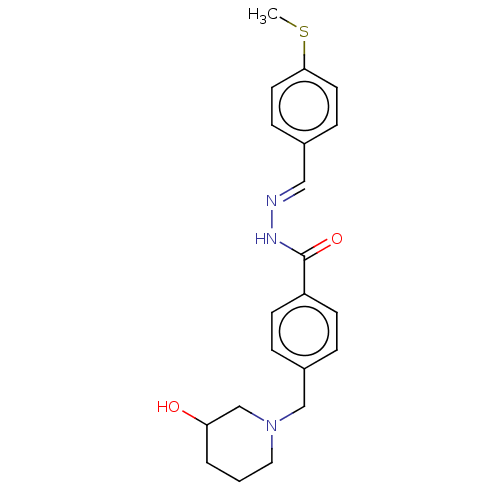 (CHEMBL4170841) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 8.42E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Apparent affinity to inhibit binding of [3H]-pCCK-8 to Cholecystokinin type A receptor of guinea pig pancreatic membranes | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139050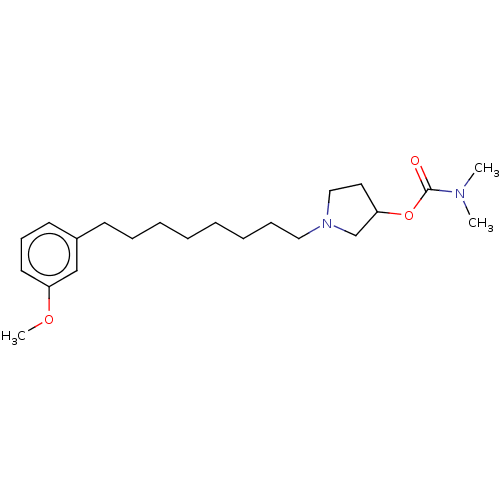 (CHEMBL3753906) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292510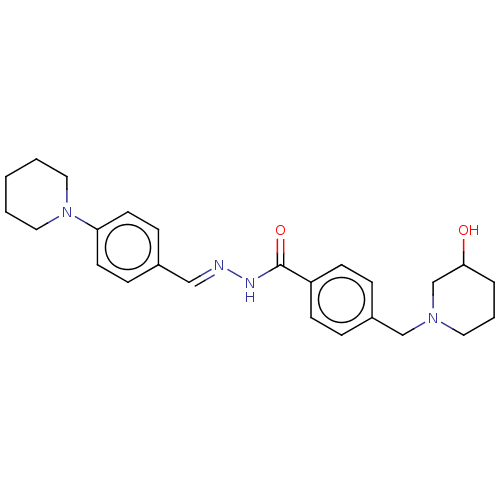 (CHEMBL4172314) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 9.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Affinity of compound to Sigma opioid receptor labelled with [3H](+)-3-PPP | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139006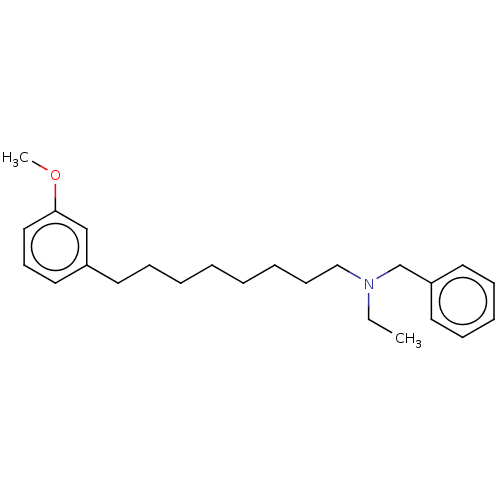 (CHEMBL3753607) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292423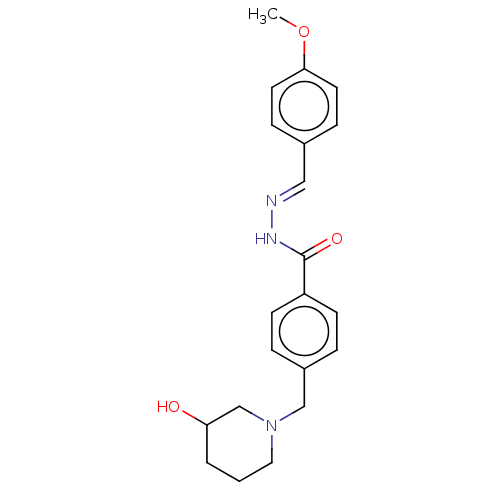 (CHEMBL4167867) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292442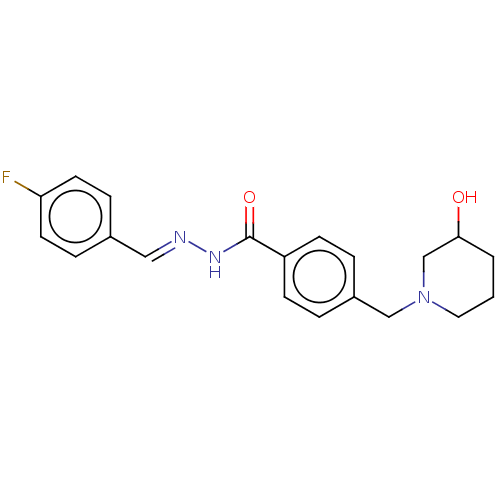 (CHEMBL4171226) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139052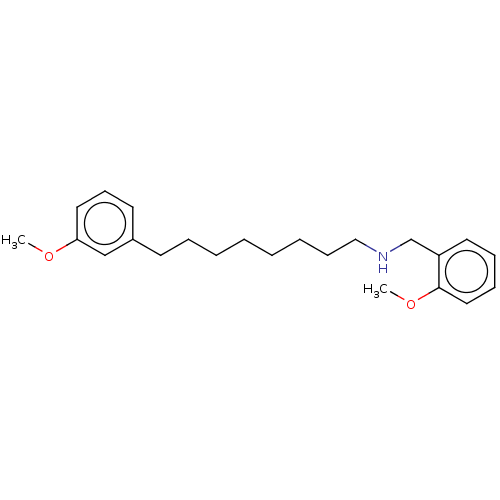 (CHEMBL3754700) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139049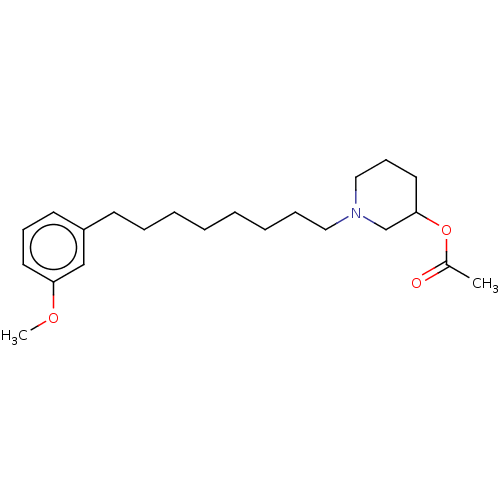 (CHEMBL3753732) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292526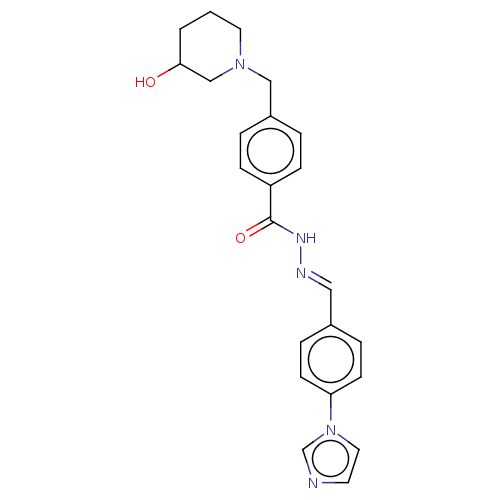 (CHEMBL4164436) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition b... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139051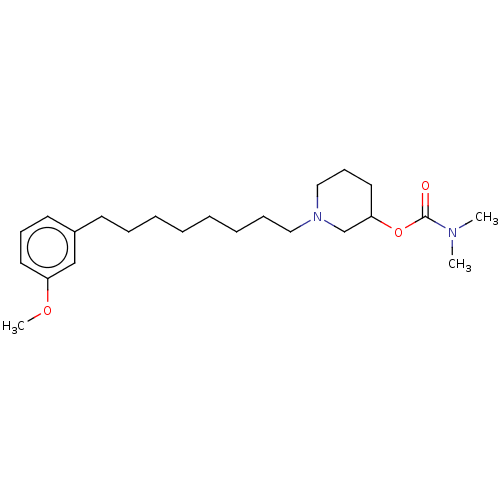 (CHEMBL3753070) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139007 (CHEMBL3754287) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.24E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Mixed inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139053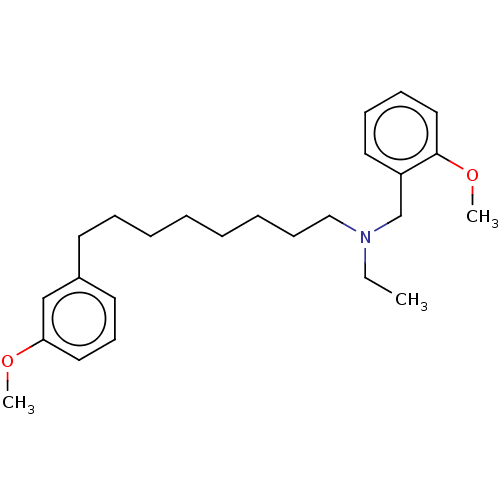 (CHEMBL3752227) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Non-competitive inhibition of electric eel acetylcholine esterase using acetylcholine iodide as substrate by Ellman's method | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Apparent affinity to inhibit binding of [3H]-pCCK-8 to Cholecystokinin type A receptor of guinea pig pancreatic membranes | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
