 Found 205 hits in this display
Found 205 hits in this display Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM22857
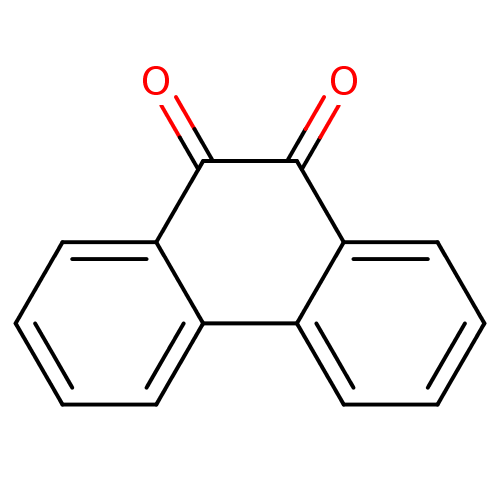
(1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...)Show InChI InChI=1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM22857

(1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...)Show InChI InChI=1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human CE1 using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM16510
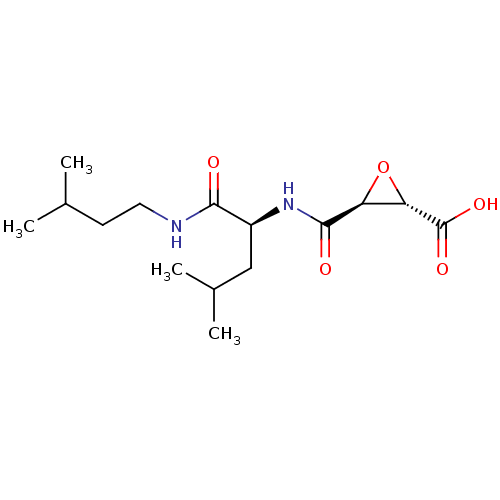
((2S,3S)-3-[[(1S)-1-(isoamylcarbamoyl)-3-methyl-but...)Show SMILES CC(C)CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H]1O[C@@H]1C(O)=O |r| Show InChI InChI=1S/C15H26N2O5/c1-8(2)5-6-16-13(18)10(7-9(3)4)17-14(19)11-12(22-11)15(20)21/h8-12H,5-7H2,1-4H3,(H,16,18)(H,17,19)(H,20,21)/t10-,11-,12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 3.36 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Chemical Laboratory (CSIR-NCL)
Curated by ChEMBL
| Assay Description
Inhibition of cathepsin B |
Bioorg Med Chem 19: 7129-35 (2011)
Article DOI: 10.1016/j.bmc.2011.09.058
BindingDB Entry DOI: 10.7270/Q2KW5GGT |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cocaine esterase
(Homo sapiens (Human)) | BDBM50009219

(2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenant...)Show SMILES CC(C)C1=Cc2ccc3c(CCCC3(C)C)c2C(=O)C1=O |t:3| Show InChI InChI=1S/C19H22O2/c1-11(2)14-10-12-7-8-15-13(6-5-9-19(15,3)4)16(12)18(21)17(14)20/h7-8,10-11H,5-6,9H2,1-4H3 | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human iCE using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Cocaine esterase
(Homo sapiens (Human)) | BDBM22857

(1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...)Show InChI InChI=1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human iCE using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM22857

