 Found 310 hits for monomerid = 60917,81348,50009001,50193145,50197063,50395076,50395077,50395081,50395083,50396018,50418773,26122,26121
Found 310 hits for monomerid = 60917,81348,50009001,50193145,50197063,50395076,50395077,50395081,50395083,50396018,50418773,26122,26121 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
D(1A) dopamine receptor
(RAT) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
| 1.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Dopamine receptor D1 in rat striatal membranes using [3H]SCH-23390 |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930WC4 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Dopamine receptor D4
(CANINE) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(RAT) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Displacement of [3H]fenoldopam from Dopamine receptor D1 of rat striatum membranes |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
D(1B) dopamine receptor
(RAT) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 11 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Rattus norvegicus (rat)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Binding affinity against Dopamine receptor D2 in rat striatal membranes using [3H]spiperone |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930WC4 |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 15 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
Alpha-2A adrenergic receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University
Curated by PDSP Ki Database
| |
Proc Natl Acad Sci U S A 88: 7491-5 (1991)
Article DOI: 10.1073/pnas.88.17.7491
BindingDB Entry DOI: 10.7270/Q2DB80B9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2C adrenergic receptor
(OK) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 22 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 28 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Adenosine receptor A2b
(Rattus norvegicus) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| | 35 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its binding affinity against Alpha-2 adrenergic receptor |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930WC4 |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D1 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Alpha-2B adrenergic receptor
(NEONATAL RAT) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Nebraska
Curated by PDSP Ki Database
| |
J Pharmacol Exp Ther 259: 323-9 (1991)
BindingDB Entry DOI: 10.7270/Q2Z899WV |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM81348

(β-Lapachone (A3) | Beta lapachone | R115 (Rea...)Show InChI InChI=1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
SIB Swiss Institute of Bioinformatics
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant IDO1 expressed in Escherichia coli by Michaelis-Menton nonlinear regression plot analysis in presence ... |
J Med Chem 58: 9421-37 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00326
BindingDB Entry DOI: 10.7270/Q28K7D3X |
More data for this
Ligand-Target Pair | |
Indoleamine 2,3-dioxygenase 1
(Homo sapiens (Human)) | BDBM81348

(β-Lapachone (A3) | Beta lapachone | R115 (Rea...)Show InChI InChI=1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of IDO1 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c00925
BindingDB Entry DOI: 10.7270/Q28D010N |
More data for this
Ligand-Target Pair | |
Cocaine esterase
(Homo sapiens (Human)) | BDBM81348

(β-Lapachone (A3) | Beta lapachone | R115 (Rea...)Show InChI InChI=1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3 | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 109 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human intestinal CES2 expressed in baculovirus infected sf9 cells using o-nitrophenyl acetate as substrate monitored at 15 secs interva... |
J Med Chem 60: 1568-1579 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01849
BindingDB Entry DOI: 10.7270/Q2FF3VMP |
More data for this
Ligand-Target Pair | |
Alpha-1A/Alpha-1B/Alpha-1D adrenergic receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
| 151 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Compound was tested for its binding affinity against Alpha-1 adrenergic receptor |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2930WC4 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1B1
(Homo sapiens (Human)) | BDBM50009001
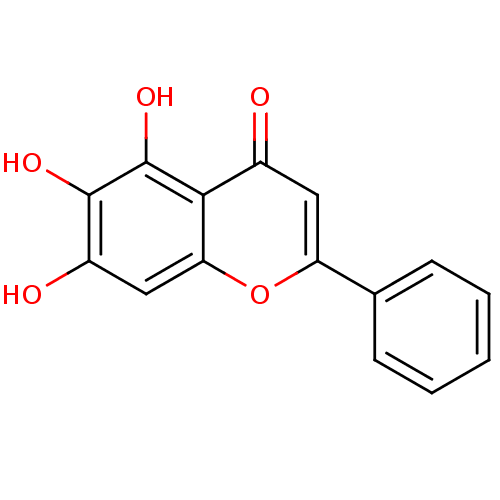
(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete
Curated by ChEMBL
| Assay Description
Inhibition of CYP1B1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin |
Bioorg Med Chem 19: 2842-9 (2011)
Article DOI: 10.1016/j.bmc.2011.03.042
BindingDB Entry DOI: 10.7270/Q2V69JXK |
More data for this
Ligand-Target Pair | |
Dopamine receptor D4
(CANINE) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 321 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 610-4 (1991)
Article DOI: 10.1038/350610a0
BindingDB Entry DOI: 10.7270/Q24T6GVD |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX15
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human 15-lipoxygenase |
J Med Chem 54: 5485-97 (2011)
Article DOI: 10.1021/jm2005089
BindingDB Entry DOI: 10.7270/Q25T3KVT |
More data for this
Ligand-Target Pair | |
Prolyl 4-hydroxylase
(Paramecium bursaria Chlorella virus 1) | BDBM50193145

