 Found 575 hits for monomerid = 55151,96613,50005711,103537,50003616,50015112,50015142,50015152,50015094,50015184,50061625,50207561,50220259,50229193,50238632
Found 575 hits for monomerid = 55151,96613,50005711,103537,50003616,50015112,50015142,50015152,50015094,50015184,50061625,50207561,50220259,50229193,50238632 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC1 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC1 (unknown origin) |
Eur J Med Chem 158: 620-706 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.073
BindingDB Entry DOI: 10.7270/Q2HT2STK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC3 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 0.580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human N-terminal GST-tagged HDAC6 expressed in baculovirus-infected Sf9 insect cells using RHKK(Ac)AMC as subst... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.640 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal FLAG/His-tagged HDAC1 expressed in baculovirus-infected Sf21 insect cells using RHKK(Ac)AMC as substrate a... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC2 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.840 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human C-terminal His-tagged HDAC3/N-terminal GST-tagged recombinant human NCOR2 (395 to 489 residues) expressed... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 1
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 0.870 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC1 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC6 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC2 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Histone deacetylase 3/Nuclear receptor corepressor 2 (HDAC3/NCoR2)
(Homo sapiens (Human)) | BDBM50238632
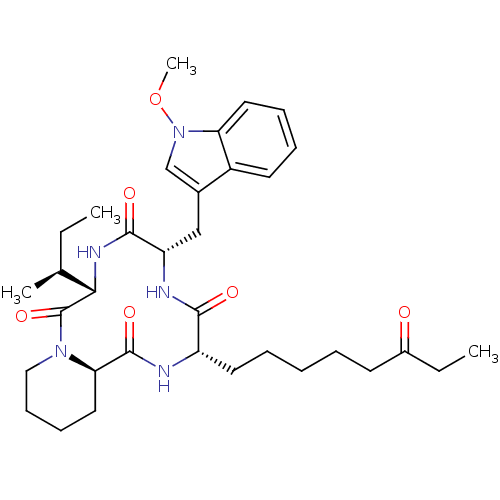
((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute of Harvard and MIT
Curated by ChEMBL
| Assay Description
Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3... |
Bioorg Med Chem Lett 18: 2809-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.007
BindingDB Entry DOI: 10.7270/Q2ST7QR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC6 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 2
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 3.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of full length recombinant human C-terminal GST-tagged HDAC2 expressed in baculovirus-infected insect cells using RHKK(Ac)AMC as substrate... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Polyamine deacetylase HDAC10
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human HDAC10 expressed in baculovirus-infected insect cells using fluorogenic peptide RHKKAc as substrate by fluorimeter |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor alpha
(Homo sapiens (Human)) | BDBM50061625
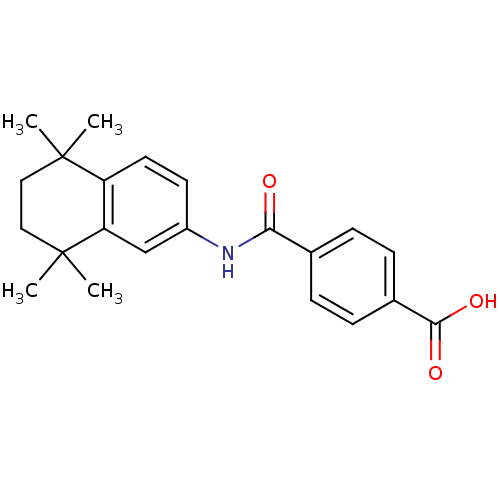
(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 6.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-alpha |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Cryptosporidium parvum) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | KEGG
UniProtKB/TrEMBL
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Binding affinity towards HDAC enzyme derived from Eimeria tenella protozoa |
Bioorg Med Chem Lett 11: 113-7 (2001)
BindingDB Entry DOI: 10.7270/Q2M045ZB |
More data for this
Ligand-Target Pair | |
Retinoic acid receptor beta
(Homo sapiens (Human)) | BDBM50061625

