 Found 8621 hits Enz. Inhib. hit(s) with Target = 'RAC-alpha serine/threonine-protein kinase'
Found 8621 hits Enz. Inhib. hit(s) with Target = 'RAC-alpha serine/threonine-protein kinase' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278693
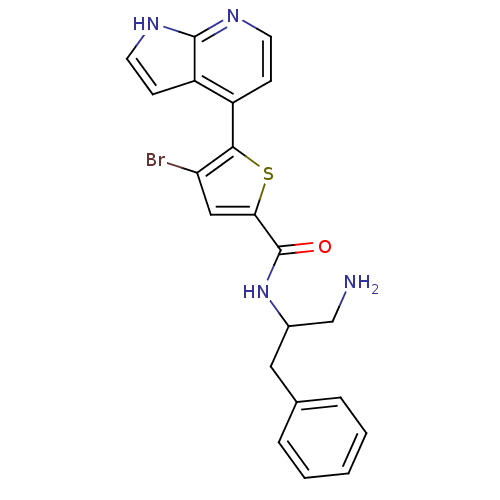
((+/-)-N-(1-amino-3-phenylpropan-2-yl)-4-bromo-5-(1...)Show SMILES NCC(Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098
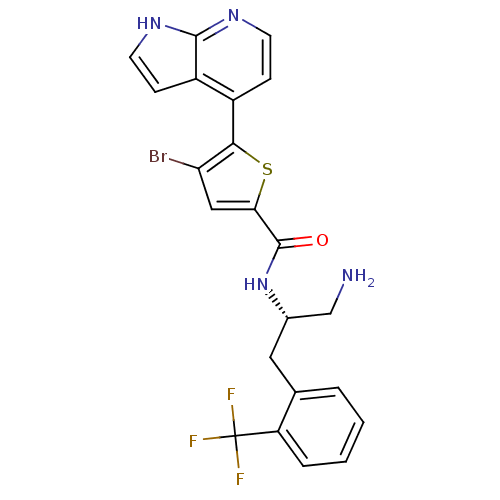
(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278837
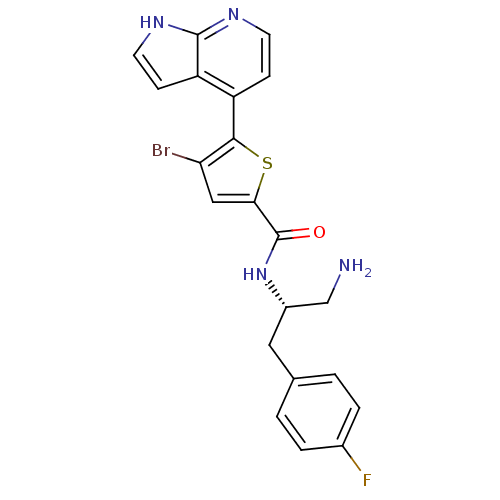
(CHEMBL496690 | N-((S)-1-amino-3-(4-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-5-7-25-20-16(15)6-8-26-20)21(28)27-14(11-24)9-12-1-3-13(23)4-2-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50502477
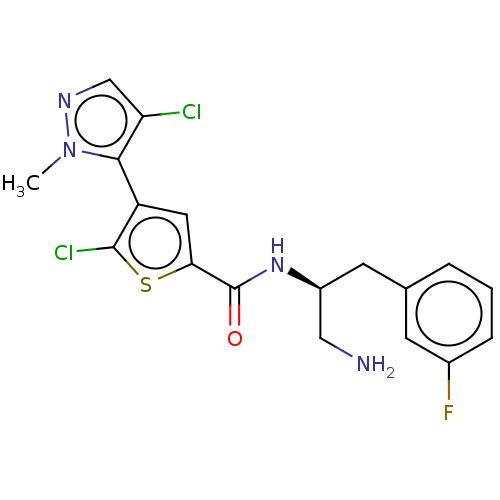
(ASB-183 | ASB183 | Afuresertib | GSK-2110183C | GS...)Show SMILES [H][C@@](CN)(Cc1cccc(F)c1)NC(=O)c1cc(c(Cl)s1)-c1c(Cl)cnn1C |r,wU:1.1,wD:1.0,(11.52,-5.94,;10.83,-4.68,;10.06,-6.01,;10.83,-7.35,;12.17,-3.88,;13.49,-4.64,;13.48,-6.2,;14.82,-6.95,;16.15,-6.19,;16.19,-4.64,;17.46,-3.84,;14.79,-3.91,;9.41,-3.84,;8.08,-4.6,;8.11,-6.13,;6.85,-3.92,;5.41,-4.51,;4.4,-3.4,;5.15,-2.04,;4.33,-.64,;6.62,-2.35,;2.85,-3.54,;1.85,-2.41,;2.36,-.88,;.42,-3.01,;.5,-4.52,;2.04,-4.83,;2.44,-6.32,)| Show InChI InChI=1S/C18H17Cl2FN4OS/c1-25-16(14(19)9-23-25)13-7-15(27-17(13)20)18(26)24-12(8-22)6-10-3-2-4-11(21)5-10/h2-5,7,9,12H,6,8,22H2,1H3,(H,24,26)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Hangzhou Institute of Innovative Medicine
Curated by ChEMBL
| Assay Description
Inhibition of Akt1 (unknown origin) |
Eur J Med Chem 180: 72-85 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.017
BindingDB Entry DOI: 10.7270/Q2Q243H9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099
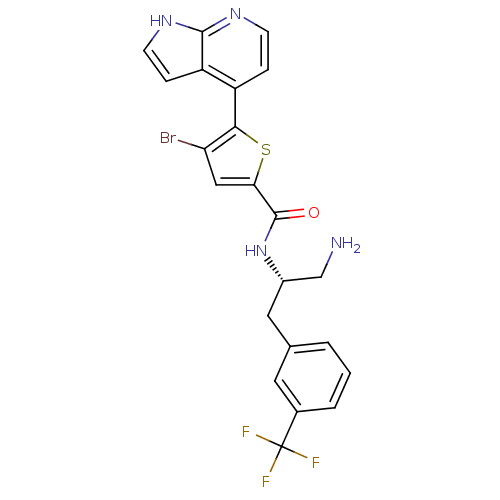
(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099