(1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...)Show InChI InChI=1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 52 | -9.93 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
St. Jude Research Hospital
| Assay Description
CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Cocaine esterase
(Homo sapiens (Human)) | BDBM83922
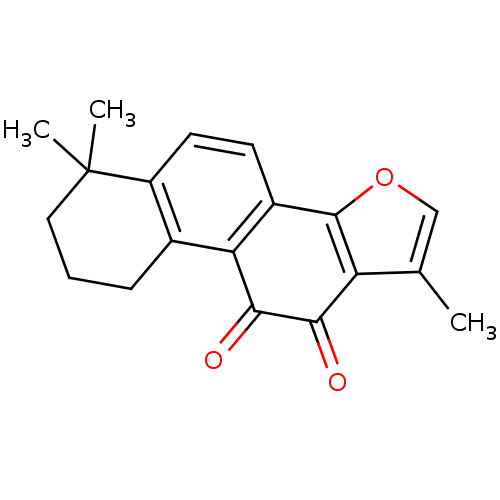
(1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...)Show InChI InChI=1S/C19H18O3/c1-10-9-22-18-12-6-7-13-11(5-4-8-19(13,2)3)15(12)17(21)16(20)14(10)18/h6-7,9H,4-5,8H2,1-3H3 | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 69 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human iCE using CPT-11 as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Cocaine esterase
(Homo sapiens (Human)) | BDBM50009219

(2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenant...)Show SMILES CC(C)C1=Cc2ccc3c(CCCC3(C)C)c2C(=O)C1=O |t:3| Show InChI InChI=1S/C19H22O2/c1-11(2)14-10-12-7-8-15-13(6-5-9-19(15,3)4)16(12)18(21)17(14)20/h7-8,10-11H,5-6,9H2,1-4H3 | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 84 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human iCE using CPT-11 as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Cocaine esterase
(Homo sapiens (Human)) | BDBM53072

((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...)Show InChI InChI=1S/C15H14N2OS3/c1-3-9-17-13(18)12(21-15(17)19)14-16(4-2)10-7-5-6-8-11(10)20-14/h3,5-8H,1,4,9H2,2H3/b14-12- | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 141 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human iCE using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM22857

(1,2-Dione-Based Compound, 14 | 9,10-dihydrophenant...)Show InChI InChI=1S/C14H8O2/c15-13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)14(13)16/h1-8H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50042386
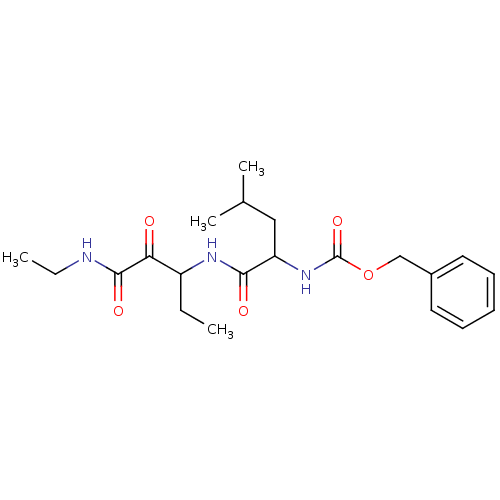
(CHEMBL325147 | [1-(1-Ethylaminooxalyl-propylcarbam...)Show SMILES CCNC(=O)C(=O)C(CC)NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C21H31N3O5/c1-5-16(18(25)20(27)22-6-2)23-19(26)17(12-14(3)4)24-21(28)29-13-15-10-8-7-9-11-15/h7-11,14,16-17H,5-6,12-13H2,1-4H3,(H,22,27)(H,23,26)(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity of alpha-keto esters towards calpain 2 at pH 8.0 |
J Med Chem 36: 3472-80 (1993)
BindingDB Entry DOI: 10.7270/Q2F76BK3 |
More data for this
Ligand-Target Pair | |
Calpain-2 catalytic subunit
(Homo sapiens (Human)) | BDBM50042386

(CHEMBL325147 | [1-(1-Ethylaminooxalyl-propylcarbam...)Show SMILES CCNC(=O)C(=O)C(CC)NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C21H31N3O5/c1-5-16(18(25)20(27)22-6-2)23-19(26)17(12-14(3)4)24-21(28)29-13-15-10-8-7-9-11-15/h7-11,14,16-17H,5-6,12-13H2,1-4H3,(H,22,27)(H,23,26)(H,24,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition of Cysteine protease Calpain 2 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Calpain-1 catalytic subunit
(Homo sapiens (Human)) | BDBM50042386

