 Found 152 hits Enz. Inhib. hit(s) with Target = 'Urokinase plasminogen activator surface receptor'
Found 152 hits Enz. Inhib. hit(s) with Target = 'Urokinase plasminogen activator surface receptor' Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005397
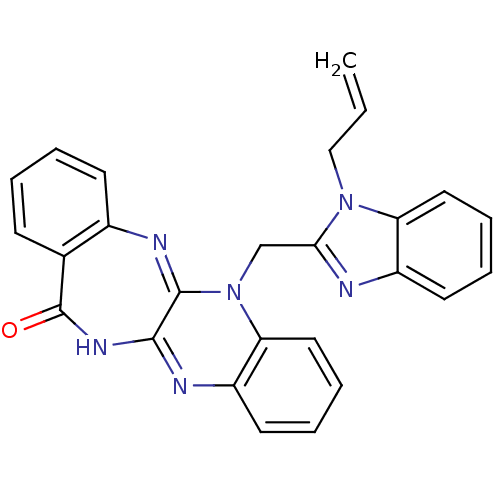
(CHEMBL2206684)Show SMILES C=CCn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:20,t:7| Show InChI InChI=1S/C26H20N6O/c1-2-15-31-21-13-7-5-11-19(21)27-23(31)16-32-22-14-8-6-12-20(22)28-24-25(32)29-18-10-4-3-9-17(18)26(33)30-24/h2-14H,1,15-16H2,(H,28,30,33) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50005398
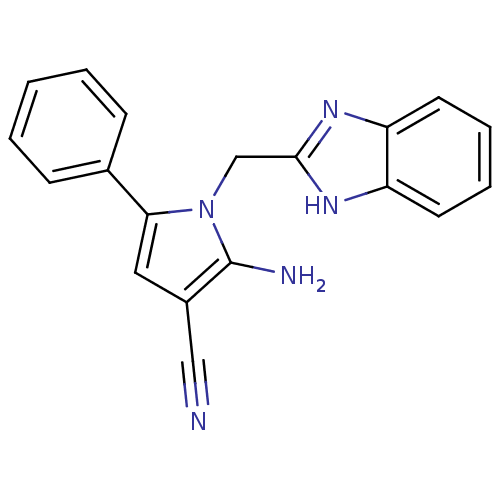
(CHEMBL2206694)Show InChI InChI=1S/C19H15N5/c20-11-14-10-17(13-6-2-1-3-7-13)24(19(14)21)12-18-22-15-8-4-5-9-16(15)23-18/h1-10H,12,21H2,(H,22,23) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402366

(CHEMBL2206696)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccncc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-17(22-25-18-8-4-5-9-19(18)26-22)14-20(15-6-2-1-3-7-15)27(21)16-10-12-24-13-11-16/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402361
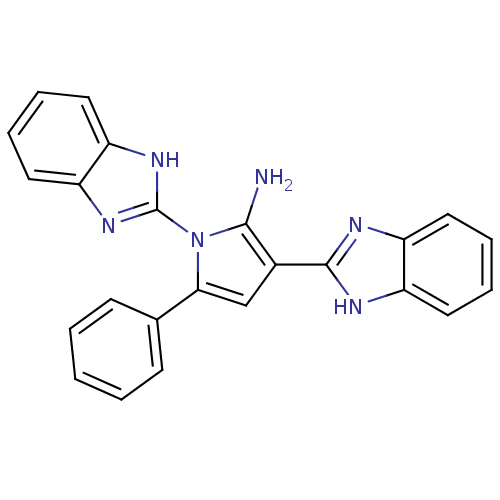
(CHEMBL2206680)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C24H18N6/c25-22-16(23-26-17-10-4-5-11-18(17)27-23)14-21(15-8-2-1-3-9-15)30(22)24-28-19-12-6-7-13-20(19)29-24/h1-14H,25H2,(H,26,27)(H,28,29) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402373
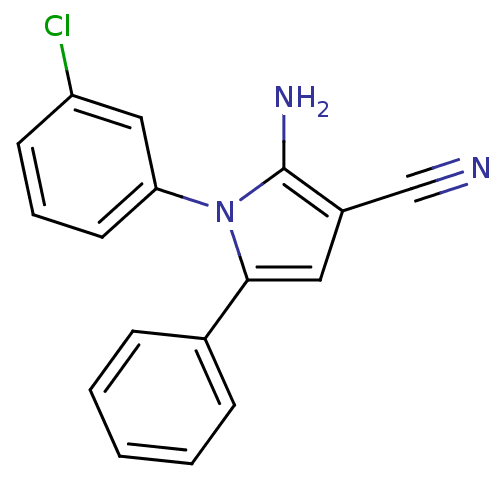
(CHEMBL2206691)Show InChI InChI=1S/C17H12ClN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402360

(CHEMBL2206681)Show SMILES Nc1c(cc(-c2ccccc2)n1Cc1nc2ccccc2[nH]1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C25H20N6/c26-24-17(25-29-20-12-6-7-13-21(20)30-25)14-22(16-8-2-1-3-9-16)31(24)15-23-27-18-10-4-5-11-19(18)28-23/h1-14H,15,26H2,(H,27,28)(H,29,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402378
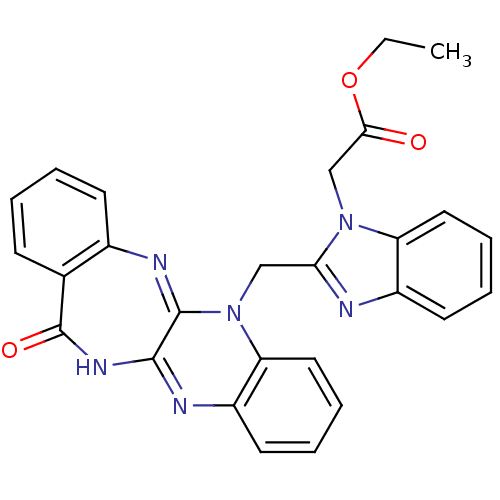
(CHEMBL2206685)Show SMILES CCOC(=O)Cn1c(CN2C3=Nc4ccccc4C(=O)NC3=Nc3ccccc23)nc2ccccc12 |c:23,t:10| Show InChI InChI=1S/C27H22N6O3/c1-2-36-24(34)16-32-21-13-7-5-11-19(21)28-23(32)15-33-22-14-8-6-12-20(22)29-25-26(33)30-18-10-4-3-9-17(18)27(35)31-25/h3-14H,2,15-16H2,1H3,(H,29,31,35) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402371
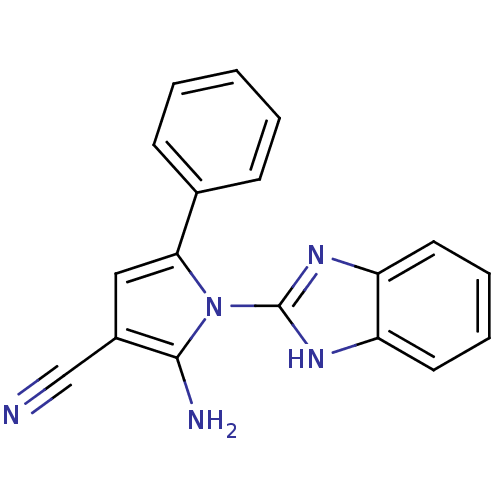
(CHEMBL2206693)Show InChI InChI=1S/C18H13N5/c19-11-13-10-16(12-6-2-1-3-7-12)23(17(13)20)18-21-14-8-4-5-9-15(14)22-18/h1-10H,20H2,(H,21,22) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402372
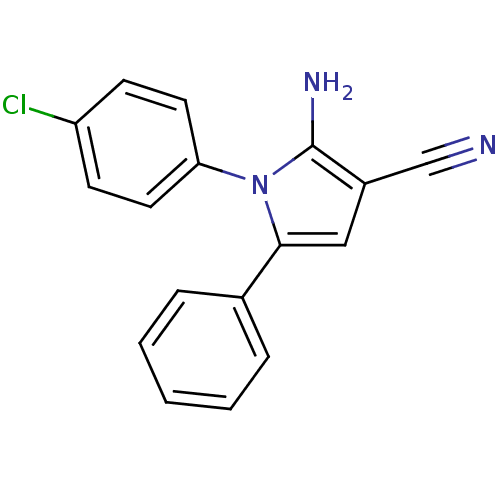