(4-[(5,5,8,8-tetramethyl-5,6,7,8-tetrahydronaphthal...)Show SMILES CC1(C)CCC(C)(C)c2cc(NC(=O)c3ccc(cc3)C(O)=O)ccc12 Show InChI InChI=1S/C22H25NO3/c1-21(2)11-12-22(3,4)18-13-16(9-10-17(18)21)23-19(24)14-5-7-15(8-6-14)20(25)26/h5-10,13H,11-12H2,1-4H3,(H,23,24)(H,25,26) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tokyo
Curated by ChEMBL
| Assay Description
Agonistic activity of the compound towards retinoic acid receptor-beta |
J Med Chem 40: 4222-34 (1998)
Article DOI: 10.1021/jm9704309
BindingDB Entry DOI: 10.7270/Q21J98VC |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Catania
Curated by ChEMBL
| Assay Description
Inhibition of C-terminal His-tagged human recombinant full-length HDAC8 expressed in baculovirus expression system assessed as reduction in 7-amino-4... |
Eur J Med Chem 141: 188-196 (2017)
Article DOI: 10.1016/j.ejmech.2017.09.075
BindingDB Entry DOI: 10.7270/Q2QZ2DGS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 45 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC8 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC8 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 6
(Homo sapiens (Human)) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 123 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Broad Institute of Harvard and MIT
Curated by ChEMBL
| Assay Description
Displacement of fluorescent 5-(3-(3-(4-((4-((7-(hydroxyamino)-7-oxoheptyl)carbamoyl)phenylamino)methyl)-1H-1,2,3-triazol-1-yl)propyl)thioureido)-2-(3... |
Bioorg Med Chem Lett 18: 2809-12 (2008)
Article DOI: 10.1016/j.bmcl.2008.04.007
BindingDB Entry DOI: 10.7270/Q2ST7QR7 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 8
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human C-terminal His-tagged HDAC8 (1 to 377 residues) expressed in baculovirus-infected insect cells using RHK(Ac)K(Ac)AMC ... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 7
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 195 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC7 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC5 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 11
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 401 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Pharmacy, Faculty of Chemistry and Pharmacy , University of Regensburg , 93040 Regensburg , Germany.
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human N-terminal His-tagged HDAC11 (1 to 347 residues) expressed in baculovirus-infected insect cells using RHKK(Ac)AMC as ... |
J Med Chem 61: 3454-3477 (2018)
Article DOI: 10.1021/acs.jmedchem.7b01593
BindingDB Entry DOI: 10.7270/Q24B33S0 |
More data for this
Ligand-Target Pair | |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 504 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hacettepe University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human Hela cells nuclear extracts by fluorimetric assay |
Bioorg Med Chem 17: 5219-28 (2009)
Article DOI: 10.1016/j.bmc.2009.05.042
BindingDB Entry DOI: 10.7270/Q22F7R82 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 9
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC9 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Indian CSIR-Central Drug Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of HDAC4 (unknown origin) |
Eur J Med Chem 158: 620-706 (2018)
Article DOI: 10.1016/j.ejmech.2018.08.073
BindingDB Entry DOI: 10.7270/Q2HT2STK |
More data for this
Ligand-Target Pair | |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human HDAC4 |
Bioorg Med Chem 23: 5151-5 (2015)
Article DOI: 10.1016/j.bmc.2014.12.066
BindingDB Entry DOI: 10.7270/Q2B859V9 |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacylase sirtuin-6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bayreuth
Curated by ChEMBL
| Assay Description
Inhibition of full length SIRT6 (unknown origin) using acetylated H3K9 peptide as substrate after 2 hrs in presence of NAD |
J Med Chem 61: 10922-10928 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01455
BindingDB Entry DOI: 10.7270/Q2GQ71GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
NAD-dependent protein deacylase sirtuin-6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 2.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at adrenergic Alpha-1D receptor in rat aorta |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 5
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC5 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacylase sirtuin-6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
| 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Antagonist activity at adrenergic Alpha-1D receptor in rat aorta |
Citation and Details
|
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase-like amidohydrolase
(Alcaligenes sp. (strain DSM 11172) (Bordetella sp....) | BDBM50238632