(CHEMBL325147 | [1-(1-Ethylaminooxalyl-propylcarbam...)Show SMILES CCNC(=O)C(=O)C(CC)NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C21H31N3O5/c1-5-16(18(25)20(27)22-6-2)23-19(26)17(12-14(3)4)24-21(28)29-13-15-10-8-7-9-11-15/h7-11,14,16-17H,5-6,12-13H2,1-4H3,(H,22,27)(H,23,26)(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of the cysteine protease human Calpain 1 |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Calpain small subunit 1/1 catalytic subunit
(Homo sapiens (Human)) | BDBM50042386

(CHEMBL325147 | [1-(1-Ethylaminooxalyl-propylcarbam...)Show SMILES CCNC(=O)C(=O)C(CC)NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C21H31N3O5/c1-5-16(18(25)20(27)22-6-2)23-19(26)17(12-14(3)4)24-21(28)29-13-15-10-8-7-9-11-15/h7-11,14,16-17H,5-6,12-13H2,1-4H3,(H,22,27)(H,23,26)(H,24,28) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibitory activity of alpha-keto esters towards calpain 1 at pH 8.0. |
J Med Chem 36: 3472-80 (1993)
BindingDB Entry DOI: 10.7270/Q2F76BK3 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM20095
(dipeptide-derived nitrile, 16 | tert-butyl N-[(1S)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N |r| Show InChI InChI=1S/C16H21N3O3/c1-16(2,3)22-15(21)19-13(14(20)18-10-9-17)11-12-7-5-4-6-8-12/h4-8,13H,10-11H2,1-3H3,(H,18,20)(H,19,21)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 260 | -8.98 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by spectrophotometric detection of the product p-nitroaniline (pNA) at waveleng... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |
Cocaine esterase
(Homo sapiens (Human)) | BDBM53072

((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...)Show InChI InChI=1S/C15H14N2OS3/c1-3-9-17-13(18)12(21-15(17)19)14-16(4-2)10-7-5-6-8-11(10)20-14/h3,5-8H,1,4,9H2,2H3/b14-12- | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 290 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human iCE using CPT-11 as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM22851
(1,2-Dione-Based Compound, 8 | 1,2-dihydronaphthale...)Show InChI InChI=1S/C10H6O2/c11-9-6-5-7-3-1-2-4-8(7)10(9)12/h1-6H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM20094

(dipeptide-derived nitrile, 15 | tert-butyl N-[(S)-...)Show SMILES CC(C)(C)OC(=O)N[C@H](C(=O)NCC#N)c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O3/c1-15(2,3)21-14(20)18-12(13(19)17-10-9-16)11-7-5-4-6-8-11/h4-8,12H,10H2,1-3H3,(H,17,19)(H,18,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| >330 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Cbz-Arg-Arg-pNA as substrate at pH 6 incubated for 30 mins measured for 20 mins by photometrical analysis |
ACS Med Chem Lett 7: 211-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00474
BindingDB Entry DOI: 10.7270/Q2TH8PMM |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Bos taurus (bovine)) | BDBM20095

(dipeptide-derived nitrile, 16 | tert-butyl N-[(1S)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N |r| Show InChI InChI=1S/C16H21N3O3/c1-16(2,3)22-15(21)19-13(14(20)18-10-9-17)11-12-7-5-4-6-8-12/h4-8,13H,10-11H2,1-3H3,(H,18,20)(H,19,21)/t13-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 520 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by fluorimetric detection of the product AMC at the wavelengths for excitation ... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM53072

((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...)Show InChI InChI=1S/C15H14N2OS3/c1-3-9-17-13(18)12(21-15(17)19)14-16(4-2)10-7-5-6-8-11(10)20-14/h3,5-8H,1,4,9H2,2H3/b14-12- | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 544 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human CE1 using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM20095

(dipeptide-derived nitrile, 16 | tert-butyl N-[(1S)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N |r| Show InChI InChI=1S/C16H21N3O3/c1-16(2,3)22-15(21)19-13(14(20)18-10-9-17)11-12-7-5-4-6-8-12/h4-8,13H,10-11H2,1-3H3,(H,18,20)(H,19,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 620 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by fluorimetric detection of the product AMC at the wavelengths for excitation ... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20095

