

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538919 (CHEMBL4633233) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393375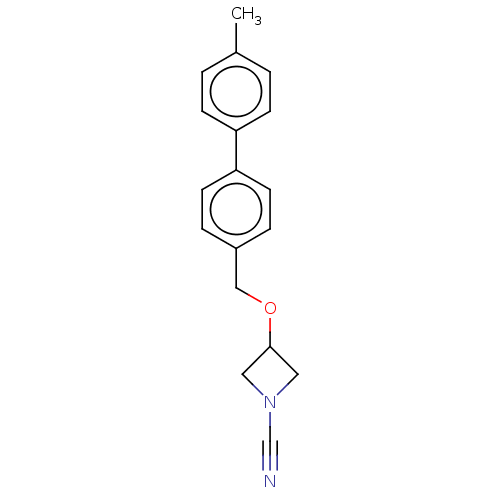 (3-((4'-Methyl-[1,1'-biphenyl]-4-yl)methoxy)azetidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393377 (3-((3-Fluoro-3'-methoxy-[1,1'-biphenyl]-4-yl)metho...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393376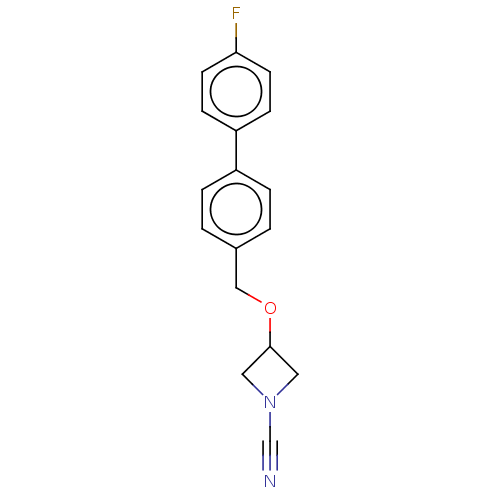 (3-((4'-Fluoro-[1,1'-biphenyl]-4-yl)methoxy)azetidi...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.680 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393378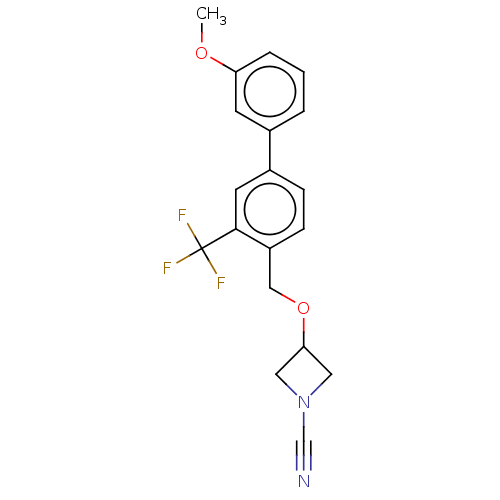 (3-((3'-Methoxy-3-(trifluoromethyl)-[1,1'-biphenyl]...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.850 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393368 (3-((2',3'-Dimethoxy-[1,1'-biphenyl]-4-yl)methoxy)a...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393366 (3-((3'-Methoxy-[1,1'-biphenyl]-4-yl)methoxy)azetid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.890 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393366 (3-((3'-Methoxy-[1,1'-biphenyl]-4-yl)methoxy)azetid...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of NAAA (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00076 BindingDB Entry DOI: 10.7270/Q2C25175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393362 (US9963444, Example 63) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.940 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538909 (CHEMBL4637701) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393380 (3-((4-Phenoxybenzyl)oxy)azetidine-1-carbonitrile |...