(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131
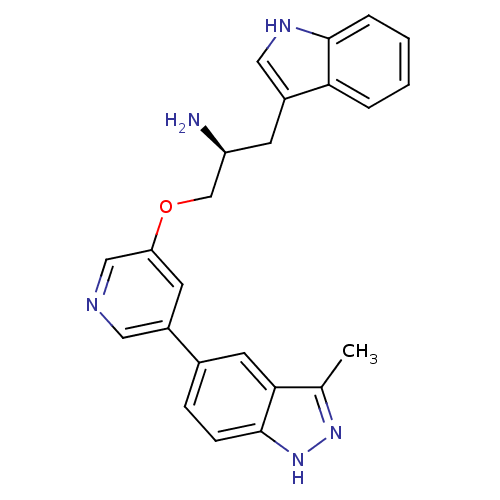
(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.0c01467
BindingDB Entry DOI: 10.7270/Q24Q801K |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15131

(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | -55.3 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM15131

(5-indazolyl pyridine 3 | 5-{5-[(2S)-2-amino-3-(1H-...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C24H23N5O/c1-15-22-10-16(6-7-24(22)29-28-15)17-9-20(13-26-11-17)30-14-19(25)8-18-12-27-23-5-3-2-4-21(18)23/h2-7,9-13,19,27H,8,14,25H2,1H3,(H,28,29)/t19-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of California
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Nat Chem Biol 5: 484-93 (2009)
Article DOI: 10.1038/nchembio.183
BindingDB Entry DOI: 10.7270/Q2D21XTB |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183
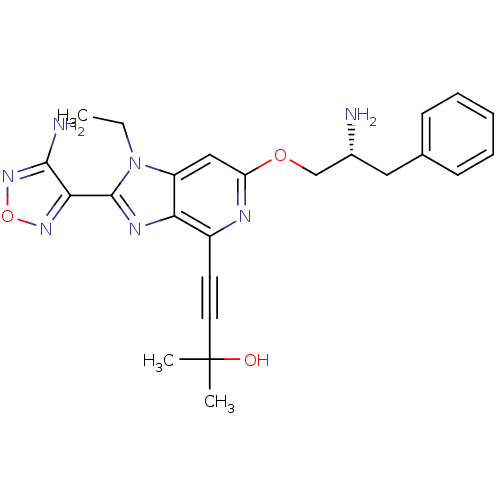
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192
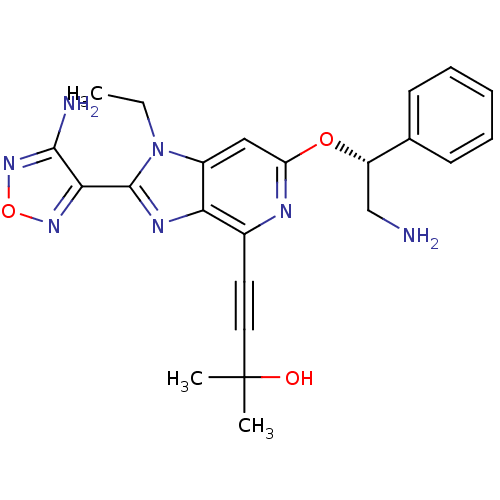
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278836
(CHEMBL523586 | N-((S)-1-amino-3-(3-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1cccc(F)c1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-4-6-25-20-16(15)5-7-26-20)21(28)27-14(11-24)9-12-2-1-3-13(23)8-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.630 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM431867
(US10550114, Compound 1a)Show SMILES C=CC(=O)Nc1ccc2[nH]c(=O)n(C3CCN(Cc4ccc(cc4)-c4nc5cc[nH]c(=O)c5cc4-c4ccccc4)CC3)c2c1 Show InChI InChI=1S/C36H32N6O3/c1-2-33(43)38-26-12-13-31-32(20-26)42(36(45)40-31)27-15-18-41(19-16-27)22-23-8-10-25(11-9-23)34-28(24-6-4-3-5-7-24)21-29-30(39-34)14-17-37-35(29)44/h2-14,17,20-21,27H,1,15-16,18-19,22H2,(H,37,44)(H,38,43)(H,40,45) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND
US Patent
| Assay Description
iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... |
US Patent US10550114 (2020)
BindingDB Entry DOI: 10.7270/Q2DV1N97 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316184