(CHEMBL2206692)Show InChI InChI=1S/C17H12ClN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402374

(CHEMBL2206690)Show InChI InChI=1S/C17H12BrN3/c18-14-6-8-15(9-7-14)21-16(10-13(11-19)17(21)20)12-4-2-1-3-5-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402363
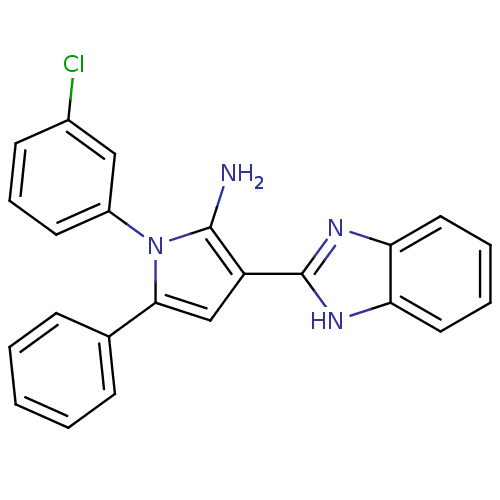
(CHEMBL2206699)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Cl)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402362
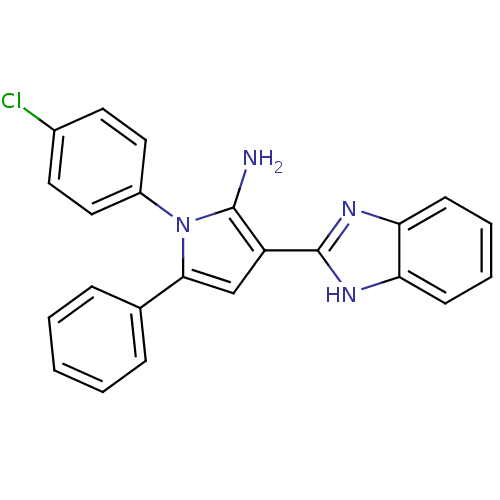
(CHEMBL2206700)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Cl)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17ClN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402375
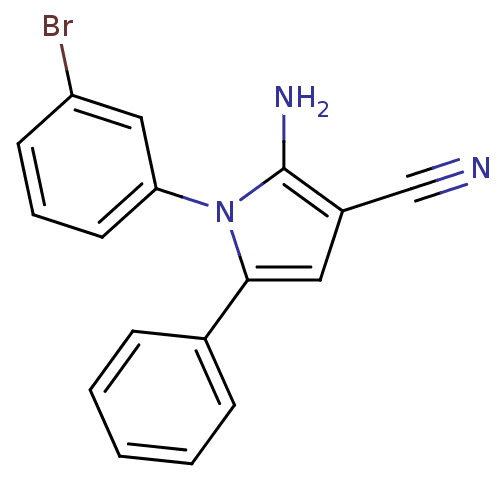
(CHEMBL2206689)Show InChI InChI=1S/C17H12BrN3/c18-14-7-4-8-15(10-14)21-16(9-13(11-19)17(21)20)12-5-2-1-3-6-12/h1-10H,20H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402370
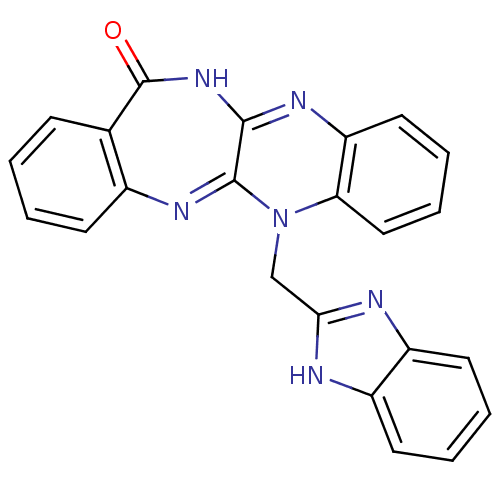
(CHEMBL2206683)Show SMILES O=C1NC2=Nc3ccccc3N(Cc3nc4ccccc4[nH]3)C2=Nc2ccccc12 |c:26,t:3| Show InChI InChI=1S/C23H16N6O/c30-23-14-7-1-2-8-15(14)27-22-21(28-23)26-18-11-5-6-12-19(18)29(22)13-20-24-16-9-3-4-10-17(16)25-20/h1-12H,13H2,(H,24,25)(H,26,28,30) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402367

(CHEMBL2206695)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccccn1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C22H17N5/c23-21-16(22-25-17-10-4-5-11-18(17)26-22)14-19(15-8-2-1-3-9-15)27(21)20-12-6-7-13-24-20/h1-14H,23H2,(H,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00700 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402376
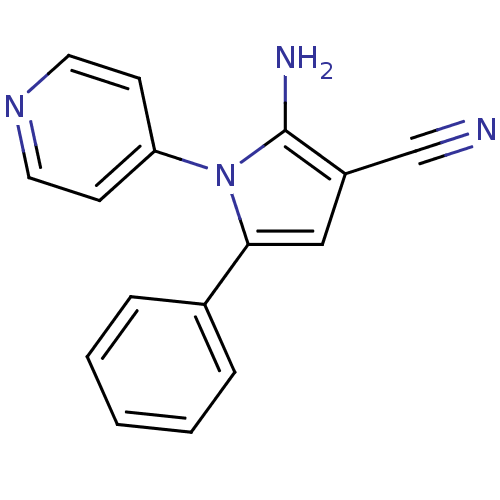
(CHEMBL2206688)Show InChI InChI=1S/C16H12N4/c17-11-13-10-15(12-4-2-1-3-5-12)20(16(13)18)14-6-8-19-9-7-14/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.00800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402364
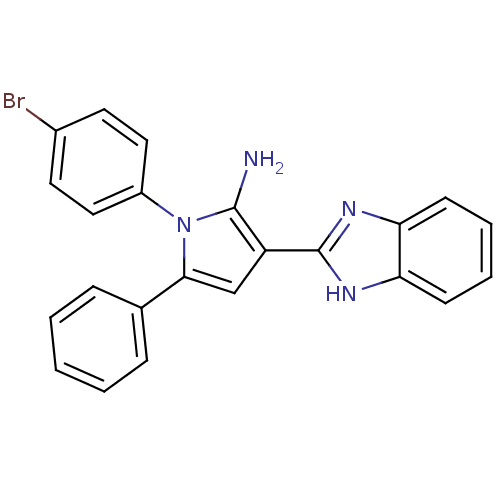
(CHEMBL2206698)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1ccc(Br)cc1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-10-12-17(13-11-16)28-21(15-6-2-1-3-7-15)14-18(22(28)25)23-26-19-8-4-5-9-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0110 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402365
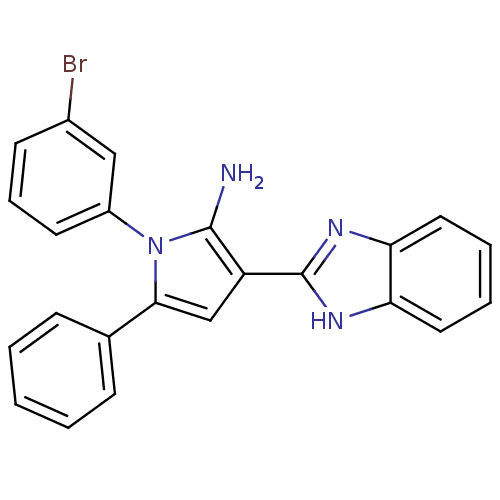
(CHEMBL2206697)Show SMILES Nc1c(cc(-c2ccccc2)n1-c1cccc(Br)c1)-c1nc2ccccc2[nH]1 Show InChI InChI=1S/C23H17BrN4/c24-16-9-6-10-17(13-16)28-21(15-7-2-1-3-8-15)14-18(22(28)25)23-26-19-11-4-5-12-20(19)27-23/h1-14H,25H2,(H,26,27) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 0.0120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402377