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393384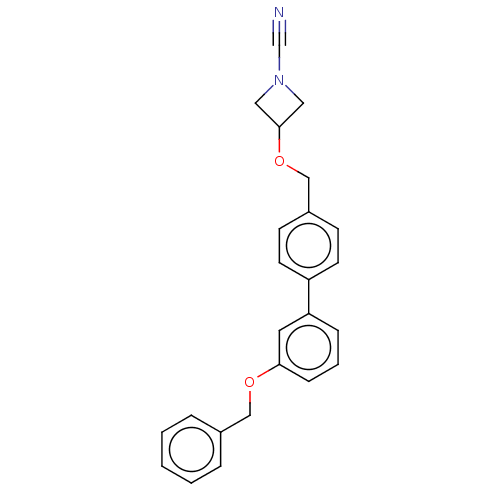 (US9963444, Example 85) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538910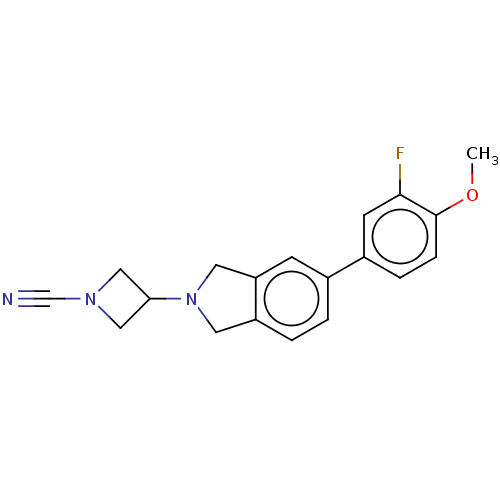 (CHEMBL4645560) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538911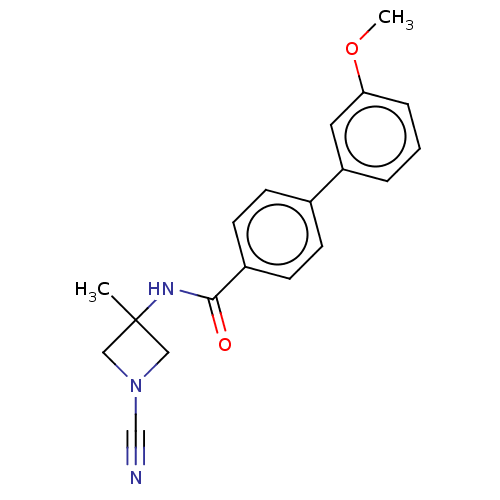 (CHEMBL4635238) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538916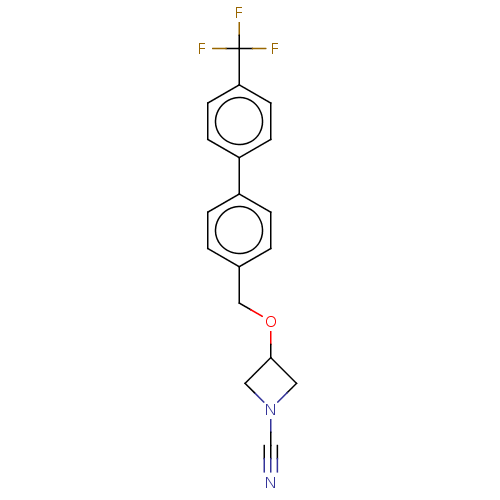 (CHEMBL4646586) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393394 (US9963444, Example 95) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM447488 (US10689357, Example 25) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NAAA using N-(4-methyl coumarin)-palmitamide as fluorogenic substrate preincubated for 90 mins followed by substrate addition by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00076 BindingDB Entry DOI: 10.7270/Q2C25175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393391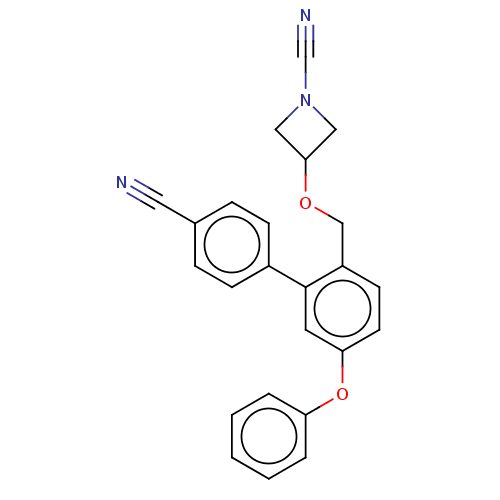 (3-((4'-Cyano-5-phenoxy-[1,1'-biphenyl]-2-yl)methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393363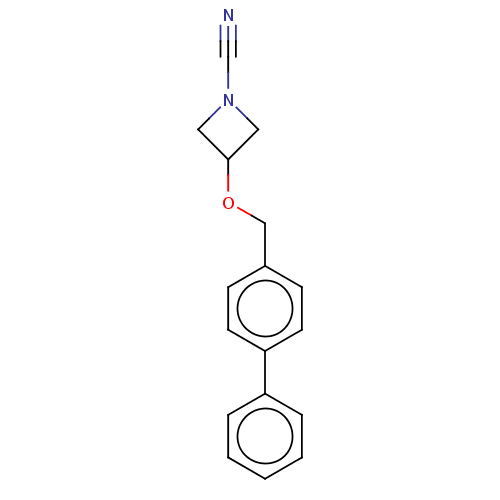 (3-([1,1'-Biphenyl]-4-ylmethoxy)azetidine-1-carboni...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538914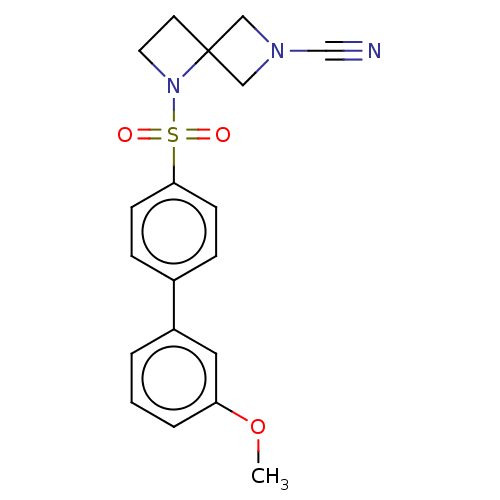 (CHEMBL4637755) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393379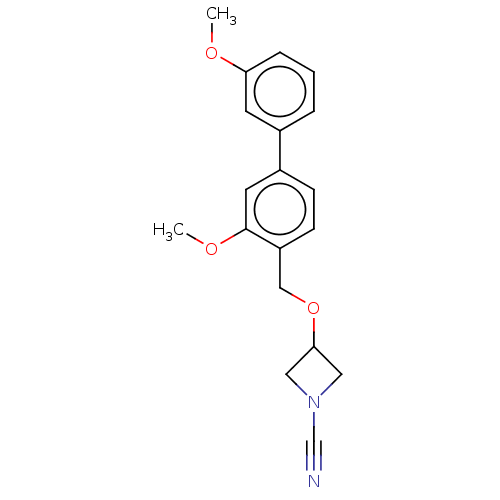 (3-((3,3'-Dimethoxy-[1,1'-biphenyl]-4-yl)methoxy)az...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393363 (3-([1,1'-Biphenyl]-4-ylmethoxy)azetidine-1-carboni...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.80 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of NAAA (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00076 BindingDB Entry DOI: 10.7270/Q2C25175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538912 (CHEMBL4643588) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM447493 (US10689357, Example 30) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NAAA using N-(4-methyl coumarin)-palmitamide as fluorogenic substrate