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013
(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15136
(5-indazolyl pyridine 11e | 5-{5-[(2S)-2-amino-3-(2...)Show SMILES COc1ccc2ccccc2c1C[C@H](N)COc1cncc(c1)-c1ccc2[nH]nc(C)c2c1 |r| Show InChI InChI=1S/C27H26N4O2/c1-17-24-12-19(7-9-26(24)31-30-17)20-11-22(15-29-14-20)33-16-21(28)13-25-23-6-4-3-5-18(23)8-10-27(25)32-2/h3-12,14-15,21H,13,16,28H2,1-2H3,(H,30,31)/t21-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.10 | -50.6 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316185
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-3-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CCN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-17-14-19(33-18(11-13-25)15-8-6-5-7-9-15)27-16(10-12-24(2,3)32)20(17)28-23(31)21-22(26)30-34-29-21/h5-9,14,18,32H,4,11,13,25H2,1-3H3,(H2,26,30)/t18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM431879
(US10550114, Compound 33)Show SMILES Clc1cc2[nH]c(=O)n(N3CCN(Cc4ccc(cc4)-c4nc5cc[nH]c(=O)c5cc4-c4ccccc4)CC3)c2cc1NC(=O)C=C Show InChI InChI=1S/C35H30ClN7O3/c1-2-32(44)38-29-20-31-30(19-27(29)36)40-35(46)43(31)42-16-14-41(15-17-42)21-22-8-10-24(11-9-22)33-25(23-6-4-3-5-7-23)18-26-28(39-33)12-13-37-34(26)45/h2-13,18-20H,1,14-17,21H2,(H,37,45)(H,38,44)(H,40,46) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND
US Patent
| Assay Description
iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... |
US Patent US10550114 (2020)
BindingDB Entry DOI: 10.7270/Q2DV1N97 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278833
(CHEMBL498051 | N-((S)-1-amino-3-(pyridin-4-yl)prop...)Show SMILES NC[C@H](Cc1ccncc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C20H18BrN5OS/c21-16-10-17(20(27)26-13(11-22)9-12-1-5-23-6-2-12)28-18(16)14-3-7-24-19-15(14)4-8-25-19/h1-8,10,13H,9,11,22H2,(H,24,25)(H,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278837

(CHEMBL496690 | N-((S)-1-amino-3-(4-fluorophenyl)pr...)Show SMILES NC[C@H](Cc1ccc(F)cc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H18BrFN4OS/c22-17-10-18(29-19(17)15-5-7-25-20-16(15)6-8-26-20)21(28)27-14(11-24)9-12-1-3-13(23)4-2-12/h1-8,10,14H,9,11,24H2,(H,25,26)(H,27,28)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15067
((2S)-1-(1H-indol-3-yl)-3-(5-isoquinolin-6-ylpyridi...)Show SMILES N[C@H](COc1cncc(c1)-c1ccc2cnccc2c1)Cc1c[nH]c2ccccc12 |r| Show InChI InChI=1S/C25H22N4O/c26-22(10-21-14-29-25-4-2-1-3-24(21)25)16-30-23-11-20(13-28-15-23)17-5-6-19-12-27-8-7-18(19)9-17/h1-9,11-15,22,29H,10,16,26H2/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| 2 | -49.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278100
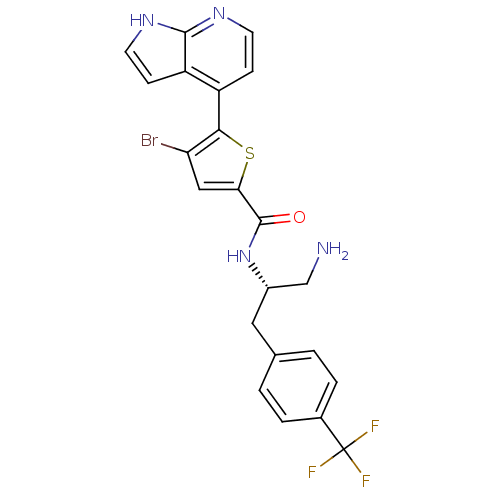
(CHEMBL482537 | N-((S)-1-amino-3-(4-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccc(cc1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-5-7-28-20-16(15)6-8-29-20)21(31)30-14(11-27)9-12-1-3-13(4-2-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT1 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316183