((6S,9S,12S,14aR)-6-((S)-sec-Butyl)-9-(1-methoxy-1H...)Show SMILES CC[C@H](C)[C@@H]1NC(=O)[C@H](Cc2cn(OC)c3ccccc23)NC(=O)[C@H](CCCCCC(=O)CC)NC(=O)[C@H]2CCCCN2C1=O |r| Show InChI InChI=1S/C34H49N5O6/c1-5-22(3)30-34(44)38-19-13-12-18-29(38)33(43)35-26(16-9-7-8-14-24(40)6-2)31(41)36-27(32(42)37-30)20-23-21-39(45-4)28-17-11-10-15-25(23)28/h10-11,15,17,21-22,26-27,29-30H,5-9,12-14,16,18-20H2,1-4H3,(H,35,43)(H,36,41)(H,37,42)/t22-,26-,27-,29+,30-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of G£ttingen
Curated by ChEMBL
| Assay Description
Displacement of Atto700-HA from Bordetella/Alcaligenes strain FB188 HDAH by fluorescence anisotropy |
Bioorg Med Chem Lett 19: 3651-6 (2009)
Article DOI: 10.1016/j.bmcl.2009.04.102
BindingDB Entry DOI: 10.7270/Q24T6JFV |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 12
(Homo sapiens (Human)) | BDBM50015184
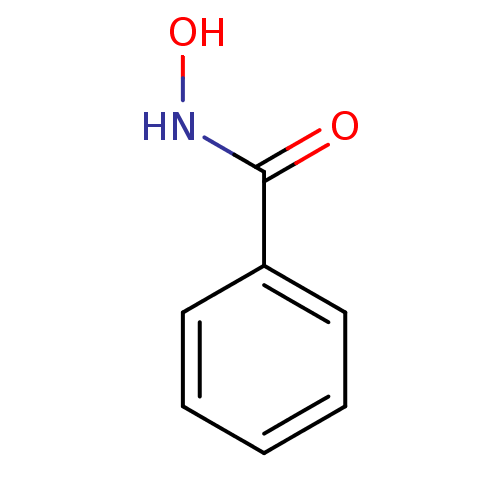
(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 9.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 12 catalytic domain preincubated for 15 mins prior to testing by phenol red based stopped-flow CO2... |
J Med Chem 60: 6428-6439 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00766
BindingDB Entry DOI: 10.7270/Q2GT5QG3 |
More data for this
Ligand-Target Pair | |
Sigma non-opioid intracellular receptor 1
(Cavia porcellus (Guinea pig)) | BDBM50003616
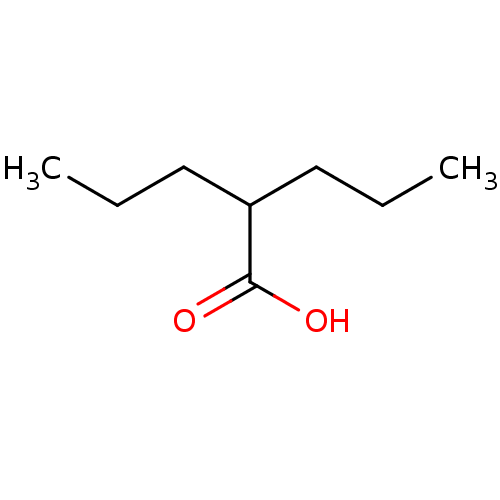
(2-n-propyl-n-valeric acid | CHEMBL109 | DPA | Di-n...)Show InChI InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Displacement of [3H]-pentazocine from sigma1 receptor in guinea pig brain membranes by scintillation counting |
Citation and Details
BindingDB Entry DOI: 10.7270/Q2RR22W8 |
More data for this
Ligand-Target Pair | |
Mandelate racemase
(Pseudomonas putida (g-Proteobacteria)) | BDBM50015184

(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Dalhousie University
Curated by ChEMBL
| Assay Description
Inhibition of mandelate racemase in Pseudomonas putida |
Bioorg Med Chem Lett 17: 105-8 (2006)
Article DOI: 10.1016/j.bmcl.2006.09.079
BindingDB Entry DOI: 10.7270/Q29W0G96 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase 4
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 1.45E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Connecticut
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant HDAC4 by Michaelis-Menten equation analysis |
ACS Med Chem Lett 4: 757-61 (2013)
Article DOI: 10.1021/ml400158k
BindingDB Entry DOI: 10.7270/Q28055HS |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 9
(Homo sapiens (Human)) | BDBM50015184

(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 4.59E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was tested for inhibitory constant of Gamma-amino-N-butyrate transaminase in pig brain at pH of 8.5 and 25 degree C |
J Med Chem 60: 6428-6439 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00766
BindingDB Entry DOI: 10.7270/Q2GT5QG3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 1
(Homo sapiens (Human)) | BDBM50015184

(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 8.31E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human carbonic anhydrase 1 preincubated for 15 mins prior to testing by phenol red based stopped-flow CO2 hydration assay |
J Med Chem 60: 6428-6439 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00766
BindingDB Entry DOI: 10.7270/Q2GT5QG3 |
More data for this
Ligand-Target Pair | |
Carbonic anhydrase 2
(Homo sapiens (Human)) | BDBM50015184