(CHEMBL2206687)Show InChI InChI=1S/C16H12N4/c17-11-13-10-14(12-6-2-1-3-7-12)20(16(13)18)15-8-4-5-9-19-15/h1-10H,18H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402379
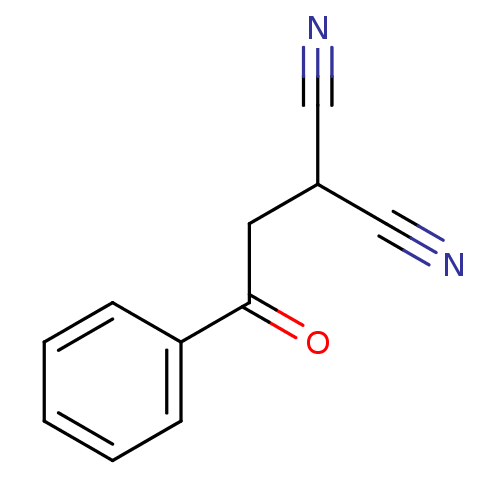
(CHEMBL2206686)Show InChI InChI=1S/C11H8N2O/c12-7-9(8-13)6-11(14)10-4-2-1-3-5-10/h1-5,9H,6H2 | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| 0.296 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402368
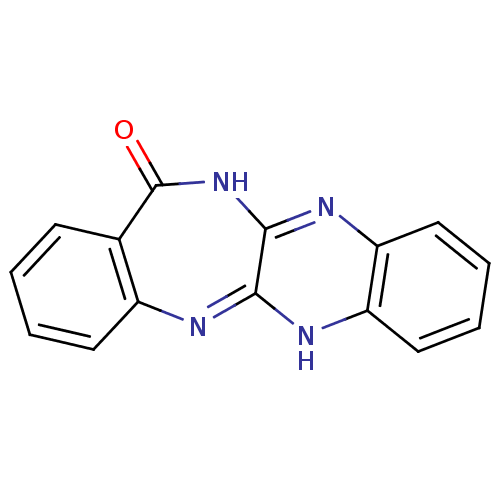
(CHEMBL2206682)Show InChI InChI=1S/C15H10N4O/c20-15-9-5-1-2-6-10(9)16-13-14(19-15)18-12-8-4-3-7-11(12)17-13/h1-8H,(H,16,17)(H,18,19,20) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98600
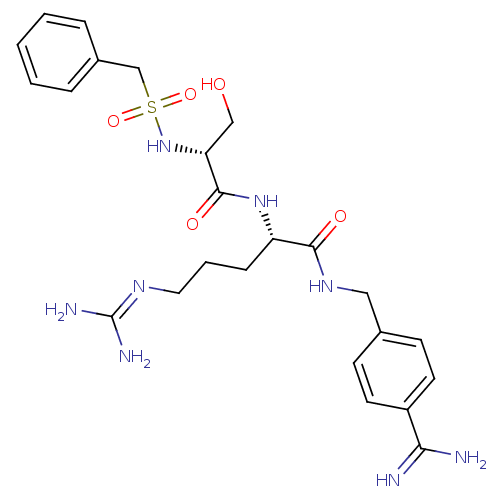
((2S)-2-[[(2R)-2-(benzylsulfonylamino)-3-hydroxy-pr...)Show SMILES [#7]\[#6](-[#7])=[#7]/[#6]-[#6]-[#6]-[#6@H](-[#7]-[#6](=O)-[#6@@H](-[#6]-[#8])-[#7]S(=O)(=O)[#6]-c1ccccc1)-[#6](=O)-[#7]-[#6]-c1ccc(cc1)-[#6](-[#7])=[#7] Show InChI InChI=1S/C24H34N8O5S/c25-21(26)18-10-8-16(9-11-18)13-30-22(34)19(7-4-12-29-24(27)28)31-23(35)20(14-33)32-38(36,37)15-17-5-2-1-3-6-17/h1-3,5-6,8-11,19-20,32-33H,4,7,12-15H2,(H3,25,26)(H,30,34)(H,31,35)(H4,27,28,29)/t19-,20+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 8.90 | -45.8 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Homo sapiens (Human)) | BDBM50402369

(CHEMBL1652555)Show InChI InChI=1S/C8H7N3O/c9-7-8(12)11-6-4-2-1-3-5(6)10-7/h1-4H,(H2,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 20.8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
National Research Center
Curated by ChEMBL
| Assay Description
Binding affinity to uPAR |
Bioorg Med Chem 20: 6989-7001 (2012)
Article DOI: 10.1016/j.bmc.2012.10.010
BindingDB Entry DOI: 10.7270/Q26W9C80 |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98599
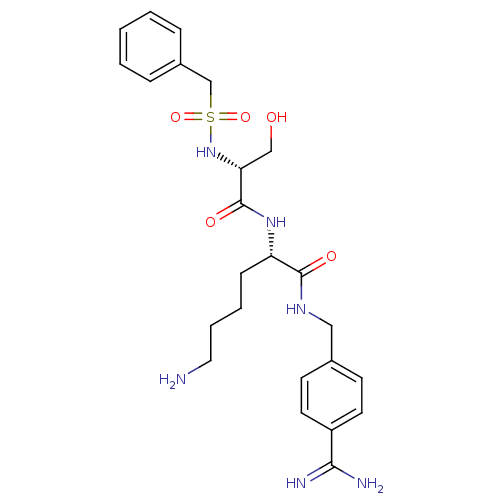
((2S)-6-amino-2-[[(2R)-2-(benzylsulfonylamino)-3-hy...)Show SMILES NCCCC[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C24H34N6O5S/c25-13-5-4-8-20(23(32)28-14-17-9-11-19(12-10-17)22(26)27)29-24(33)21(15-31)30-36(34,35)16-18-6-2-1-3-7-18/h1-3,6-7,9-12,20-21,30-31H,4-5,8,13-16,25H2,(H3,26,27)(H,28,32)(H,29,33)/t20-,21+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 24 | -43.3 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM50231520
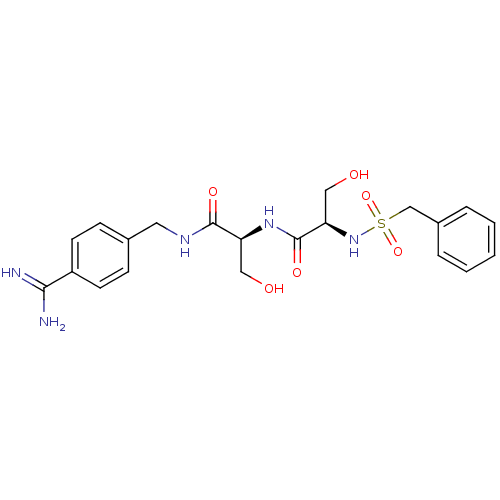