(dipeptide-derived nitrile, 16 | tert-butyl N-[(1S)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N |r| Show InChI InChI=1S/C16H21N3O3/c1-16(2,3)22-15(21)19-13(14(20)18-10-9-17)11-12-7-5-4-6-8-12/h4-8,13H,10-11H2,1-3H3,(H,18,20)(H,19,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by fluorimetric detection of the product AMC at the wavelengths for excitation ... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM22851

(1,2-Dione-Based Compound, 8 | 1,2-dihydronaphthale...)Show InChI InChI=1S/C10H6O2/c11-9-6-5-7-3-1-2-4-8(7)10(9)12/h1-6H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 930 | -8.22 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
St. Jude Research Hospital
| Assay Description
CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Homo sapiens (Human)) | BDBM20095

(dipeptide-derived nitrile, 16 | tert-butyl N-[(1S)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N |r| Show InChI InChI=1S/C16H21N3O3/c1-16(2,3)22-15(21)19-13(14(20)18-10-9-17)11-12-7-5-4-6-8-12/h4-8,13H,10-11H2,1-3H3,(H,18,20)(H,19,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.26E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human cathepsin L using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substrate addition by fluor... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] B
(Homo sapiens (Human)) | BDBM24776
(1,4-Naphthoquinone | 1,4-Naphthoquinone (5a) | 1,4...)Show InChI InChI=1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Rio de Janeiro
Curated by ChEMBL
| Assay Description
Competitive inhibition of human recombinant MAO-B expressed in baculovirus infected insect cells using benzylamine as substrate by Lineweaver-Burk pl... |
Bioorg Med Chem 19: 7416-24 (2011)
Article DOI: 10.1016/j.bmc.2011.10.049
BindingDB Entry DOI: 10.7270/Q2571CFB |
More data for this
Ligand-Target Pair | |
Cathepsin S
(Homo sapiens (Human)) | BDBM20094

(dipeptide-derived nitrile, 15 | tert-butyl N-[(S)-...)Show SMILES CC(C)(C)OC(=O)N[C@H](C(=O)NCC#N)c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O3/c1-15(2,3)21-14(20)18-12(13(19)17-10-9-16)11-7-5-4-6-8-11/h4-8,12H,10H2,1-3H3,(H,17,19)(H,18,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by fluorimetric detection of the product AMC at the wavelengths for excitation ... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |
Cholinesterase
(Homo sapiens (Human)) | BDBM22851

(1,2-Dione-Based Compound, 8 | 1,2-dihydronaphthale...)Show InChI InChI=1S/C10H6O2/c11-9-6-5-7-3-1-2-4-8(7)10(9)12/h1-6H | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Research Hospital
| Assay Description
The cholinesterase assays were performed using colorimetric method reported by Ellman. Estimates of the competitive inhibition constants (Ki) were ob... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Coagulation factor XII
(Homo sapiens (Human)) | BDBM22851

(1,2-Dione-Based Compound, 8 | 1,2-dihydronaphthale...)Show InChI InChI=1S/C10H6O2/c11-9-6-5-7-3-1-2-4-8(7)10(9)12/h1-6H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.40E+3 | -7.66 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
St. Jude Research Hospital
| Assay Description
CE inhibition was determined using a spectrophotometric multiwell plate assay with o-NPA as a substrate. The rate of change in absorbance at 420 nm w... |
J Med Chem 50: 5727-34 (2007)
Article DOI: 10.1021/jm0706867
BindingDB Entry DOI: 10.7270/Q2Q52MWQ |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Bos taurus (bovine)) | BDBM50042386