(BENZHYDROXAMIC ACID | BENZOHYDROXAMATE | CHEMBL163...)Show InChI InChI=1S/C7H7NO2/c9-7(8-10)6-4-2-1-3-5-6/h1-5,10H,(H,8,9) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.79E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Firenze
Curated by ChEMBL
| Assay Description
Compound was tested for inhibition of purine nucleoside phosphorylase using human erythro lysate |
J Med Chem 60: 6428-6439 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00766
BindingDB Entry DOI: 10.7270/Q2GT5QG3 |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50003616

(2-n-propyl-n-valeric acid | CHEMBL109 | DPA | Di-n...)Show InChI InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 3.63E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CES1 |
Drug Metab Dispos 41: 698-703 (2013)
Article DOI: 10.1124/dmd.112.050252
BindingDB Entry DOI: 10.7270/Q25D8TKW |
More data for this
Ligand-Target Pair | |
NAD-dependent protein deacylase sirtuin-6
(Homo sapiens (Human)) | BDBM50005711

(CHEBI:46024 | GNF-Pf-1011 | TRICHOSTATIN | Trichos...)Show SMILES C[C@H](\C=C(/C)\C=C\C(=O)NO)C(=O)c1ccc(cc1)N(C)C |r| Show InChI InChI=1S/C17H22N2O3/c1-12(5-10-16(20)18-22)11-13(2)17(21)14-6-8-15(9-7-14)19(3)4/h5-11,13,22H,1-4H3,(H,18,20)/b10-5+,12-11+/t13-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 4.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bayreuth
Curated by ChEMBL
| Assay Description
Inhibition of human SIRT6 preincubated for 5 mins in presence of substrate followed enzyme addition measured after 5 to 10 mins in presence of NAD by... |
J Med Chem 61: 10922-10928 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01455
BindingDB Entry DOI: 10.7270/Q2GQ71GJ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Histone deacetylase
(Homo sapiens (Human)) | BDBM50003616

(2-n-propyl-n-valeric acid | CHEMBL109 | DPA | Di-n...)Show InChI InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 5.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hacettepe University
Curated by ChEMBL
| Assay Description
Inhibition of HDAC in human Hela cells nuclear extracts by fluorimetric assay |
Bioorg Med Chem 17: 5219-28 (2009)
Article DOI: 10.1016/j.bmc.2009.05.042
BindingDB Entry DOI: 10.7270/Q22F7R82 |
More data for this
Ligand-Target Pair | |
UDP-glucuronosyltransferase 2B7
(Homo sapiens (Human)) | BDBM50003616

(2-n-propyl-n-valeric acid | CHEMBL109 | DPA | Di-n...)Show InChI InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 1.60E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The University of British Columbia
Curated by ChEMBL
| Assay Description
Inhibition of AZT glucuronidation by human recombinant UGT2B7 |
Pharmacol Ther 106: 97-132 (2005)
Article DOI: 10.1016/j.pharmthera.2004.10.013
BindingDB Entry DOI: 10.7270/Q2959HZ9 |
More data for this
Ligand-Target Pair | |
Cocaine esterase
(Homo sapiens (Human)) | BDBM50003616

(2-n-propyl-n-valeric acid | CHEMBL109 | DPA | Di-n...)Show InChI InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 7.90E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Eisai Inc.
Curated by ChEMBL
| Assay Description
Inhibition of human CES2 |
Drug Metab Dispos 41: 698-703 (2013)
Article DOI: 10.1124/dmd.112.050252
BindingDB Entry DOI: 10.7270/Q25D8TKW |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2A6
(Homo sapiens (Human)) | BDBM50003616