((R)-N-[(S)-1-(4-carbamimidoyl-benzylcarbamoyl)-2-h...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CO)NC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C21H27N5O6S/c22-19(23)16-8-6-14(7-9-16)10-24-20(29)17(11-27)25-21(30)18(12-28)26-33(31,32)13-15-4-2-1-3-5-15/h1-9,17-18,26-28H,10-13H2,(H3,22,23)(H,24,29)(H,25,30)/t17-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| 25 | -43.2 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98587
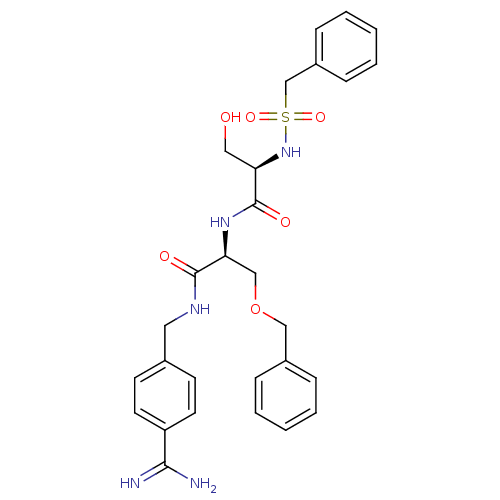
((2R)-N-[(1S)-1-(benzyloxymethyl)-2-[(4-carbamimido...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](COCc2ccccc2)NC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C28H33N5O6S/c29-26(30)23-13-11-20(12-14-23)15-31-27(35)25(18-39-17-21-7-3-1-4-8-21)32-28(36)24(16-34)33-40(37,38)19-22-9-5-2-6-10-22/h1-14,24-25,33-34H,15-19H2,(H3,29,30)(H,31,35)(H,32,36)/t24-,25+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 28 | -43.0 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98584

(3-[[(1R)-2-[[(1S)-2-[(4-carbamimidoylphenyl)methyl...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1cccc(c1)C(O)=O)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H27N5O7S/c1-13(20(29)25-10-14-5-7-16(8-6-14)19(23)24)26-21(30)18(11-28)27-35(33,34)12-15-3-2-4-17(9-15)22(31)32/h2-9,13,18,27-28H,10-12H2,1H3,(H3,23,24)(H,25,29)(H,26,30)(H,31,32)/t13-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 30 | -42.8 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98596

((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccc(F)cc2)cc1 Show InChI InChI=1S/C20H24FN5O5S/c21-16-7-3-14(4-8-16)12-32(30,31)26-17(11-27)20(29)25-10-18(28)24-9-13-1-5-15(6-2-13)19(22)23/h1-8,17,26-27H,9-12H2,(H3,22,23)(H,24,28)(H,25,29)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 31 | -42.7 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98594
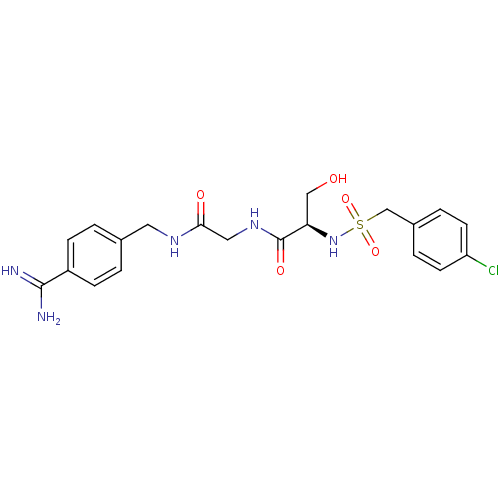
((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccc(Cl)cc2)cc1 Show InChI InChI=1S/C20H24ClN5O5S/c21-16-7-3-14(4-8-16)12-32(30,31)26-17(11-27)20(29)25-10-18(28)24-9-13-1-5-15(6-2-13)19(22)23/h1-8,17,26-27H,9-12H2,(H3,22,23)(H,24,28)(H,25,29)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 32 | -42.6 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98588
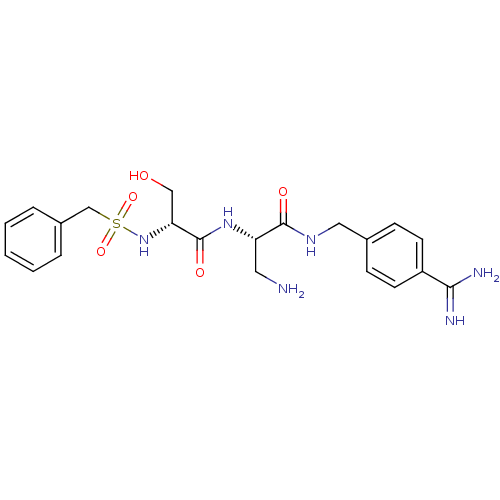
((2S)-3-amino-2-[[(2R)-2-(benzylsulfonylamino)-3-hy...)Show SMILES NC[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C21H28N6O5S/c22-10-17(20(29)25-11-14-6-8-16(9-7-14)19(23)24)26-21(30)18(12-28)27-33(31,32)13-15-4-2-1-3-5-15/h1-9,17-18,27-28H,10-13,22H2,(H3,23,24)(H,25,29)(H,26,30)/t17-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 36 | -42.3 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM50110015