(2-(1-chloro-4-hydroxyisoquinoline-3-carboxamido)ac...)Show InChI InChI=1S/C12H9ClN2O4/c13-11-7-4-2-1-3-6(7)10(18)9(15-11)12(19)14-5-8(16)17/h1-4,18H,5H2,(H,14,19)(H,16,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/TrEMBL
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 710 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Inhibition of N-terminal His6-tagged recombinant Paramecium bursaria chlorella virus 1 CPH expressed in Escherichia coli Rosetta 2 (DE3) cells pre-in... |
Bioorg Med Chem 27: 2405-2412 (2019)
Article DOI: 10.1016/j.bmc.2019.01.018
BindingDB Entry DOI: 10.7270/Q2KK9G4R |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(BOVINE) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PubMed
| 790 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of [3H]spiroperidol binding against Dopamine receptor D2 |
J Med Chem 29: 1904-12 (1986)
BindingDB Entry DOI: 10.7270/Q2TQ623R |
More data for this
Ligand-Target Pair | |
D(1B) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | |
D(2) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 810 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Texas at Austin
Curated by ChEMBL
| Assay Description
Affinity towards Dopamine receptor D2 |
J Med Chem 41: 4385-99 (1998)
Article DOI: 10.1021/jm9800292
BindingDB Entry DOI: 10.7270/Q2R21227 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A1
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Crete
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A1 EROD activity assessed as inhibition of deethylation of 7-ethoxyresorufin to resorufin |
Bioorg Med Chem 19: 2842-9 (2011)
Article DOI: 10.1016/j.bmc.2011.03.042
BindingDB Entry DOI: 10.7270/Q2V69JXK |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM81348