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(OC[C@H](N)Cc3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C24H27N7O3/c1-4-31-18-13-19(33-14-16(25)12-15-8-6-5-7-9-15)27-17(10-11-24(2,3)32)20(18)28-23(31)21-22(26)30-34-29-21/h5-9,13,16,32H,4,12,14,25H2,1-3H3,(H2,26,30)/t16-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15132
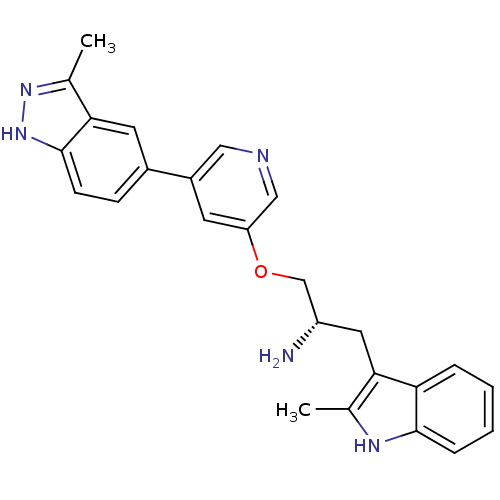
(5-indazolyl pyridine 11a | 5-{5-[(2S)-2-amino-3-(2...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2c(C)[nH]c3ccccc23)c1 |r| Show InChI InChI=1S/C25H25N5O/c1-15-22(21-5-3-4-6-24(21)28-15)11-19(26)14-31-20-9-18(12-27-13-20)17-7-8-25-23(10-17)16(2)29-30-25/h3-10,12-13,19,28H,11,14,26H2,1-2H3,(H,29,30)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 2.10 | -49.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278770

(CHEMBL470597 | N-((S)-1-amino-3-phenylpropan-2-yl)...)Show SMILES NC[C@H](Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316182
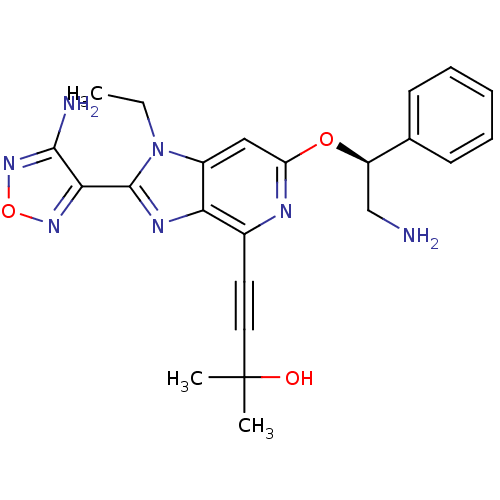
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((S)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15134

(5-indazolyl pyridine 11c | 5-{5-[(2S)-2-amino-3-(n...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2ccc3ccccc3c2)c1 |r| Show InChI InChI=1S/C26H24N4O/c1-17-25-13-21(8-9-26(25)30-29-17)22-12-24(15-28-14-22)31-16-23(27)11-18-6-7-19-4-2-3-5-20(19)10-18/h2-10,12-15,23H,11,16,27H2,1H3,(H,29,30)/t23-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.20 | -48.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15125
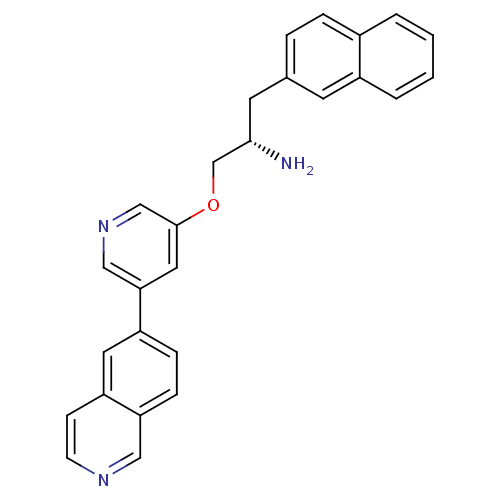
(5-isoquinolinyl pyridine 10d | 6-{5-[(2S)-2-amino-...)Show SMILES N[C@H](COc1cncc(c1)-c1ccc2cnccc2c1)Cc1ccc2ccccc2c1 |r| Show InChI InChI=1S/C27H23N3O/c28-26(12-19-5-6-20-3-1-2-4-21(20)11-19)18-31-27-14-25(16-30-17-27)22-7-8-24-15-29-10-9-23(24)13-22/h1-11,13-17,26H,12,18,28H2/t26-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 3.5 | -47.8 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098

(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM25013

(4-[2-(4-amino-1,2,5-oxadiazol-3-yl)-1-ethyl-7-[(3S...)Show SMILES CCn1c(nc2c(ncc(OC[C@H]3CCCNC3)c12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C21H27N7O3/c1-4-28-18-15(30-12-13-6-5-9-23-10-13)11-24-14(7-8-21(2,3)29)16(18)25-20(28)17-19(22)27-31-26-17/h11,13,23,29H,4-6,9-10,12H2,1-3H3,(H2,22,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50310456
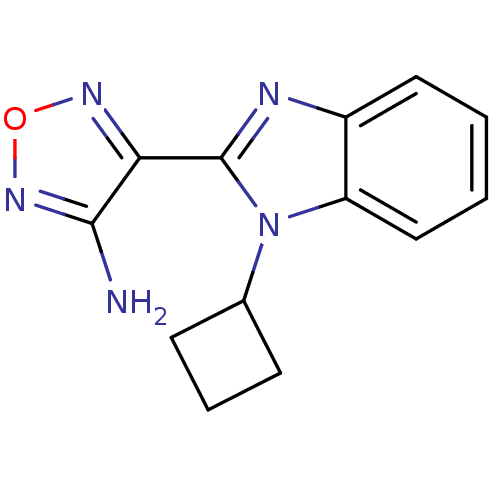
(4-(1-cyclobutyl-1H-benzo[d]imidazol-2-yl)-1,2,5-ox...)Show InChI InChI=1S/C13H13N5O/c14-12-11(16-19-17-12)13-15-9-6-1-2-7-10(9)18(13)8-4-3-5-8/h1-2,6-8H,3-5H2,(H2,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K assessed as decrease in NADH absorbance at 340 nm in the presence of |
Bioorg Med Chem Lett 19: 5191-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.022
BindingDB Entry DOI: 10.7270/Q2KP8287 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278098