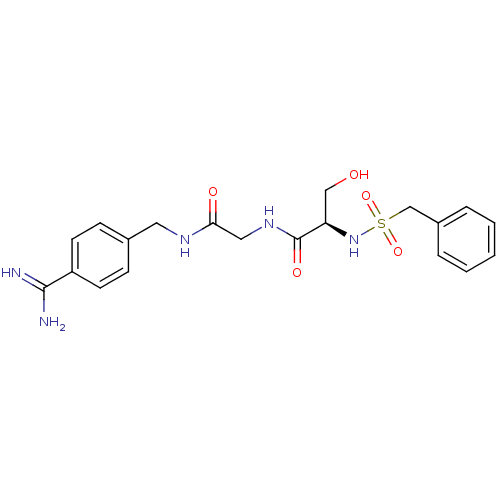
(CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C20H25N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h1-9,17,25-26H,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 36 | -42.3 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98583
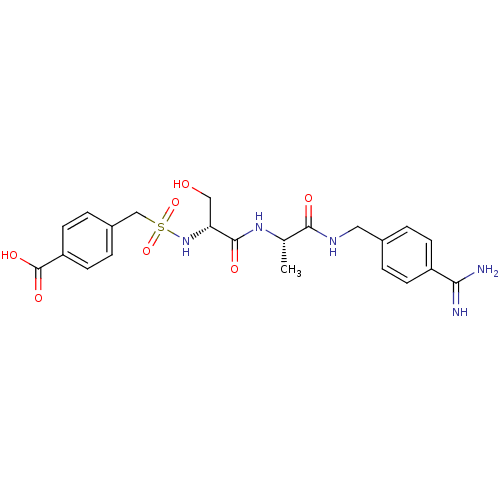
(4-[[(1R)-2-[[(1S)-2-[(4-carbamimidoylphenyl)methyl...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccc(cc1)C(O)=O)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H27N5O7S/c1-13(20(29)25-10-14-2-6-16(7-3-14)19(23)24)26-21(30)18(11-28)27-35(33,34)12-15-4-8-17(9-5-15)22(31)32/h2-9,13,18,27-28H,10-12H2,1H3,(H3,23,24)(H,25,29)(H,26,30)(H,31,32)/t13-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 38 | -42.2 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98601
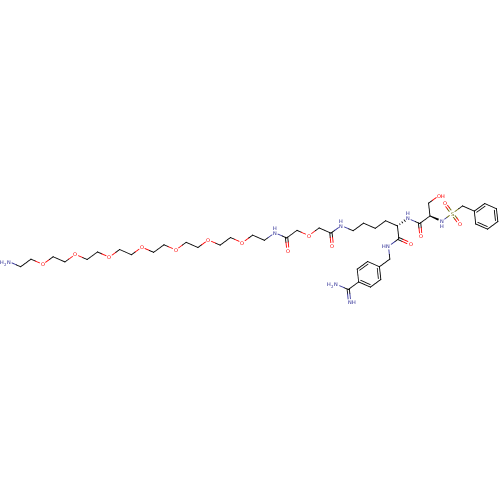
((2S)-6-[[2-[2-[2-[2-[2-[2-[2-[2-[2-(2-aminoethoxy)...)Show SMILES NCCOCCOCCOCCOCCOCCOCCOCCNC(=O)COCC(=O)NCCCC[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C44H72N8O15S/c45-13-16-60-18-20-62-22-24-64-26-28-66-29-27-65-25-23-63-21-19-61-17-15-49-41(55)33-67-32-40(54)48-14-5-4-8-38(43(56)50-30-35-9-11-37(12-10-35)42(46)47)51-44(57)39(31-53)52-68(58,59)34-36-6-2-1-3-7-36/h1-3,6-7,9-12,38-39,52-53H,4-5,8,13-34,45H2,(H3,46,47)(H,48,54)(H,49,55)(H,50,56)(H,51,57)/t38-,39+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 42 | -42.0 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98595
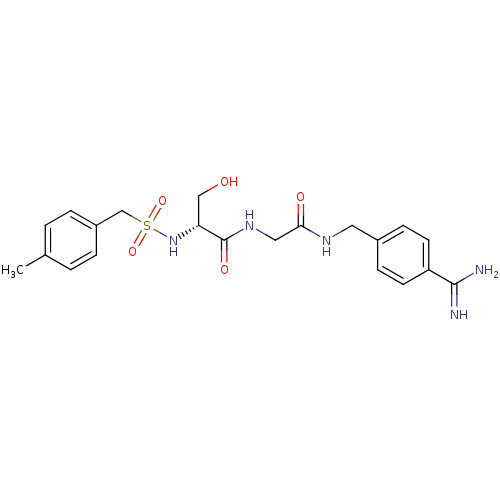
((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...)Show SMILES Cc1ccc(CS(=O)(=O)N[C@H](CO)C(=O)NCC(=O)NCc2ccc(cc2)C(N)=N)cc1 Show InChI InChI=1S/C21H27N5O5S/c1-14-2-4-16(5-3-14)13-32(30,31)26-18(12-27)21(29)25-11-19(28)24-10-15-6-8-17(9-7-15)20(22)23/h2-9,18,26-27H,10-13H2,1H3,(H3,22,23)(H,24,28)(H,25,29)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 58 | -41.2 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98590
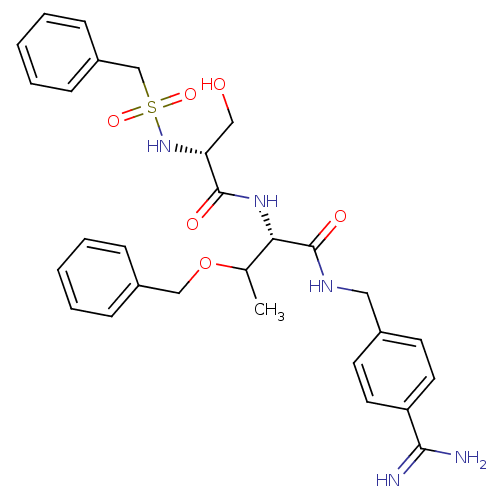
((2S)-3-benzyloxy-2-[[(2R)-2-(benzylsulfonylamino)-...)Show SMILES CC(OCc1ccccc1)[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C29H35N5O6S/c1-20(40-18-22-8-4-2-5-9-22)26(29(37)32-16-21-12-14-24(15-13-21)27(30)31)33-28(36)25(17-35)34-41(38,39)19-23-10-6-3-7-11-23/h2-15,20,25-26,34-35H,16-19H2,1H3,(H3,30,31)(H,32,37)(H,33,36)/t20?,25-,26+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 61 | -41.0 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98591
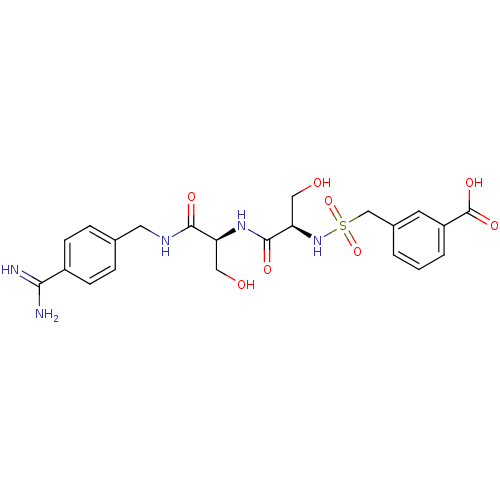