preincubated for 90 mins followed by substrate addition by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00076 BindingDB Entry DOI: 10.7270/Q2C25175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393385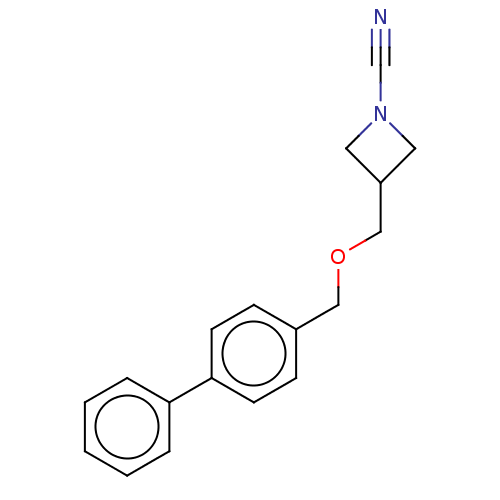 (US9963444, Example 86) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM233790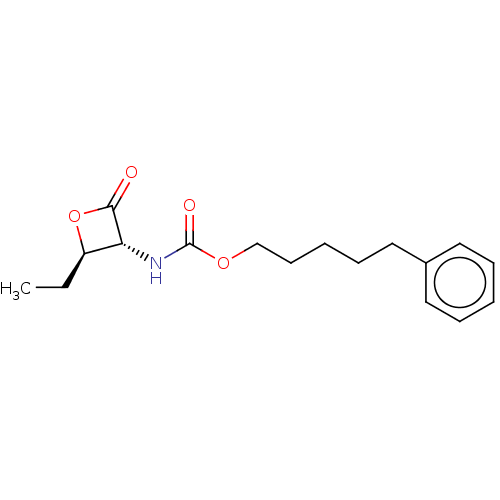 (US9353075, 47) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4 | n/a | n/a | n/a | n/a | 4.5 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50576137 (CHEMBL4863412) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NAAA using N-(4-methyl coumarin)-palmitamide as fluorogenic substrate preincubated for 90 mins followed by substrate addition by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00076 BindingDB Entry DOI: 10.7270/Q2C25175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538918 (CHEMBL4638402) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439649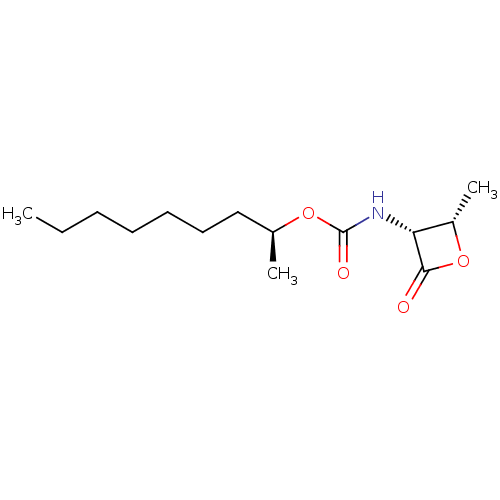 (CHEMBL2419811 | US9353075, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439649 (CHEMBL2419811 | US9353075, 7) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cells | J Med Chem 56: 6917-34 (2013) Article DOI: 10.1021/jm400739u BindingDB Entry DOI: 10.7270/Q2F47QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439653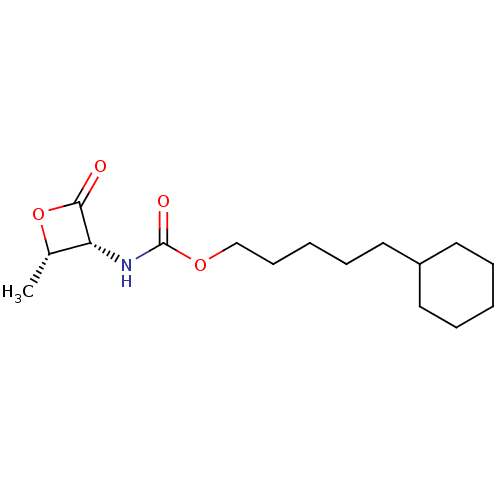 (CHEMBL2419830 | US9353075, 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439653 (CHEMBL2419830 | US9353075, 35) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cells | J Med Chem 56: 6917-34 (2013) Article DOI: 10.1021/jm400739u BindingDB Entry DOI: 10.7270/Q2F47QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393389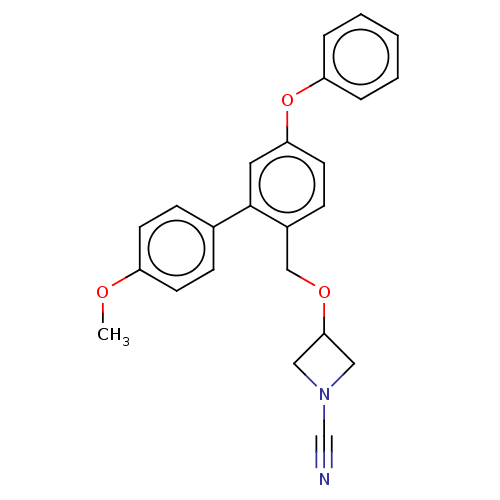 (3-((4'-Methoxy-5-phenoxy-[1,1'-biphenyl]-2-yl)meth...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393373 (3-((4-(2-Methoxypyridin-3-yl)benzyl)oxy)azetidine-...