(CHEMBL325147 | [1-(1-Ethylaminooxalyl-propylcarbam...)Show SMILES CCNC(=O)C(=O)C(CC)NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C21H31N3O5/c1-5-16(18(25)20(27)22-6-2)23-19(26)17(12-14(3)4)24-21(28)29-13-15-10-8-7-9-11-15/h7-11,14,16-17H,5-6,12-13H2,1-4H3,(H,22,27)(H,23,26)(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 5.2 | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of alpha-keto esters towards cathepsin B at pH 5.2 |
J Med Chem 36: 3472-80 (1993)
BindingDB Entry DOI: 10.7270/Q2F76BK3 |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM16510

((2S,3S)-3-[[(1S)-1-(isoamylcarbamoyl)-3-methyl-but...)Show SMILES CC(C)CCNC(=O)[C@H](CC(C)C)NC(=O)[C@H]1O[C@@H]1C(O)=O |r| Show InChI InChI=1S/C15H26N2O5/c1-8(2)5-6-16-13(18)10(7-9(3)4)17-14(19)11-12(22-11)15(20)21/h8-12H,5-7H2,1-4H3,(H,16,18)(H,17,19)(H,20,21)/t10-,11-,12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Wisconsin
Curated by ChEMBL
| Assay Description
Kinetic constant Apparent binding constant (Ki`) for the inhibition of papain conducted in 0.1 M phosphate, pH 6.8, at 30 degree C |
J Med Chem 39: 3357-66 (1996)
Article DOI: 10.1021/jm950445b
BindingDB Entry DOI: 10.7270/Q2B56HTP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cathepsin B
(Homo sapiens (Human)) | BDBM50042386

(CHEMBL325147 | [1-(1-Ethylaminooxalyl-propylcarbam...)Show SMILES CCNC(=O)C(=O)C(CC)NC(=O)C(CC(C)C)NC(=O)OCc1ccccc1 Show InChI InChI=1S/C21H31N3O5/c1-5-16(18(25)20(27)22-6-2)23-19(26)17(12-14(3)4)24-21(28)29-13-15-10-8-7-9-11-15/h7-11,14,16-17H,5-6,12-13H2,1-4H3,(H,22,27)(H,23,26)(H,24,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
School of Chemistry and Biochemistry
Curated by ChEMBL
| Assay Description
Inhibition of human cathepsin B |
J Med Chem 39: 4089-98 (1996)
Article DOI: 10.1021/jm950541c
BindingDB Entry DOI: 10.7270/Q2TX3DGR |
More data for this
Ligand-Target Pair | |
Cocaine esterase
(Homo sapiens (Human)) | BDBM83922

(1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...)Show InChI InChI=1S/C19H18O3/c1-10-9-22-18-12-6-7-13-11(5-4-8-19(13,2)3)15(12)17(21)16(20)14(10)18/h6-7,9H,4-5,8H2,1-3H3 | NCI pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.45E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human iCE using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Cruzipain
(Trypanosoma cruzi) | BDBM20095

(dipeptide-derived nitrile, 16 | tert-butyl N-[(1S)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N |r| Show InChI InChI=1S/C16H21N3O3/c1-16(2,3)22-15(21)19-13(14(20)18-10-9-17)11-12-7-5-4-6-8-12/h4-8,13H,10-11H2,1-3H3,(H,18,20)(H,19,21)/t13-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of recombinant Trypanosoma cruzi cruzain using Z-Phe-Arg-7-amido-4-methylcoumarin as substrate preincubated for 2 mins followed by substra... |
Citation and Details
Article DOI: 10.1016/j.bmc.2020.115743
BindingDB Entry DOI: 10.7270/Q27H1P7R |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50009219

(2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenant...)Show SMILES CC(C)C1=Cc2ccc3c(CCCC3(C)C)c2C(=O)C1=O |t:3| Show InChI InChI=1S/C19H22O2/c1-11(2)14-10-12-7-8-15-13(6-5-9-19(15,3)4)16(12)18(21)17(14)20/h7-8,10-11H,5-6,9H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human liver carboxylesterase 1 using o-nitrophenyl acetate as substrate |
Eur J Med Chem 149: 79-89 (2018)
Article DOI: 10.1016/j.ejmech.2018.02.052
BindingDB Entry DOI: 10.7270/Q2K076WP |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM50009219