(3-[[(1R)-2-[[(1S)-2-[(4-carbamimidoylphenyl)methyl...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CO)NC(=O)[C@@H](CO)NS(=O)(=O)Cc2cccc(c2)C(O)=O)cc1 Show InChI InChI=1S/C22H27N5O8S/c23-19(24)15-6-4-13(5-7-15)9-25-20(30)17(10-28)26-21(31)18(11-29)27-36(34,35)12-14-2-1-3-16(8-14)22(32)33/h1-8,17-18,27-29H,9-12H2,(H3,23,24)(H,25,30)(H,26,31)(H,32,33)/t17-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 75 | -40.5 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98581
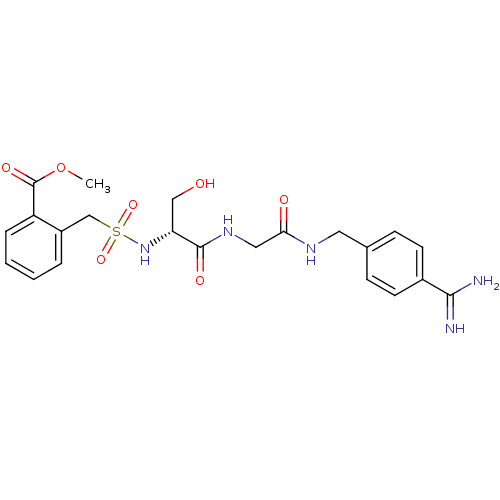
(US8476306, 6.6 | methyl 2-[[(1R)-2-[[2-[(4-carbami...)Show SMILES COC(=O)c1ccccc1CS(=O)(=O)N[C@H](CO)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H27N5O7S/c1-34-22(31)17-5-3-2-4-16(17)13-35(32,33)27-18(12-28)21(30)26-11-19(29)25-10-14-6-8-15(9-7-14)20(23)24/h2-9,18,27-28H,10-13H2,1H3,(H3,23,24)(H,25,29)(H,26,30)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 83 | -40.3 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98585
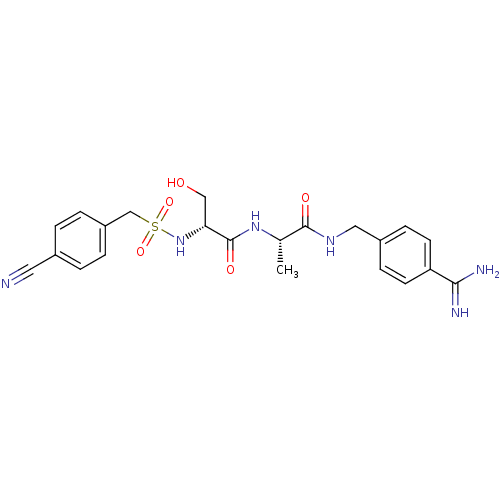
((2R)-N-[(1S)-2-[(4-carbamimidoylphenyl)methylamino...)Show SMILES C[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccc(cc1)C#N)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H26N6O5S/c1-14(21(30)26-11-16-6-8-18(9-7-16)20(24)25)27-22(31)19(12-29)28-34(32,33)13-17-4-2-15(10-23)3-5-17/h2-9,14,19,28-29H,11-13H2,1H3,(H3,24,25)(H,26,30)(H,27,31)/t14-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 89 | -40.1 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
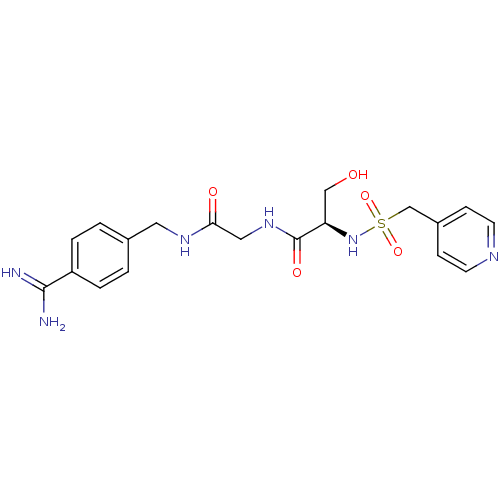
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98598
((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccncc2)cc1 Show InChI InChI=1S/C19H24N6O5S/c20-18(21)15-3-1-13(2-4-15)9-23-17(27)10-24-19(28)16(11-26)25-31(29,30)12-14-5-7-22-8-6-14/h1-8,16,25-26H,9-12H2,(H3,20,21)(H,23,27)(H,24,28)/t16-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 100 | -39.8 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98603
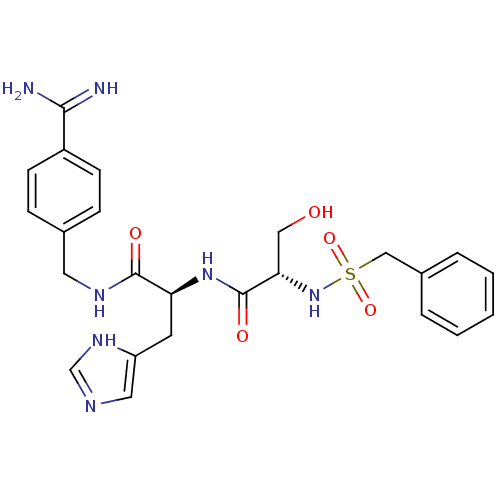
(3-amino-N-[4-[[(2S)-2-[[(2R)-2-(benzylsulfonylamin...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](Cc2cnc[nH]2)NC(=O)[C@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C24H29N7O5S/c25-22(26)18-8-6-16(7-9-18)11-28-23(33)20(10-19-12-27-15-29-19)30-24(34)21(13-32)31-37(35,36)14-17-4-2-1-3-5-17/h1-9,12,15,20-21,31-32H,10-11,13-14H2,(H3,25,26)(H,27,29)(H,28,33)(H,30,34)/t20-,21-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 110 | -39.6 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98589
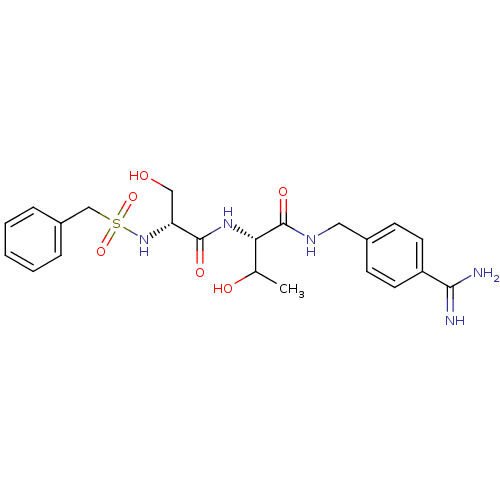