PeQuiM- Laboratory of Research in Medicinal Chemistry, Institute of Chemistry, Federal University of Alfenas, 37130-000, Alfenas, MG, Brazil. Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measure... | Eur J Med Chem 130: 440-457 (2017) Article DOI: 10.1016/j.ejmech.2017.02.043 BindingDB Entry DOI: 10.7270/Q2VX0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50030474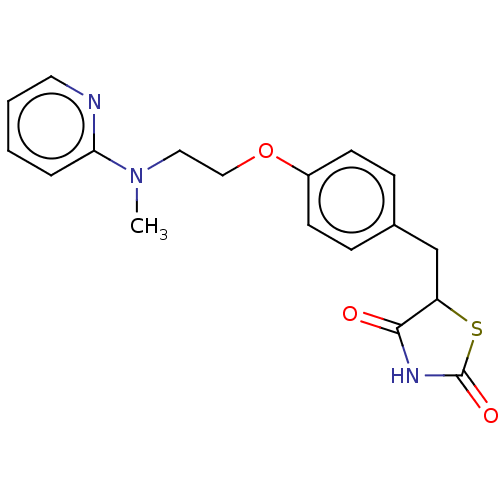 (Avandamet | Avandaryl | Avandia | BRL-49653 | CHEB...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE PC cid PC sid PDB UniChem Similars | DrugBank PDB Article PubMed | n/a | n/a | 159 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do ABC Curated by ChEMBL | Assay Description Displacement of fluormone from human PPARgamma LBD expressed in Escherichia coli BL21 DE3 by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5795-802 (2013) Article DOI: 10.1016/j.bmcl.2013.09.010 BindingDB Entry DOI: 10.7270/Q2D221JC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50256407 (CHEMBL4088197) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | n/a | n/a |
PeQuiM- Laboratory of Research in Medicinal Chemistry, Institute of Chemistry, Federal University of Alfenas, 37130-000, Alfenas, MG, Brazil. Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measure... | Eur J Med Chem 130: 440-457 (2017) Article DOI: 10.1016/j.ejmech.2017.02.043 BindingDB Entry DOI: 10.7270/Q2VX0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM53804 (4-({2-[(1,3-dioxo-1,3-dihydro-2H-inden-2-ylidene)m...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 1.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do ABC Curated by ChEMBL | Assay Description Displacement of fluormone from human PPARgamma LBD expressed in Escherichia coli BL21 DE3 by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5795-802 (2013) Article DOI: 10.1016/j.bmcl.2013.09.010 BindingDB Entry DOI: 10.7270/Q2D221JC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10404 ((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 2.