(2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenant...)Show SMILES CC(C)C1=Cc2ccc3c(CCCC3(C)C)c2C(=O)C1=O |t:3| Show InChI InChI=1S/C19H22O2/c1-11(2)14-10-12-7-8-15-13(6-5-9-19(15,3)4)16(12)18(21)17(14)20/h7-8,10-11H,5-6,9H2,1-4H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.53E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human CE1 using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM83922

(1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...)Show InChI InChI=1S/C19H18O3/c1-10-9-22-18-12-6-7-13-11(5-4-8-19(13,2)3)15(12)17(21)16(20)14(10)18/h6-7,9H,4-5,8H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| | 2.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM20095

(dipeptide-derived nitrile, 16 | tert-butyl N-[(1S)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N |r| Show InChI InChI=1S/C16H21N3O3/c1-16(2,3)22-15(21)19-13(14(20)18-10-9-17)11-12-7-5-4-6-8-12/h4-8,13H,10-11H2,1-3H3,(H,18,20)(H,19,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by fluorimetric detection of the product AMC at the wavelengths for excitation ... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |
Acetylcholinesterase
(Homo sapiens (Human)) | BDBM53072

((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...)Show InChI InChI=1S/C15H14N2OS3/c1-3-9-17-13(18)12(21-15(17)19)14-16(4-2)10-7-5-6-8-11(10)20-14/h3,5-8H,1,4,9H2,2H3/b14-12- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Liver carboxylesterase 1
(Homo sapiens (Human)) | BDBM83922

(1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...)Show InChI InChI=1S/C19H18O3/c1-10-9-22-18-12-6-7-13-11(5-4-8-19(13,2)3)15(12)17(21)16(20)14(10)18/h6-7,9H,4-5,8H2,1-3H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.89E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
St. Jude Children's Research Hospital
Curated by ChEMBL
| Assay Description
Inhibition of human CE1 using o-NPA as substrate by spectrophotometric assay |
J Nat Prod 76: 36-44 (2013)
Article DOI: 10.1021/np300628a
BindingDB Entry DOI: 10.7270/Q2VX0HWJ |
More data for this
Ligand-Target Pair | |
Amine oxidase [flavin-containing] A
(Homo sapiens (Human)) | BDBM24776

(1,4-Naphthoquinone | 1,4-Naphthoquinone (5a) | 1,4...)Show InChI InChI=1S/C10H6O2/c11-9-5-6-10(12)8-4-2-1-3-7(8)9/h1-6H | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Federal University of Rio de Janeiro
Curated by ChEMBL
| Assay Description
Noncompetitive inhibition of human recombinant MAO-A expressed in baculovirus infected insect cells using p-tyramine as substrate by Lineweaver-Burk ... |
Bioorg Med Chem 19: 7416-24 (2011)
Article DOI: 10.1016/j.bmc.2011.10.049
BindingDB Entry DOI: 10.7270/Q2571CFB |
More data for this
Ligand-Target Pair | |
Streptopain
(Streptococcus pyogenes (Firmicutes)) | BDBM152724
(2477)Show InChI InChI=1S/C10H10N2O2/c11-6-7-12-10(13)14-8-9-4-2-1-3-5-9/h1-5H,7-8H2,(H,12,13) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| 8.00E+3 | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Scripps Research Institute
| Assay Description
SpeB was incubated at 50 nM in the presence of increasing amounts of inhibitor (3 μM to 200 μM) in a reaction buffer consisting of PBS at p... |
Biochemistry 54: 4365-73 (2015)
Article DOI: 10.1021/acs.biochem.5b00607
BindingDB Entry DOI: 10.7270/Q2KD1WNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM53072