(β-Lapachone (A3) | Beta lapachone | R115 (Rea...)Show InChI InChI=1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 1.22E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human liver CES1 expressed in baculovirus infected sf9 cells using oseltamivir as substrate |
J Med Chem 60: 1568-1579 (2017)
Article DOI: 10.1021/acs.jmedchem.6b01849
BindingDB Entry DOI: 10.7270/Q2FF3VMP |
More data for this
Ligand-Target Pair | |
Polyunsaturated fatty acid lipoxygenase ALOX12
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Human Genome Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human platelet-type 12-lipoxygenase |
J Med Chem 54: 5485-97 (2011)
Article DOI: 10.1021/jm2005089
BindingDB Entry DOI: 10.7270/Q25T3KVT |
More data for this
Ligand-Target Pair | |
D(1A) dopamine receptor
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| DrugBank
PDB
Article
PubMed
| 1.82E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Toronto
Curated by PDSP Ki Database
| |
Nature 350: 614-9 (1991)
Article DOI: 10.1038/350614a0
BindingDB Entry DOI: 10.7270/Q2NV9GQR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of JMJD2E relative to alpha-ketoglutarate |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 2A
(Homo sapiens (Human)) | BDBM50395076
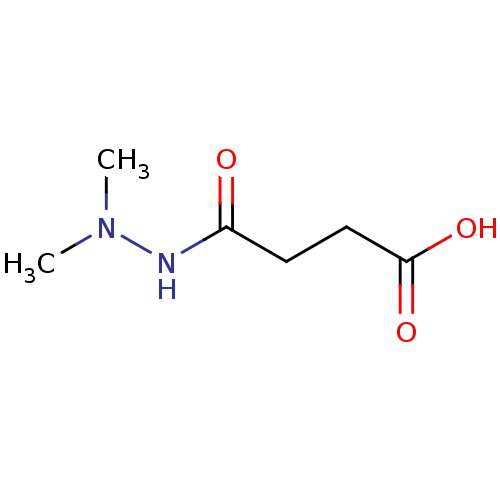
(CHEMBL2164243)Show InChI InChI=1S/C6H12N2O3/c1-8(2)7-5(9)3-4-6(10)11/h3-4H2,1-2H3,(H,7,9)(H,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.97E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Competitive inhibition of human KDM2A expressed in Escherichia coli using 2-oxoglutarate by enzyme kinetic assay |
J Med Chem 55: 6639-43 (2012)
Article DOI: 10.1021/jm300677j
BindingDB Entry DOI: 10.7270/Q2JH3N9S |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50418773
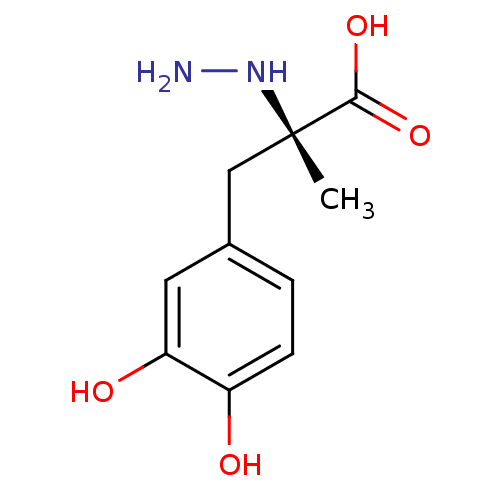
(CARBIDOPA)Show InChI InChI=1S/C10H14N2O4/c1-10(12-11,9(15)16)5-6-2-3-7(13)8(14)4-6/h2-4,12-14H,5,11H2,1H3,(H,15,16)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Uncompetitive inhibition of JMJD2E relative to alpha-ketoglutarate |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM81348

(β-Lapachone (A3) | Beta lapachone | R115 (Rea...)Show InChI InChI=1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| 3.55E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of JMJD2E relative to alpha-ketoglutarate |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.33E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Copenhagen
Curated by ChEMBL
| Assay Description
Non-competitive inhibition of JMJD2E relative to alpha-ketoglutarate |
Bioorg Med Chem 19: 3625-36 (2011)
Article DOI: 10.1016/j.bmc.2011.01.046
BindingDB Entry DOI: 10.7270/Q23X870S |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 2A
(Homo sapiens (Human)) | BDBM50395076