(CHEMBL520788 | N-((S)-1-amino-3-(2-(trifluoromethy...)Show SMILES NC[C@H](Cc1ccccc1C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)14-5-7-28-20-15(14)6-8-29-20)21(31)30-13(11-27)9-12-3-1-2-4-16(12)22(24,25)26/h1-8,10,13H,9,11,27H2,(H,28,29)(H,30,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278099

(CHEMBL482536 | N-((S)-1-amino-3-(3-(trifluoromethy...)Show SMILES NC[C@H](Cc1cccc(c1)C(F)(F)F)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C22H18BrF3N4OS/c23-17-10-18(32-19(17)15-4-6-28-20-16(15)5-7-29-20)21(31)30-14(11-27)9-12-2-1-3-13(8-12)22(24,25)26/h1-8,10,14H,9,11,27H2,(H,28,29)(H,30,31)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM431877

(US10550114, Compound 32)Show SMILES C=CC(=O)Nc1cccc(c1)C(=O)NC1CCN(Cc2ccc(cc2)-c2nc3cc[nH]c(=O)c3cc2-c2ccccc2)CC1 Show InChI InChI=1S/C36H33N5O3/c1-2-33(42)38-29-10-6-9-27(21-29)35(43)39-28-16-19-41(20-17-28)23-24-11-13-26(14-12-24)34-30(25-7-4-3-5-8-25)22-31-32(40-34)15-18-37-36(31)44/h2-15,18,21-22,28H,1,16-17,19-20,23H2,(H,37,44)(H,38,42)(H,39,43) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 4.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND
US Patent
| Assay Description
iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... |
US Patent US10550114 (2020)
BindingDB Entry DOI: 10.7270/Q2DV1N97 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316192

(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-((R)-2-amino...)Show SMILES CCn1c(nc2c(nc(O[C@@H](CN)c3ccccc3)cc12)C#CC(C)(C)O)-c1nonc1N |r| Show InChI InChI=1S/C23H25N7O3/c1-4-30-16-12-18(32-17(13-24)14-8-6-5-7-9-14)26-15(10-11-23(2,3)31)19(16)27-22(30)20-21(25)29-33-28-20/h5-9,12,17,31H,4,13,24H2,1-3H3,(H2,25,29)/t17-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278771
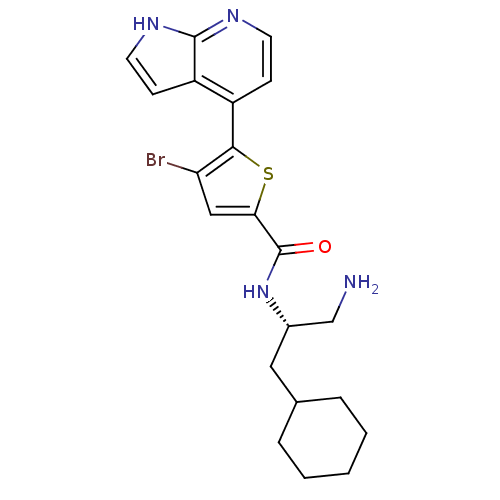
(CHEMBL470598 | N-((S)-1-amino-3-cyclohexylpropan-2...)Show SMILES NC[C@H](CC1CCCCC1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H25BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h6-9,11,13-14H,1-5,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50316189
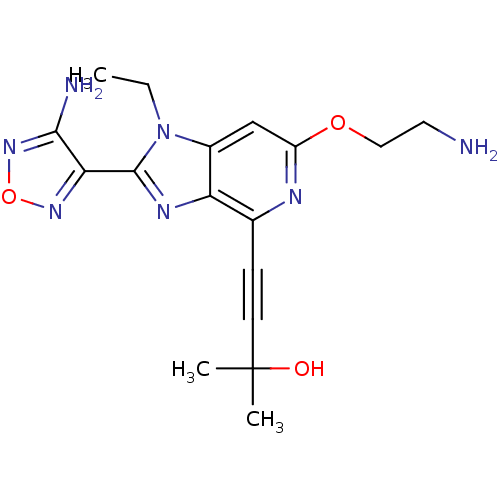
(4-(2-(4-amino-1,2,5-oxadiazol-3-yl)-6-(2-aminoetho...)Show SMILES CCn1c(nc2c(nc(OCCN)cc12)C#CC(C)(C)O)-c1nonc1N Show InChI InChI=1S/C17H21N7O3/c1-4-24-11-9-12(26-8-7-18)20-10(5-6-17(2,3)25)13(11)21-16(24)14-15(19)23-27-22-14/h9,25H,4,7-8,18H2,1-3H3,(H2,19,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT |
Bioorg Med Chem Lett 19: 1508-11 (2009)
Article DOI: 10.1016/j.bmcl.2009.01.002
BindingDB Entry DOI: 10.7270/Q2XK8FP7 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278771