(2-n-propyl-n-valeric acid | CHEMBL109 | DPA | Di-n...)Show InChI InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PubMed
| 9.15E+6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
Mechanism based inhibition of human cytochrome P450 2A6 measured by coumarin 7-hydroxylation |
Curr Drug Metab 6: 413-54 (2005)
BindingDB Entry DOI: 10.7270/Q2VQ33X3 |
More data for this
Ligand-Target Pair | |
M18 aspartyl aminopeptidase
(Plasmodium falciparum 3D7) | BDBM55151
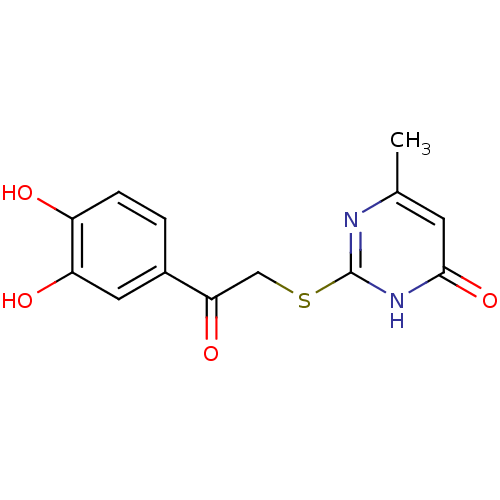
(1-(3,4-Dihydroxy-phenyl)-2-(4-hydroxy-6-methyl-pyr...)Show InChI InChI=1S/C13H12N2O4S/c1-7-4-12(19)15-13(14-7)20-6-11(18)8-2-3-9(16)10(17)5-8/h2-5,16-17H,6H2,1H3,(H,14,15,19) | PDB
KEGG
UniProtKB/TrEMBL
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PCBioAssay
| n/a | n/a | 1.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| Assay Description
Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provide... |
PubChem Bioassay (2009)
BindingDB Entry DOI: 10.7270/Q24B2ZQ2 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM55151