((5Z)-3-allyl-5-(3-ethyl-1,3-benzothiazol-2-ylidene...)Show InChI InChI=1S/C15H14N2OS3/c1-3-9-17-13(18)12(21-15(17)19)14-16(4-2)10-7-5-6-8-11(10)20-14/h3,5-8H,1,4,9H2,2H3/b14-12- | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... |
Bioorg Med Chem 20: 5928-35 (2012)
Article DOI: 10.1016/j.bmc.2012.07.038
BindingDB Entry DOI: 10.7270/Q2QF8TZ4 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM83922

(1,6,6-trimethyl-8,9-dihydro-7H-naphtho[1,2-g][1]be...)Show InChI InChI=1S/C19H18O3/c1-10-9-22-18-12-6-7-13-11(5-4-8-19(13,2)3)15(12)17(21)16(20)14(10)18/h6-7,9H,4-5,8H2,1-3H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.12E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... |
Bioorg Med Chem 20: 5928-35 (2012)
Article DOI: 10.1016/j.bmc.2012.07.038
BindingDB Entry DOI: 10.7270/Q2QF8TZ4 |
More data for this
Ligand-Target Pair | |
Replicase polyprotein 1a
(Human SARS coronavirus (SARS-CoV)) | BDBM50009219

(2-isopropyl-8,8-dimethyl-5,6,7,8-tetrahydrophenant...)Show SMILES CC(C)C1=Cc2ccc3c(CCCC3(C)C)c2C(=O)C1=O |t:3| Show InChI InChI=1S/C19H22O2/c1-11(2)14-10-12-7-8-15-13(6-5-9-19(15,3)4)16(12)18(21)17(14)20/h7-8,10-11H,5-6,9H2,1-4H3 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Korea Research Institute of Bioscience and Biotechnology
Curated by ChEMBL
| Assay Description
Time dependent inhibition of SARS-CoV PLpro expressed in Escherichia coli BL21 (DE3) using Arg-Leu-Arg-Gly-Gly-AMC as substrate at 3 to 100 uM up to ... |
Bioorg Med Chem 20: 5928-35 (2012)
Article DOI: 10.1016/j.bmc.2012.07.038
BindingDB Entry DOI: 10.7270/Q2QF8TZ4 |
More data for this
Ligand-Target Pair | |
Procathepsin L
(Bos taurus (bovine)) | BDBM20094

(dipeptide-derived nitrile, 15 | tert-butyl N-[(S)-...)Show SMILES CC(C)(C)OC(=O)N[C@H](C(=O)NCC#N)c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O3/c1-15(2,3)21-14(20)18-12(13(19)17-10-9-16)11-7-5-4-6-8-11/h4-8,12H,10H2,1-3H3,(H,17,19)(H,18,20)/t12-/m0/s1 | Reactome pathway
KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by fluorimetric detection of the product AMC at the wavelengths for excitation ... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |
Cathepsin B
(Homo sapiens (Human)) | BDBM20095

(dipeptide-derived nitrile, 16 | tert-butyl N-[(1S)...)Show SMILES CC(C)(C)OC(=O)N[C@@H](Cc1ccccc1)C(=O)NCC#N |r| Show InChI InChI=1S/C16H21N3O3/c1-16(2,3)22-15(21)19-13(14(20)18-10-9-17)11-12-7-5-4-6-8-12/h4-8,13H,10-11H2,1-3H3,(H,18,20)(H,19,21)/t13-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.0 | n/a |
University of Bonn
Curated by ChEMBL
| Assay Description
Inhibition of human liver cathepsin B using Cbz-Arg-Arg-pNA as substrate at pH 6 incubated for 30 mins measured for 20 mins by photometrical analysis |
ACS Med Chem Lett 7: 211-6 (2016)
Article DOI: 10.1021/acsmedchemlett.5b00474
BindingDB Entry DOI: 10.7270/Q2TH8PMM |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM152724