(CHEMBL2164243)Show InChI InChI=1S/C6H12N2O3/c1-8(2)7-5(9)3-4-6(10)11/h3-4H2,1-2H3,(H,7,9)(H,10,11) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 8.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oxford
Curated by ChEMBL
| Assay Description
Mixed type inhibition of human KDM2A expressed in Escherichia coli assessed inhibition constant for compound-enzyme-substrate complex using methyl ly... |
J Med Chem 55: 6639-43 (2012)
Article DOI: 10.1021/jm300677j
BindingDB Entry DOI: 10.7270/Q2JH3N9S |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 6
(Mus musculus) | BDBM26122
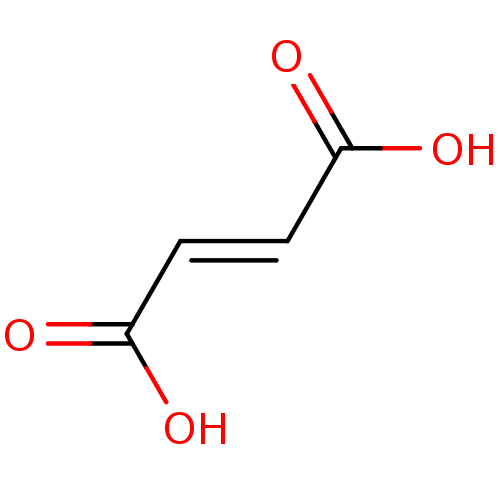
((2E)-but-2-enedioic acid | FUMARIC ACID | Fumarate...)Show InChI InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+ | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 6.17E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of mouse Oat1-mediated [3H]PAH uptake in Xenopus oocytes after 1 hr |
J Biol Chem 282: 23841-53 (2007)
Article DOI: 10.1074/jbc.M703467200
BindingDB Entry DOI: 10.7270/Q2W95B35 |
More data for this
Ligand-Target Pair | |
N(4)-(beta-N-acetylglucosaminyl)-L-asparaginase
(Homo sapiens (Human)) | BDBM26121
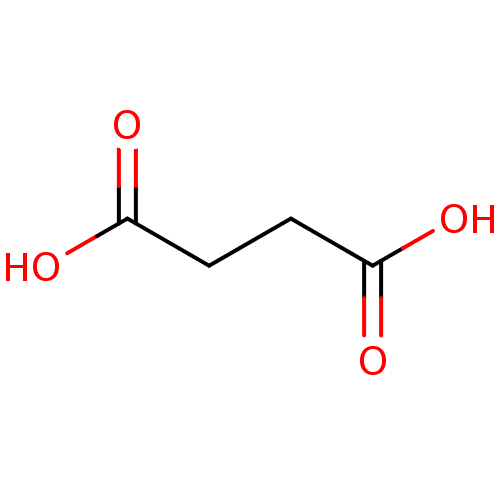
(SUCCINIC ACID | Substrate analogue, 11 | Succinate...)Show InChI InChI=1S/C4H6O4/c5-3(6)1-2-4(7)8/h1-2H2,(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.00E+6 | -3.26 | n/a | n/a | n/a | n/a | n/a | 5.8 | 37 |
University of North Carolina
| Assay Description
Glycosylasparaginase activity was measured in citrate-phosphate buffer at pH 5.8 at 37 C. N-Acetyl-D-glucosamine released during the reaction was me... |
J Enzym Inhib 16: 269-74 (2001)
Article DOI: 10.1080/14756360109162375
BindingDB Entry DOI: 10.7270/Q2P55M2S |
More data for this
Ligand-Target Pair | |
Delta-aminolevulinic acid dehydratase
(Escherichia coli) | BDBM26121

(SUCCINIC ACID | Substrate analogue, 11 | Succinate...)Show InChI InChI=1S/C4H6O4/c5-3(6)1-2-4(7)8/h1-2H2,(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.25E+7 | -2.70 | n/a | n/a | n/a | n/a | n/a | 8.1 | 37 |
University of Neuchâtel
| Assay Description
The PBGS assay is a colorimetric assay based on the reaction between PBG and 4-dimethylaminobenzaldehyde (Ehrlich's reagent). |
Chembiochem 2: 343-54 (2001)
Article DOI: 10.1002/1439-7633(20010504)2
BindingDB Entry DOI: 10.7270/Q29Z93CN |
More data for this
Ligand-Target Pair | |
Solute carrier family 22 member 20
(Mus musculus) | BDBM26122

((2E)-but-2-enedioic acid | FUMARIC ACID | Fumarate...)Show InChI InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| >3.16E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of mouse Oat6-mediated [3H]ES uptake in Xenopus oocytes after 1 hr |
J Biol Chem 282: 23841-53 (2007)
Article DOI: 10.1074/jbc.M703467200
BindingDB Entry DOI: 10.7270/Q2W95B35 |
More data for this
Ligand-Target Pair | |
Tyrosyl-DNA phosphodiesterase 2
(Homo sapiens (Human)) | BDBM81348