(1-(3,4-Dihydroxy-phenyl)-2-(4-hydroxy-6-methyl-pyr...)Show InChI InChI=1S/C13H12N2O4S/c1-7-4-12(19)15-13(14-7)20-6-11(18)8-2-3-9(16)10(17)5-8/h2-5,16-17H,6H2,1H3,(H,14,15,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PCBioAssay
| n/a | n/a | >5.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| Assay Description
Source (MLPCN Center Name): The Scripps Research Institute Molecular Screening Center Affiliation: The Scripps Research Institute, TSRI Assay Provide... |
PubChem Bioassay (2009)
BindingDB Entry DOI: 10.7270/Q20K2704 |
More data for this
Ligand-Target Pair | |
Guanine nucleotide-binding protein subunit alpha-15
(Homo sapiens (Human)) | BDBM96613
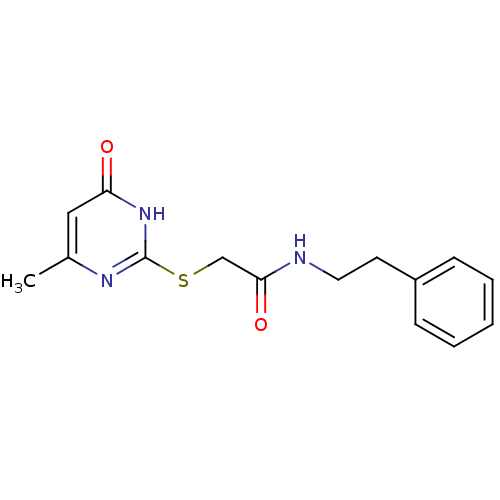
(2-[(4-keto-6-methyl-1H-pyrimidin-2-yl)thio]-N-phen...)Show InChI InChI=1S/C15H17N3O2S/c1-11-9-13(19)18-15(17-11)21-10-14(20)16-8-7-12-5-3-2-4-6-12/h2-6,9H,7-8,10H2,1H3,(H,16,20)(H,17,18,19) | UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| PCBioAssay
| n/a | n/a | n/a | n/a | >2.99E+4 | n/a | n/a | n/a | n/a |
The Scripps Research Institute Molecular Screening Center
Curated by PubChem BioAssay
| |
PubChem Bioassay (2013)
BindingDB Entry DOI: 10.7270/Q2CN72J7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
 Purchase
Purchase Purchase
Purchase Purchase
Purchase
 Purchase
Purchase Purchase
Purchase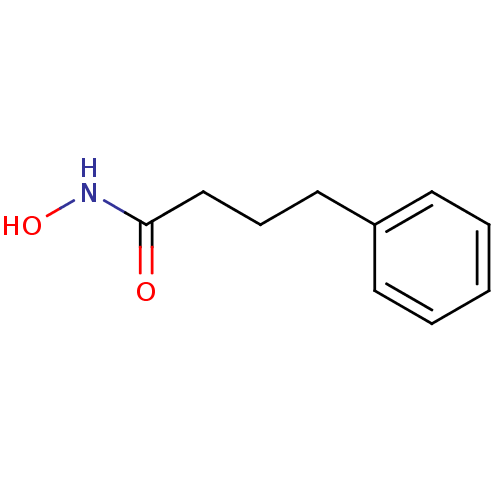 Purchase
Purchase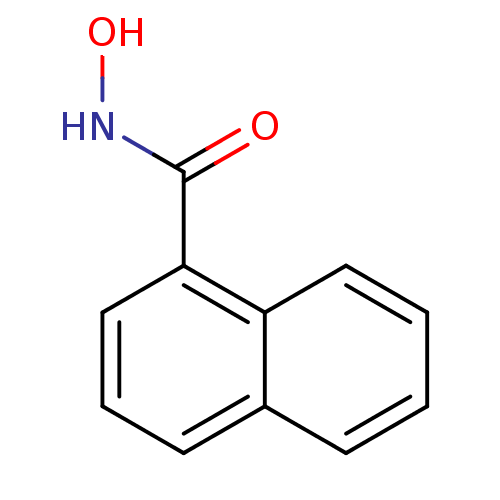 Purchase
Purchase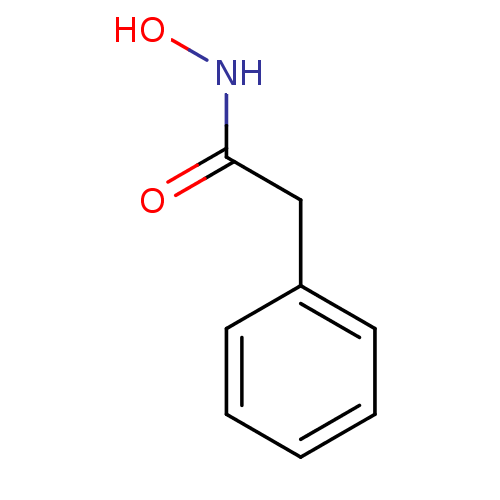 Purchase
Purchase Purchase
Purchase Purchase
Purchase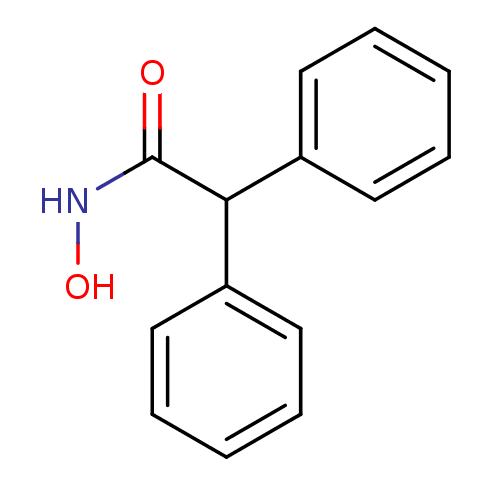 Purchase
Purchase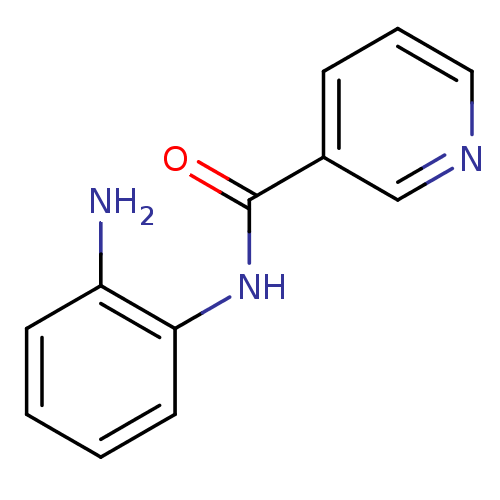 Purchase
Purchase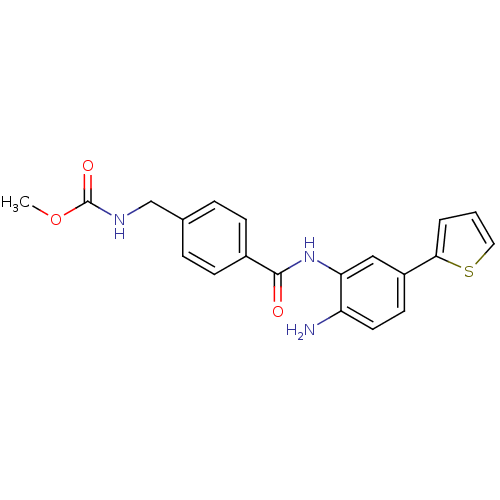 Purchase
Purchase Purchase
Purchase