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538922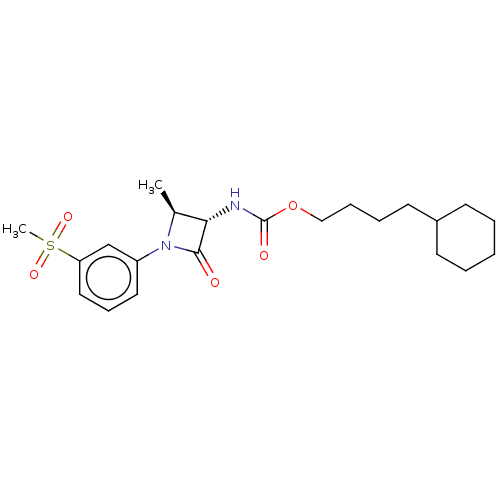 (CHEMBL4647961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of NAAA (unknown origin) | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538922 (CHEMBL4647961) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of NAAA (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00076 BindingDB Entry DOI: 10.7270/Q2C25175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM233791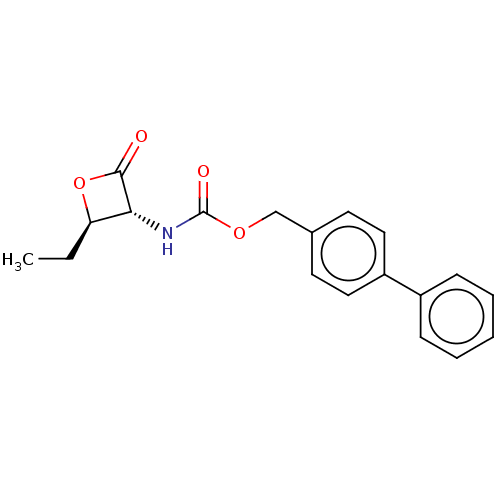 (US9353075, 48) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6 | n/a | n/a | n/a | n/a | 4.5 | n/a |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description The assay was run in Optiplate 96-wells black plates, in a total reaction volume of 200 μL. NAAA protein preparation (4.0 μg) was pre-incub... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151057 (CHEMBL3770726) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
University of California Curated by ChEMBL | Assay Description Inhibition of human NAAA | J Med Chem 63: 7475-7490 (2020) Article DOI: 10.1021/acs.jmedchem.0c00191 BindingDB Entry DOI: 10.7270/Q2V98CM4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50234746 (CHEMBL4093333) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of recombinant human spleen NAAA expressed in HEK293 cells using PAMCA as substrate preincubated for 10 mins followed by substrate additio... | Eur J Med Chem 126: 561-575 (2017) Article DOI: 10.1016/j.ejmech.2016.11.039 BindingDB Entry DOI: 10.7270/Q25H7JHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50234744 (CHEMBL4101070) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of recombinant human spleen NAAA expressed in HEK293 cells using PAMCA as substrate preincubated for 10 mins followed by substrate additio... | Eur J Med Chem 126: 561-575 (2017) Article DOI: 10.1016/j.ejmech.2016.11.039 BindingDB Entry DOI: 10.7270/Q25H7JHB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393397 (3-((4-(2,3-Dihydrobenzo[b][1,4]dioxin-6-yl)benzyl)...