(β-Lapachone (A3) | Beta lapachone | R115 (Rea...)Show InChI InChI=1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3 | PDB
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 970 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester
Curated by ChEMBL
| Assay Description
Inhibition of TDP2 (unknown origin) using 4-nitrophenyl phenylphosphonate as substrate after 60 mins |
J Med Chem 56: 6352-70 (2013)
Article DOI: 10.1021/jm400568p
BindingDB Entry DOI: 10.7270/Q2N017ZB |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM26121

(SUCCINIC ACID | Substrate analogue, 11 | Succinate...)Show InChI InChI=1S/C4H6O4/c5-3(6)1-2-4(7)8/h1-2H2,(H,5,6)(H,7,8) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.10E+5 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Oxford
| Assay Description
A coupled-assay for JMJD2E activity employing formaldehyde dehydrogenase (FDH) from Pseudomonas putida was developed. Formaldehyde release by demethy... |
J Med Chem 51: 7053-6 (2008)
Article DOI: 10.1021/jm800936s
BindingDB Entry DOI: 10.7270/Q2959FV4 |
More data for this
Ligand-Target Pair | |
Lysine-specific demethylase 4E
(Homo sapiens (Human)) | BDBM26122

((2E)-but-2-enedioic acid | FUMARIC ACID | Fumarate...)Show InChI InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.60E+6 | n/a | n/a | n/a | n/a | 7.5 | 37 |
University of Oxford
| Assay Description
A coupled-assay for JMJD2E activity employing formaldehyde dehydrogenase (FDH) from Pseudomonas putida was developed. Formaldehyde release by demethy... |
J Med Chem 51: 7053-6 (2008)
Article DOI: 10.1021/jm800936s
BindingDB Entry DOI: 10.7270/Q2959FV4 |
More data for this
Ligand-Target Pair | |
Protein-glutamine gamma-glutamyltransferase 2
(Homo sapiens (Human)) | BDBM81348

(β-Lapachone (A3) | Beta lapachone | R115 (Rea...)Show InChI InChI=1S/C15H14O3/c1-15(2)8-7-11-13(17)12(16)9-5-3-4-6-10(9)14(11)18-15/h3-6H,7-8H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | 37 |
Duke University Medical Center
| Assay Description
In order to eliminated fluorescence interference, chemicals were subjected to a secondary screening using colorimetric BP incorporation assay. TGase... |
Chem Biol 15: 969-78 (2008)
Article DOI: 10.1016/j.chembiol.2008.07.015
BindingDB Entry DOI: 10.7270/Q2DR2SZR |
More data for this
Ligand-Target Pair | |
17-beta-hydroxysteroid dehydrogenase type 3
(Homo sapiens (Human)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.30E+3 | n/a | n/a | n/a | n/a | 7.45 | 37 |
Cardiff University
| Assay Description
Inhibition assay of human testes mcrosomal 17 beta-hydroxysteroid dehydrogenase for the reduction of androstenedione. |
J Enzym Inhib 16: 35-45 (2001)
Article DOI: 10.1080/14756360109162353
BindingDB Entry DOI: 10.7270/Q2SX6BRB |
More data for this
Ligand-Target Pair | |
Linoleate 9S-lipoxygenase-4
(Glycine max (Soybean) (Glycine hispida)) | BDBM50009001

(5,6,7-Trihydroxyflavone | 5,6,7-trihydroxy-2-pheny...)Show InChI InChI=1S/C15H10O5/c16-9-6-11(8-4-2-1-3-5-8)20-12-7-10(17)14(18)15(19)13(9)12/h1-7,17-19H | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | 8.0 | 25 |
PCSIR Laboratories Complex
| Assay Description
LOX inhibiting activity was measured by modifying the spectrophotometric method developed by Tappel. |
J Enzyme Inhib Med Chem 23: 313-6 (2008)
Article DOI: 10.1080/14756360701536455
BindingDB Entry DOI: 10.7270/Q2930RRD |
More data for this
Ligand-Target Pair | |
Methyl-accepting chemotaxis protein NahY
(Pseudomonas putida (Arthrobacter siderocapsulatus)) | BDBM26122