(CHEMBL470598 | N-((S)-1-amino-3-cyclohexylpropan-2...)Show SMILES NC[C@H](CC1CCCCC1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 |r| Show InChI InChI=1S/C21H25BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h6-9,11,13-14H,1-5,10,12,23H2,(H,24,25)(H,26,27)/t14-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15133
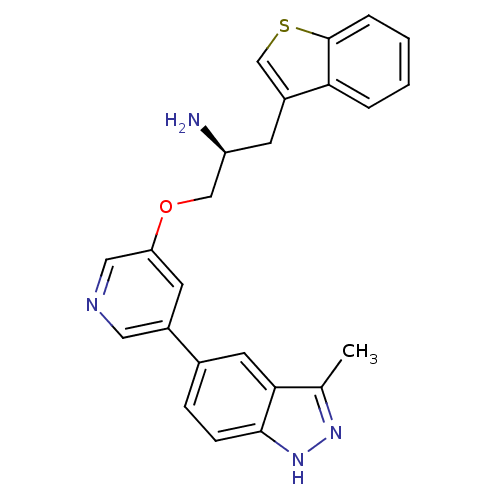
(5-indazolyl pyridine 11b | 5-{5-[(2S)-2-amino-3-(1...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2csc3ccccc23)c1 |r| Show InChI InChI=1S/C24H22N4OS/c1-15-22-10-16(6-7-23(22)28-27-15)17-9-20(12-26-11-17)29-13-19(25)8-18-14-30-24-5-3-2-4-21(18)24/h2-7,9-12,14,19H,8,13,25H2,1H3,(H,27,28)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 6.60 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15123
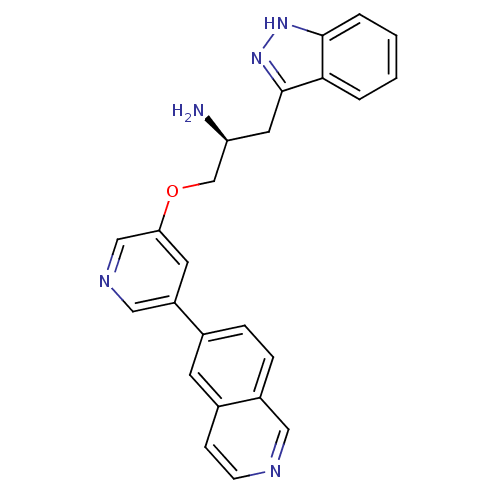
(5-isoquinolinyl pyridine 10b | 6-{5-[(2S)-2-amino-...)Show SMILES N[C@H](COc1cncc(c1)-c1ccc2cnccc2c1)Cc1n[nH]c2ccccc12 |r| Show InChI InChI=1S/C24H21N5O/c25-20(11-24-22-3-1-2-4-23(22)28-29-24)15-30-21-10-19(13-27-14-21)16-5-6-18-12-26-8-7-17(18)9-16/h1-10,12-14,20H,11,15,25H2,(H,28,29)/t20-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 6.80 | -46.1 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-beta serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50278693