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM447489 (US10689357, Example 26) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of human NAAA using N-(4-methyl coumarin)-palmitamide as fluorogenic substrate preincubated for 90 mins followed by substrate addition by ... | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00076 BindingDB Entry DOI: 10.7270/Q2C25175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50538913 (CHEMBL4649749) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Rattus norvegicus (Rat)) | BDBM50032458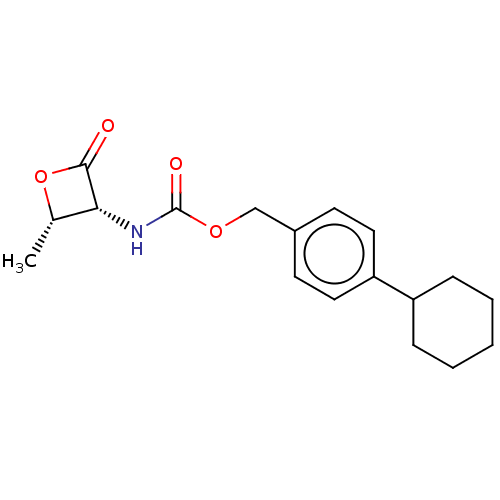 (CHEMBL3354144 | US9353075, 42) | PDB UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of NAAA in Sprague-Dawley rat lung assessed as inhibition of hydrolysis of 10-cis-heptadecenoylethanolamide by UPLC/MS method | J Med Chem 57: 10101-11 (2014) Article DOI: 10.1021/jm501455s BindingDB Entry DOI: 10.7270/Q26111W8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM393392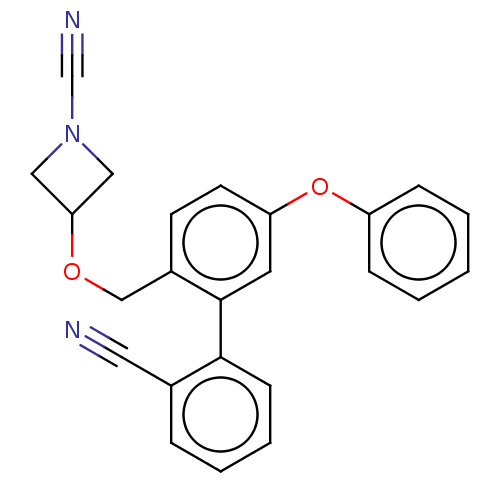 (3-((2'-Cyano-5-phenoxy-[1,1'-biphenyl]-2-yl)methox...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 6.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Northeastern University Curated by ChEMBL | Assay Description Inhibition of activated human NAAA using fluorogenic PAMCA and N-4-methylcoumarin as substrate incubated for 90 mins by fluorescence based assay | Bioorg Med Chem 28: (2020) Article DOI: 10.1016/j.bmc.2019.115195 BindingDB Entry DOI: 10.7270/Q2K35Z63 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50151154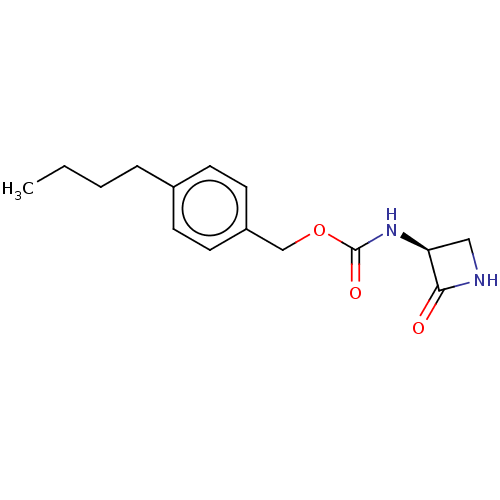 (CHEMBL3770896) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Italian