((2E)-but-2-enedioic acid | FUMARIC ACID | Fumarate...)Show InChI InChI=1S/C4H4O4/c5-3(6)1-2-4(7)8/h1-2H,(H,5,6)(H,7,8)/b2-1+ | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | 20 |
CSIC
| Assay Description
Measurements were done on a VP-microcalorimeter (MicroCal, Amherst, MA). |
J Biol Chem 285: 23126-36 (2010)
Article DOI: 10.1074/jbc.M110.110403
BindingDB Entry DOI: 10.7270/Q2K64GNS |
More data for this
Ligand-Target Pair | |
Methyl-accepting chemotaxis protein NahY
(Pseudomonas putida (Arthrobacter siderocapsulatus)) | BDBM26121

(SUCCINIC ACID | Substrate analogue, 11 | Succinate...)Show InChI InChI=1S/C4H6O4/c5-3(6)1-2-4(7)8/h1-2H2,(H,5,6)(H,7,8) | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 8.20E+4 | n/a | n/a | n/a | n/a | 20 |
CSIC
| Assay Description
Measurements were done on a VP-microcalorimeter (MicroCal, Amherst, MA). |
J Biol Chem 285: 23126-36 (2010)
Article DOI: 10.1074/jbc.M110.110403
BindingDB Entry DOI: 10.7270/Q2K64GNS |
More data for this
Ligand-Target Pair | |
Guanine nucleotide-binding protein subunit alpha-15
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | n/a | >2.99E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q2CN72J7 |
More data for this
Ligand-Target Pair | |
Trace amine-associated receptor 1
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PCBioAssay
| n/a | n/a | 8.04E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q280517J |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Trace amine-associated receptor 1
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
PCBioAssay
| n/a | n/a | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q24748HT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Guanine nucleotide-binding protein subunit alpha-15
(Homo sapiens (Human)) | BDBM60917

(9-chloranyl-5-(4-hydroxyphenyl)-2,3,4,5-tetrahydro...)Show InChI InChI=1S/C16H16ClNO3/c17-15-11-5-6-18-8-13(9-1-3-10(19)4-2-9)12(11)7-14(20)16(15)21/h1-4,7,13,18-21H,5-6,8H2 | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PCBioAssay
| n/a | n/a | >2.99E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q2QZ28KG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase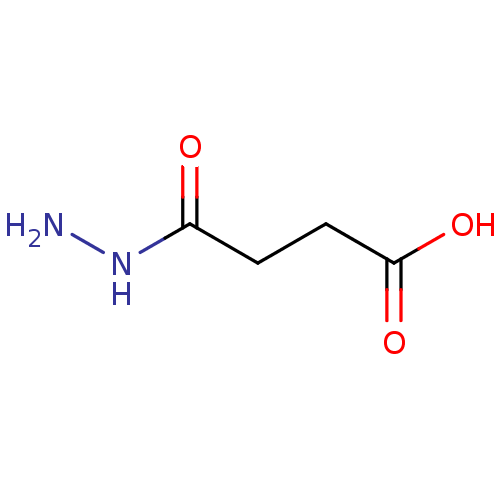 Purchase
Purchase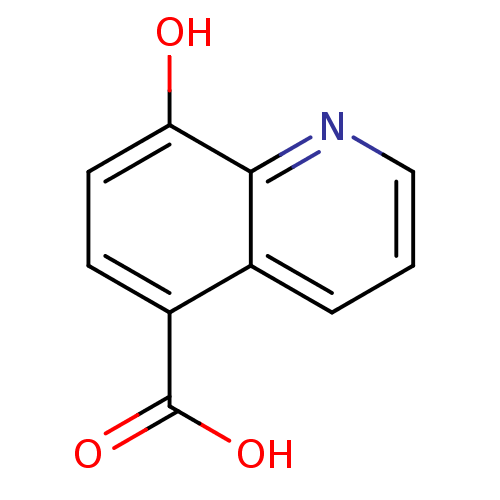 Purchase
Purchase Purchase
Purchase Purchase
Purchase Purchase
Purchase