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase preincubated for 10 mins followed by the addition of 550 uM of acetylcholine iodide as substrate measured ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50387185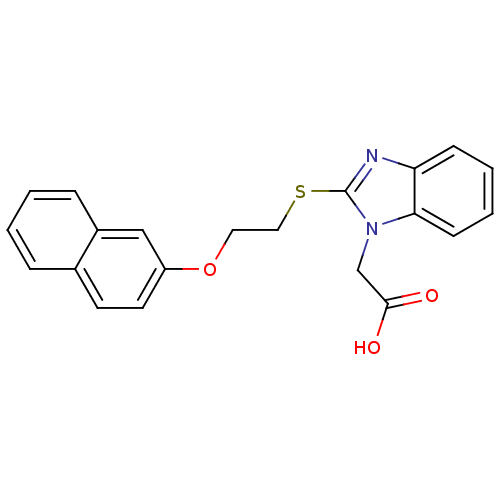 (CHEMBL2048021) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do ABC Curated by ChEMBL | Assay Description Displacement of fluormone from human PPARgamma LBD expressed in Escherichia coli BL21 DE3 by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5795-802 (2013) Article DOI: 10.1016/j.bmcl.2013.09.010 BindingDB Entry DOI: 10.7270/Q2D221JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292422 (CHEMBL4175724) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.71E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139052 (CHEMBL3754700) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50590393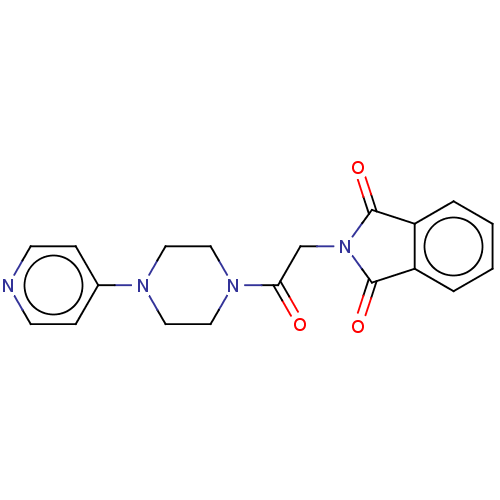 (CHEMBL5195581) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Citation and Details Article DOI: 10.1039/d1md00374g BindingDB Entry DOI: 10.7270/Q29K4G7Q | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Binding affinity of [3H]spiroperidol to striatal Dopamine receptor D2 binding sites | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM8960 ((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 4.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PeQuiM- Laboratory of Research in Medicinal Chemistry, Institute of Chemistry, Federal University of Alfenas, 37130-000, Alfenas, MG, Brazil. Curated by ChEMBL | Assay Description Inhibition of equine serum BuChE using butyrylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measured at 12 se... | Eur J Med Chem 130: 440-457 (2017) Article DOI: 10.1016/j.ejmech.2017.02.043 BindingDB Entry DOI: 10.7270/Q2VX0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139053 (CHEMBL3752227) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Peroxisome proliferator-activated receptor gamma (Homo sapiens (Human)) | BDBM50493700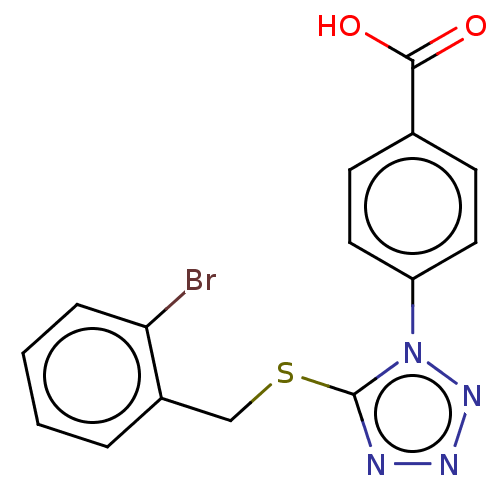 (CHEMBL1426715) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universidade Federal do ABC Curated by ChEMBL | Assay Description Displacement of fluormone from human PPARgamma LBD expressed in Escherichia coli BL21 DE3 by fluorescence polarization assay | Bioorg Med Chem Lett 23: 5795-802 (2013) Article DOI: 10.1016/j.bmcl.2013.09.010 BindingDB Entry DOI: 10.7270/Q2D221JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM50139053 (CHEMBL3752227) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of human acetylcholine esterase preincubated for 10 mins followed by the addition of 550 uM of acetylcholine iodide as substrate measured ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139053 (CHEMBL3752227) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139006 (CHEMBL3753607) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292442 (CHEMBL4171226) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition activity against AICAR formyltransferase determined against L cell | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292536 (CHEMBL4170841) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.04E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292510 (CHEMBL4172314) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.09E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292539 (CHEMBL4174394) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292423 (CHEMBL4167867) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139050 (CHEMBL3753906) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139051 (CHEMBL3753070) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292565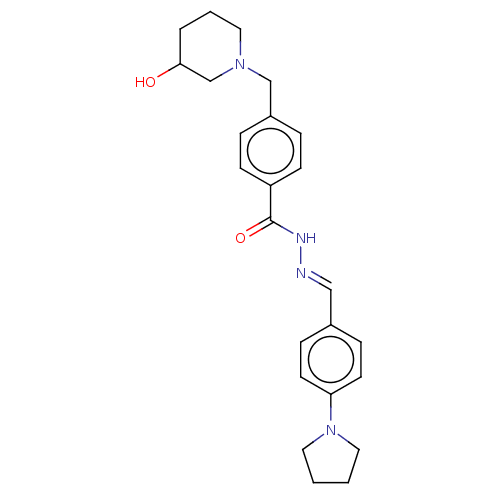 (CHEMBL4160612) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139006 (CHEMBL3753607) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139049 (CHEMBL3753732) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139007 (CHEMBL3754287) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.64E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50256425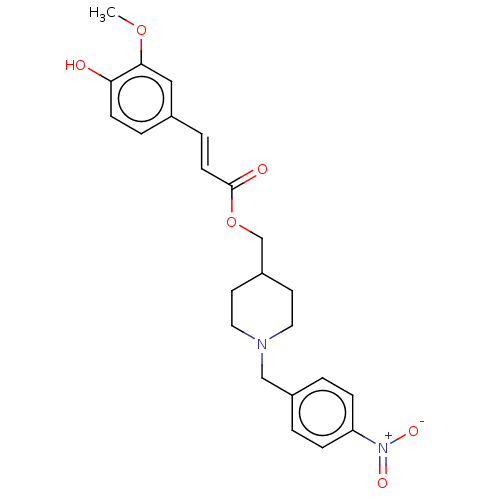 (CHEMBL4066925) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