Institute of Technology Curated by ChEMBL | Assay Description Inhibition of recombinant human NAAA expressed in HEK293 cells after 30 mins by UPLC/MS analysis | Eur J Med Chem 111: 138-59 (2016) Article DOI: 10.1016/j.ejmech.2016.01.046 BindingDB Entry DOI: 10.7270/Q2GQ70MX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50389023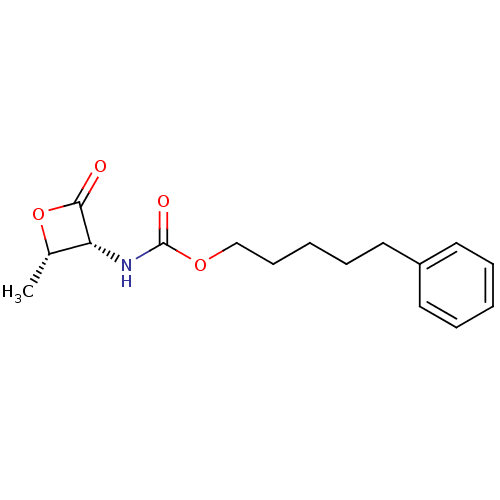 (CHEMBL2064166 | US9353075, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50439664 (CHEMBL2419814 | US9353075, 17) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 4.5 | 37 |
The Regents of the University of California; Fondazione Istituto Italiano Di Technologia; Universita Degli Studi Di Parma; Universita Degli Studi Di Urbino “Carlo Bo” US Patent | Assay Description NAAA protein preparation (10 ug) was pre-incubated with various concentrations of test compound or vehicle control in 100 mM NaH2PO4, 100 mM Tri Sodi... | US Patent US9353075 (2016) BindingDB Entry DOI: 10.7270/Q23N229Q | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50389023 (CHEMBL2064166 | US9353075, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Istituto Italiano di Tecnologia Curated by ChEMBL | Assay Description Inhibition of C-terminal His-6-tagged recombinant human spleen NAAA enzyme expressed in HEK293 cells | J Med Chem 56: 6917-34 (2013) Article DOI: 10.1021/jm400739u BindingDB Entry DOI: 10.7270/Q2F47QK0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| N-acylethanolamine-hydrolyzing acid amidase (Homo sapiens (Human)) | BDBM50389023 (CHEMBL2064166 | US9353075, 6) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description Inhibition of NAAA (unknown origin) | Citation and Details Article DOI: 10.1021/acs.jmedchem.1c00076 BindingDB Entry DOI: 10.7270/Q2C25175 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 864 total ) | Next | Last >> |