((2S)-2-[[(2R)-2-(benzylsulfonylamino)-3-hydroxy-pr...)Show SMILES CC(O)[C@H](NC(=O)[C@@H](CO)NS(=O)(=O)Cc1ccccc1)C(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C22H29N5O6S/c1-14(29)19(22(31)25-11-15-7-9-17(10-8-15)20(23)24)26-21(30)18(12-28)27-34(32,33)13-16-5-3-2-4-6-16/h2-10,14,18-19,27-29H,11-13H2,1H3,(H3,23,24)(H,25,31)(H,26,30)/t14?,18-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 110 | -39.6 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98597
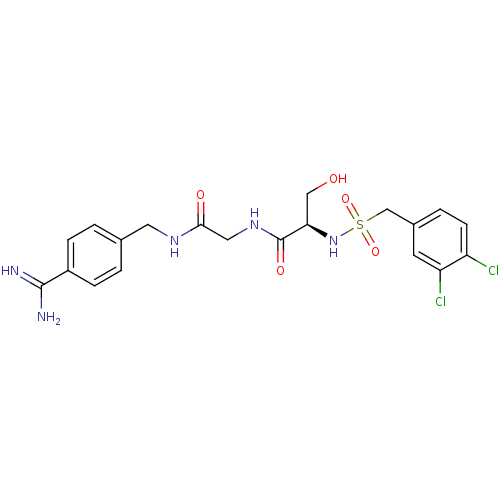
((2R)-N-[2-[(4-carbamimidoylphenyl)methylamino]-2-o...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccc(Cl)c(Cl)c2)cc1 Show InChI InChI=1S/C20H23Cl2N5O5S/c21-15-6-3-13(7-16(15)22)11-33(31,32)27-17(10-28)20(30)26-9-18(29)25-8-12-1-4-14(5-2-12)19(23)24/h1-7,17,27-28H,8-11H2,(H3,23,24)(H,25,29)(H,26,30)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 110 | -39.6 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98586
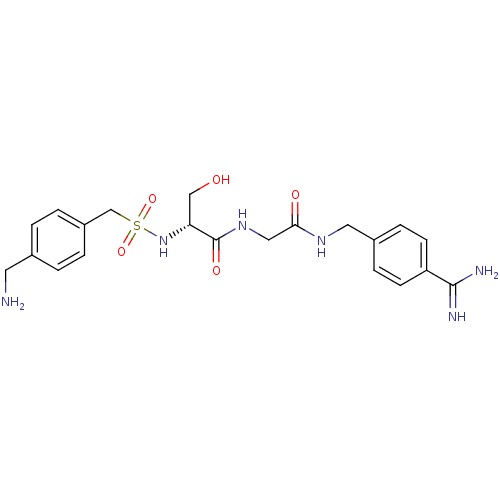
((2R)-2-[[4-(aminomethyl)phenyl]methylsulfonylamino...)Show SMILES NCc1ccc(CS(=O)(=O)N[C@H](CO)C(=O)NCC(=O)NCc2ccc(cc2)C(N)=N)cc1 Show InChI InChI=1S/C21H28N6O5S/c22-9-14-1-3-16(4-2-14)13-33(31,32)27-18(12-28)21(30)26-11-19(29)25-10-15-5-7-17(8-6-15)20(23)24/h1-8,18,27-28H,9-13,22H2,(H3,23,24)(H,25,29)(H,26,30)/t18-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 120 | -39.4 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM50110015

(CHEMBL158936 | N-(BENZYLSULFONYL)SERYL-N~1~-{4-[AM...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccccc2)cc1 Show InChI InChI=1S/C20H25N5O5S/c21-19(22)16-8-6-14(7-9-16)10-23-18(27)11-24-20(28)17(12-26)25-31(29,30)13-15-4-2-1-3-5-15/h1-9,17,25-26H,10-13H2,(H3,21,22)(H,23,27)(H,24,28)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 120 | -39.4 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98604
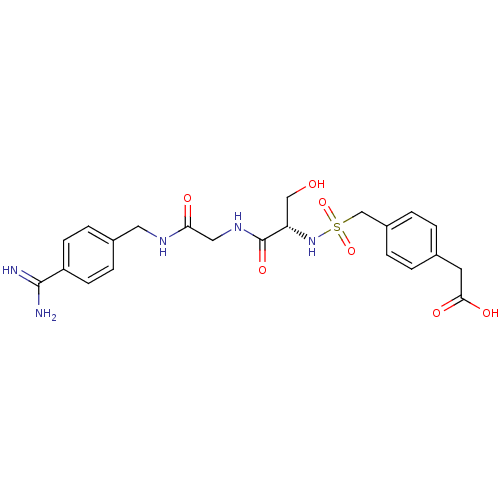
(2-[4-[[(1R)-2-[[2-[4-[(3-amino-4-methoxy-benzoyl)a...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@H](CO)NS(=O)(=O)Cc2ccc(CC(O)=O)cc2)cc1 Show InChI InChI=1S/C22H27N5O7S/c23-21(24)17-7-5-15(6-8-17)10-25-19(29)11-26-22(32)18(12-28)27-35(33,34)13-16-3-1-14(2-4-16)9-20(30)31/h1-8,18,27-28H,9-13H2,(H3,23,24)(H,25,29)(H,26,32)(H,30,31)/t18-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| US Patent
| 130 | -39.2 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98580
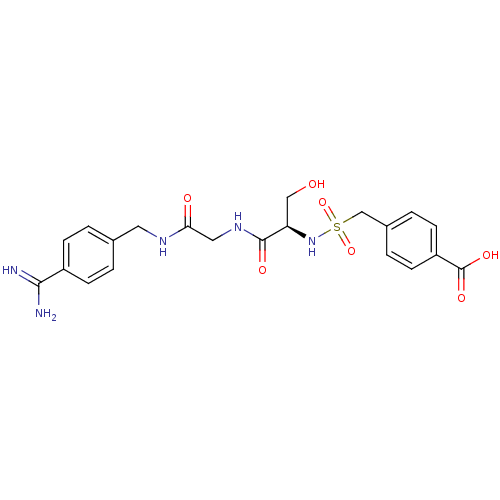