((+/-)-N-(1-amino-3-phenylpropan-2-yl)-4-bromo-5-(1...)Show SMILES NCC(Cc1ccccc1)NC(=O)c1cc(Br)c(s1)-c1ccnc2[nH]ccc12 Show InChI InChI=1S/C21H19BrN4OS/c22-17-11-18(21(27)26-14(12-23)10-13-4-2-1-3-5-13)28-19(17)15-6-8-24-20-16(15)7-9-25-20/h1-9,11,14H,10,12,23H2,(H,24,25)(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of AKT2 |
Bioorg Med Chem Lett 19: 2244-8 (2009)
Article DOI: 10.1016/j.bmcl.2009.02.094
BindingDB Entry DOI: 10.7270/Q24X57PX |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15145
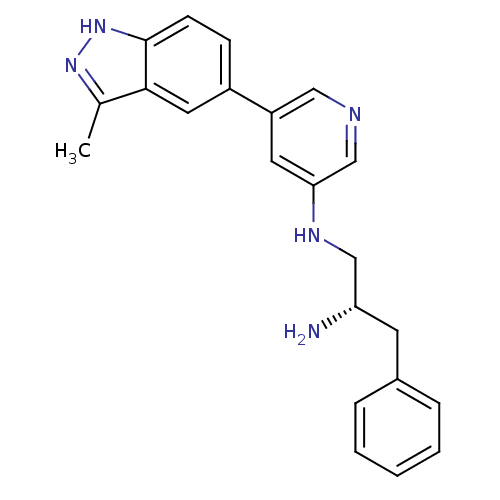
(5-indazolyl pyridine 16 | N-[(2S)-2-amino-3-phenyl...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(NC[C@@H](N)Cc2ccccc2)c1 |r| Show InChI InChI=1S/C22H23N5/c1-15-21-11-17(7-8-22(21)27-26-15)18-10-20(14-24-12-18)25-13-19(23)9-16-5-3-2-4-6-16/h2-8,10-12,14,19,25H,9,13,23H2,1H3,(H,26,27)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| 8.30 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15135
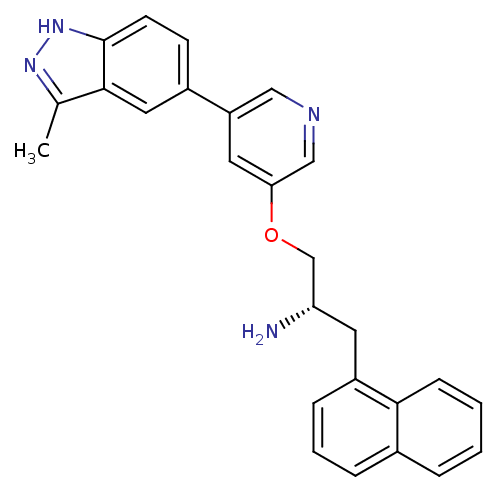
(5-indazolyl pyridine 11d | 5-{5-[(2S)-2-amino-3-(n...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2cccc3ccccc23)c1 |r| Show InChI InChI=1S/C26H24N4O/c1-17-25-13-19(9-10-26(25)30-29-17)21-12-23(15-28-14-21)31-16-22(27)11-20-7-4-6-18-5-2-3-8-24(18)20/h2-10,12-15,22H,11,16,27H2,1H3,(H,29,30)/t22-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| 8.30 | -45.7 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM50361651
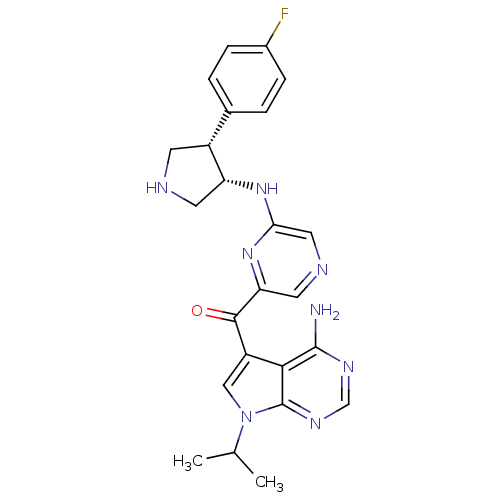
(CHEMBL1940249)Show SMILES CC(C)n1cc(C(=O)c2cncc(N[C@@H]3CNC[C@@H]3c3ccc(F)cc3)n2)c2c(N)ncnc12 |r| Show InChI InChI=1S/C24H25FN8O/c1-13(2)33-11-17(21-23(26)29-12-30-24(21)33)22(34)19-9-28-10-20(32-19)31-18-8-27-7-16(18)14-3-5-15(25)6-4-14/h3-6,9-13,16,18,27H,7-8H2,1-2H3,(H,31,32)(H2,26,29,30)/t16-,18-/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of His-tagged AKT1 using 5FAM-GRPRTSSFAEGCONH2 as substrate by fluorescence based assay |
J Med Chem 54: 8490-500 (2011)
Article DOI: 10.1021/jm201019k
BindingDB Entry DOI: 10.7270/Q23N23TV |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase
(Homo sapiens (Human)) | BDBM431882
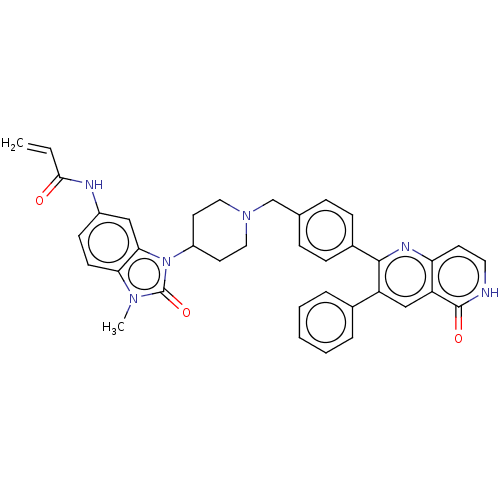
(US10550114, Compound 46)Show SMILES Cn1c2ccc(NC(=O)C=C)cc2n(C2CCN(Cc3ccc(cc3)-c3nc4cc[nH]c(=O)c4cc3-c3ccccc3)CC2)c1=O Show InChI InChI=1S/C37H34N6O3/c1-3-34(44)39-27-13-14-32-33(21-27)43(37(46)41(32)2)28-16-19-42(20-17-28)23-24-9-11-26(12-10-24)35-29(25-7-5-4-6-8-25)22-30-31(40-35)15-18-38-36(30)45/h3-15,18,21-22,28H,1,16-17,19-20,23H2,2H3,(H,38,45)(H,39,44) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 10.7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TECHNISCHE UNIVERSITÄT DORTMUND