(2477)Show InChI InChI=1S/C10H10N2O2/c11-6-7-12-10(13)14-8-9-4-2-1-3-5-9/h1-5H,7-8H2,(H,12,13) | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| 4.50E+4 | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | 7.4 | n/a |
The Scripps Research Institute
| Assay Description
SpeB was incubated at 50 nM in the presence of increasing amounts of inhibitor (3 μM to 200 μM) in a reaction buffer consisting of PBS at p... |
Biochemistry 54: 4365-73 (2015)
Article DOI: 10.1021/acs.biochem.5b00607
BindingDB Entry DOI: 10.7270/Q2KD1WNP |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM50052693
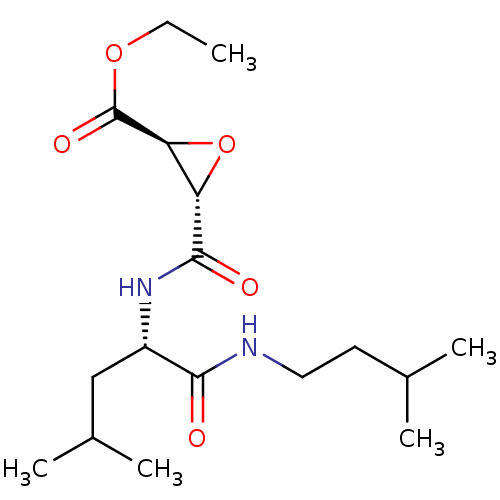
((2S,3S)-3-[(S)-3-Methyl-1-(3-methyl-butylcarbamoyl...)Show SMILES CCOC(=O)[C@H]1O[C@@H]1C(=O)N[C@@H](CC(C)C)C(=O)NCCC(C)C |r| Show InChI InChI=1S/C17H30N2O5/c1-6-23-17(22)14-13(24-14)16(21)19-12(9-11(4)5)15(20)18-8-7-10(2)3/h10-14H,6-9H2,1-5H3,(H,18,20)(H,19,21)/t12-,13-,14-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 6.8 | n/a |
University of Wisconsin
Curated by ChEMBL
| Assay Description
Kinetic constant Apparent binding constant (Ki`) for the inhibition of papain conducted in 0.1 M phosphate, pH 6.8, at 30 degree C |
J Med Chem 39: 3357-66 (1996)
Article DOI: 10.1021/jm950445b
BindingDB Entry DOI: 10.7270/Q2B56HTP |
More data for this
Ligand-Target Pair | |
Papain
(Carica papaya) | BDBM20094

(dipeptide-derived nitrile, 15 | tert-butyl N-[(S)-...)Show SMILES CC(C)(C)OC(=O)N[C@H](C(=O)NCC#N)c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O3/c1-15(2,3)21-14(20)18-12(13(19)17-10-9-16)11-7-5-4-6-8-11/h4-8,12H,10H2,1-3H3,(H,17,19)(H,18,20)/t12-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8.30E+4 | -5.56 | n/a | n/a | n/a | n/a | n/a | 6.5 | 25 |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by spectrophotometric detection of the product p-nitroaniline (pNA) at waveleng... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |
Cathepsin K
(Homo sapiens (Human)) | BDBM20094

(dipeptide-derived nitrile, 15 | tert-butyl N-[(S)-...)Show SMILES CC(C)(C)OC(=O)N[C@H](C(=O)NCC#N)c1ccccc1 |r| Show InChI InChI=1S/C15H19N3O3/c1-15(2,3)21-14(20)18-12(13(19)17-10-9-16)11-7-5-4-6-8-11/h4-8,12H,10H2,1-3H3,(H,17,19)(H,18,20)/t12-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Rheinische Friedrich-Wilhelms-Universitat Bonn
| Assay Description
Enzyme activities were calculated from kinetic measurements performed by fluorimetric detection of the product AMC at the wavelengths for excitation ... |
J Med Chem 48: 7688-707 (2005)
Article DOI: 10.1021/jm050686b
BindingDB Entry DOI: 10.7270/Q2GB22BG |
More data for this
Ligand-Target Pair | |