(4-[[(1R)-2-[[2-[(4-carbamimidoylphenyl)methylamino...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccc(cc2)C(O)=O)cc1 Show InChI InChI=1S/C21H25N5O7S/c22-19(23)15-5-1-13(2-6-15)9-24-18(28)10-25-20(29)17(11-27)26-34(32,33)12-14-3-7-16(8-4-14)21(30)31/h1-8,17,26-27H,9-12H2,(H3,22,23)(H,24,28)(H,25,29)(H,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 150 | -38.8 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98578
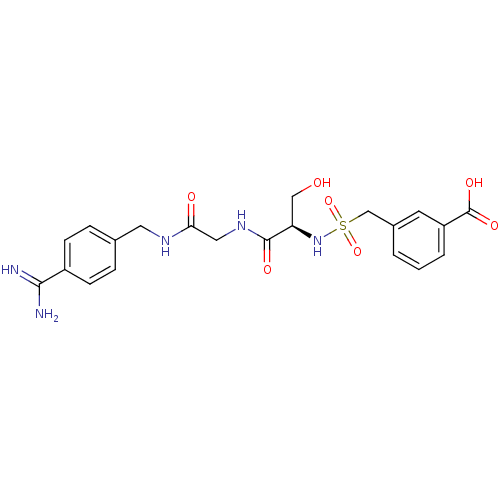
(3-[[(1R)-2-[[2-[(4-carbamimidoylphenyl)methylamino...)Show SMILES NC(=N)c1ccc(CNC(=O)CNC(=O)[C@@H](CO)NS(=O)(=O)Cc2cccc(c2)C(O)=O)cc1 Show InChI InChI=1S/C21H25N5O7S/c22-19(23)15-6-4-13(5-7-15)9-24-18(28)10-25-20(29)17(11-27)26-34(32,33)12-14-2-1-3-16(8-14)21(30)31/h1-8,17,26-27H,9-12H2,(H3,22,23)(H,24,28)(H,25,29)(H,30,31)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 160 | -38.7 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98602
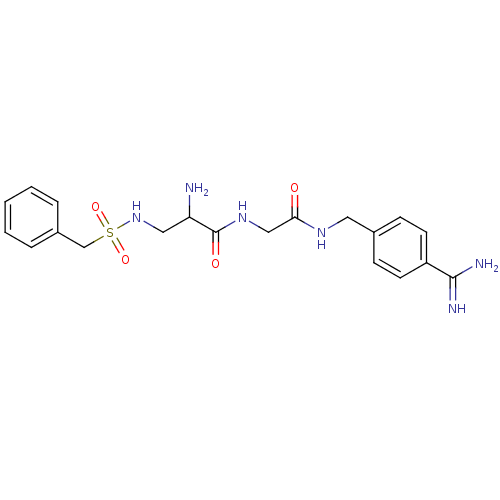
(3-amino-N-[4-[[2-[[(2R)-3-amino-2-(benzylsulfonyla...)Show SMILES NC(CNS(=O)(=O)Cc1ccccc1)C(=O)NCC(=O)NCc1ccc(cc1)C(N)=N Show InChI InChI=1S/C20H26N6O4S/c21-17(11-26-31(29,30)13-15-4-2-1-3-5-15)20(28)25-12-18(27)24-10-14-6-8-16(9-7-14)19(22)23/h1-9,17,26H,10-13,21H2,(H3,22,23)(H,24,27)(H,25,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| 180 | -38.4 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98593
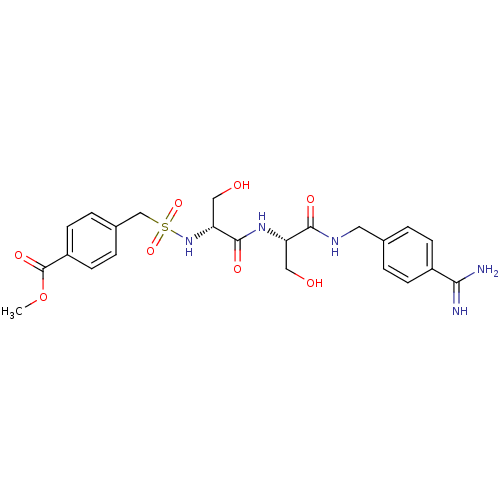
(US8476306, 6.19 | methyl 4-[[(1R)-2-[[(1S)-2-[(4-c...)Show SMILES COC(=O)c1ccc(CS(=O)(=O)N[C@H](CO)C(=O)N[C@@H](CO)C(=O)NCc2ccc(cc2)C(N)=N)cc1 Show InChI InChI=1S/C23H29N5O8S/c1-36-23(33)17-8-4-15(5-9-17)13-37(34,35)28-19(12-30)22(32)27-18(11-29)21(31)26-10-14-2-6-16(7-3-14)20(24)25/h2-9,18-19,28-30H,10-13H2,1H3,(H3,24,25)(H,26,31)(H,27,32)/t18-,19+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 230 | -37.8 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |
Urokinase plasminogen activator surface receptor
(Rattus norvegicus) | BDBM98592
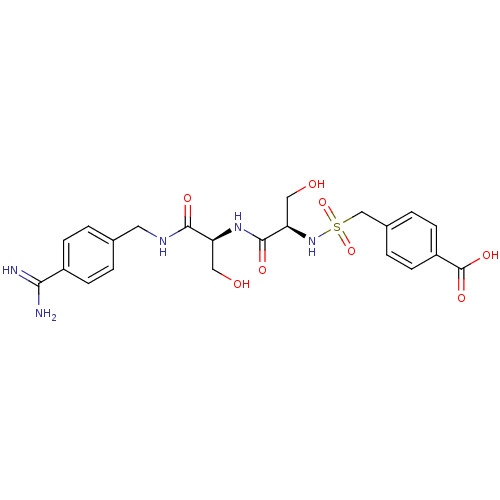
(4-[[(1R)-2-[[(1S)-2-[(4-carbamimidoylphenyl)methyl...)Show SMILES NC(=N)c1ccc(CNC(=O)[C@H](CO)NC(=O)[C@@H](CO)NS(=O)(=O)Cc2ccc(cc2)C(O)=O)cc1 Show InChI InChI=1S/C22H27N5O8S/c23-19(24)15-5-1-13(2-6-15)9-25-20(30)17(10-28)26-21(31)18(11-29)27-36(34,35)12-14-3-7-16(8-4-14)22(32)33/h1-8,17-18,27-29H,9-12H2,(H3,23,24)(H,25,30)(H,26,31)(H,32,33)/t17-,18+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 230 | -37.8 | n/a | n/a | n/a | n/a | n/a | n/a | 24 |
The Medicines Company
US Patent
| Assay Description
Inhibition constant of the compound against Plasminogen activator urokinase |
US Patent US8476306 (2013)
BindingDB Entry DOI: 10.7270/Q2154FPH |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data