US Patent
| Assay Description
iFLiK and HTRF studies were carried out as described in Z. Fang, J. R. Simard, D. Plenker, H. D. Nguyen, T. Phan, P. Wolle, S. Baumeister, D. Rauh, A... |
US Patent US10550114 (2020)
BindingDB Entry DOI: 10.7270/Q2DV1N97 |
More data for this
Ligand-Target Pair | |
RAC-alpha serine/threonine-protein kinase [139-480,S378A,S381A,T450D,S473D]
(Homo sapiens (Human)) | BDBM15138
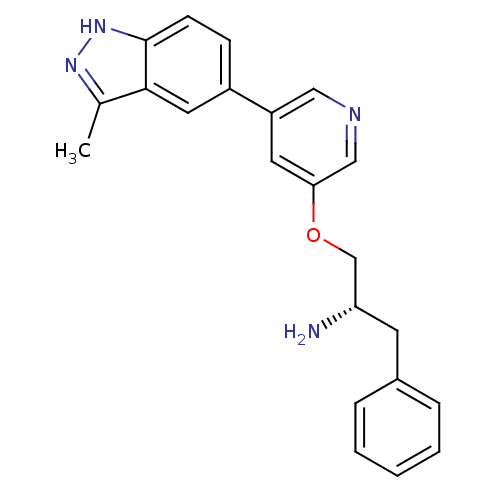
(5-indazolyl pyridine 11g | 5-{5-[(2S)-2-amino-3-ph...)Show SMILES Cc1n[nH]c2ccc(cc12)-c1cncc(OC[C@@H](N)Cc2ccccc2)c1 |r| Show InChI InChI=1S/C22H22N4O/c1-15-21-11-17(7-8-22(21)26-25-15)18-10-20(13-24-12-18)27-14-19(23)9-16-5-3-2-4-6-16/h2-8,10-13,19H,9,14,23H2,1H3,(H,25,26)/t19-/m0/s1 | PDB
MMDB
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 11 | -45.0 | n/a | n/a | n/a | n/a | n/a | 7.5 | 22 |
Abbott Laboratories
| Assay Description
The kinase assay uses His-Akt1 and a biotinylated peptide as substrate. The biotinylated peptides were immobilized on streptavidin-coated FLASH plat... |
Bioorg Med Chem Lett 16: 3740-4 (2006)
Article DOI: 10.1016/j.bmcl.2006.04.046
BindingDB Entry DOI: 10.7270/Q2ZP44C9 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50310458
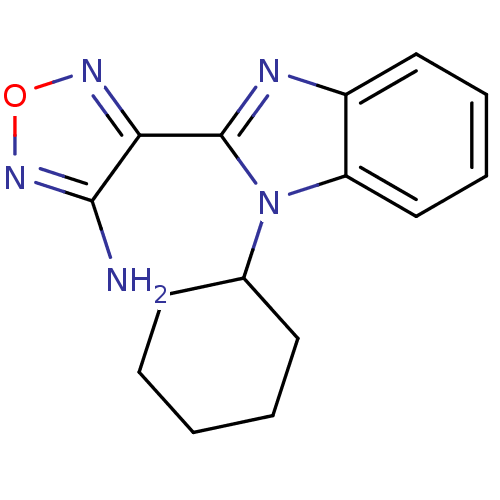
(4-(1-cyclohexyl-1H-benzo[d]imidazol-2-yl)-1,2,5-ox...)Show InChI InChI=1S/C15H17N5O/c16-14-13(18-21-19-14)15-17-11-8-4-5-9-12(11)20(15)10-6-2-1-3-7-10/h4-5,8-10H,1-3,6-7H2,(H2,16,19) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K assessed as decrease in NADH absorbance at 340 nm in the presence of |
Bioorg Med Chem Lett 19: 5191-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.022
BindingDB Entry DOI: 10.7270/Q2KP8287 |
More data for this
Ligand-Target Pair | |
Ribosomal protein S6 kinase beta-1
(Homo sapiens (Human)) | BDBM50310459
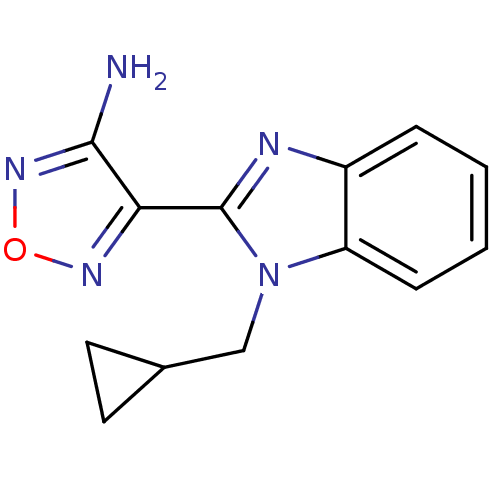
(4-(1-(cyclopropylmethyl)-1H-benzo[d]imidazol-2-yl)...)Show InChI InChI=1S/C13H13N5O/c14-12-11(16-19-17-12)13-15-9-3-1-2-4-10(9)18(13)7-8-5-6-8/h1-4,8H,5-7H2,(H2,14,17) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Vertex Pharmaceuticals, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of p70S6K assessed as decrease in NADH absorbance at 340 nm in the presence of |
Bioorg Med Chem Lett 19: 5191-4 (2009)
Article DOI: 10.1016/j.bmcl.2009.07.022
BindingDB Entry DOI: 10.7270/Q2KP8287 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data