PeQuiM- Laboratory of Research in Medicinal Chemistry, Institute of Chemistry, Federal University of Alfenas, 37130-000, Alfenas, MG, Brazil. Curated by ChEMBL | Assay Description Inhibition of Electrophorus electricus AChE using acetylthiocholine iodide as substrate pretreated for 10 mins followed by substrate addition measure... | Eur J Med Chem 130: 440-457 (2017) Article DOI: 10.1016/j.ejmech.2017.02.043 BindingDB Entry DOI: 10.7270/Q2VX0JXD | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139052 (CHEMBL3754700) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Cholinesterase (Equus caballus (Horse)) | BDBM50139049 (CHEMBL3753732) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of horse butyrylcholine esterase preincubated for 10 mins followed by the addition of butyrylcholine iodide as substrate measured after 5 ... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50139007 (CHEMBL3754287) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bras£lia Curated by ChEMBL | Assay Description Inhibition of electric eel acetylcholine esterase preincubated for 10 mins followed by the addition of acetylcholine iodide as substrate measured aft... | Eur J Med Chem 108: 687-700 (2016) Article DOI: 10.1016/j.ejmech.2015.12.024 BindingDB Entry DOI: 10.7270/Q20R9R7V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM50292566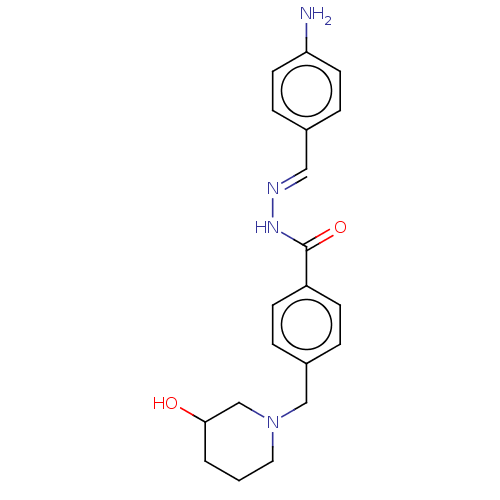 (CHEMBL4168953) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Alfenas Curated by ChEMBL | Assay Description Inhibition of electric eel AChE using acetylthiocholine iodide as substrate preincubated for 10 mins followed by substrate addition by Ellman's metho... | Eur J Med Chem 147: 48-65 (2018) Article DOI: 10.1016/j.ejmech.2018.01.066 BindingDB Entry DOI: 10.7270/Q2K64MM0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 77 total ) | Next | Last >> |