

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471710 (US10829438, Example 1L | US11174219, Example 1L) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471714 (US10829438, Example 6A | US11174219, Example 6A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471712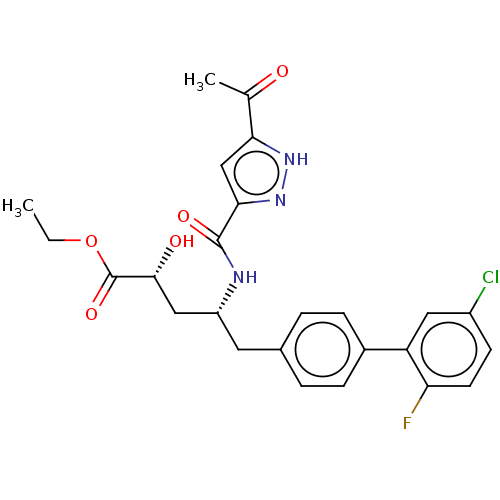 (US10829438, Example 4B | US11174219, Example 4B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471713 (US10829438, Example 5B | US11174219, Example 5B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471705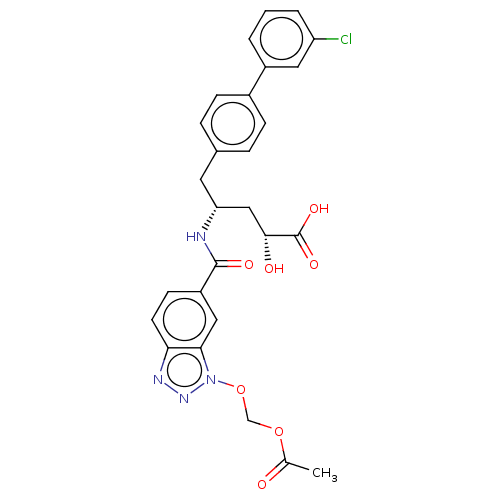 (US10829438, Example 1G | US11174219, Example 1G) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | US Patent US10829438 (2020) BindingDB Entry DOI: 10.7270/Q29C71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471710 (US10829438, Example 1L | US11174219, Example 1L) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | US Patent US10829438 (2020) BindingDB Entry DOI: 10.7270/Q29C71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471712 (US10829438, Example 4B | US11174219, Example 4B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | US Patent US10829438 (2020) BindingDB Entry DOI: 10.7270/Q29C71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471713 (US10829438, Example 5B | US11174219, Example 5B) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | US Patent US10829438 (2020) BindingDB Entry DOI: 10.7270/Q29C71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471705 (US10829438, Example 1G | US11174219, Example 1G) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471714 (US10829438, Example 6A | US11174219, Example 6A) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | <1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | US Patent US10829438 (2020) BindingDB Entry DOI: 10.7270/Q29C71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407299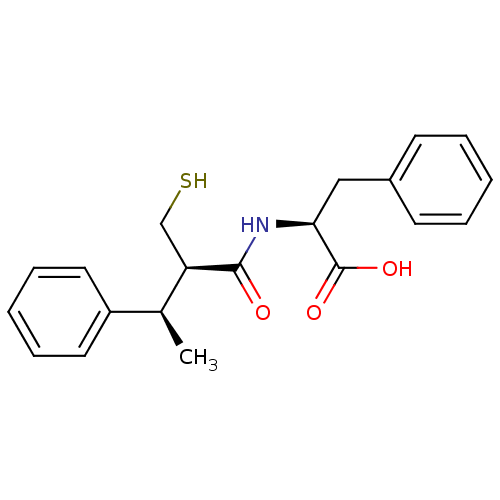 (CHEMBL2052007) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407299 (CHEMBL2052007) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471711 (US10829438, Example 1M | US11174219, Example 1M) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | US Patent US10829438 (2020) BindingDB Entry DOI: 10.7270/Q29C71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471711 (US10829438, Example 1M | US11174219, Example 1M) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 3.55 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407298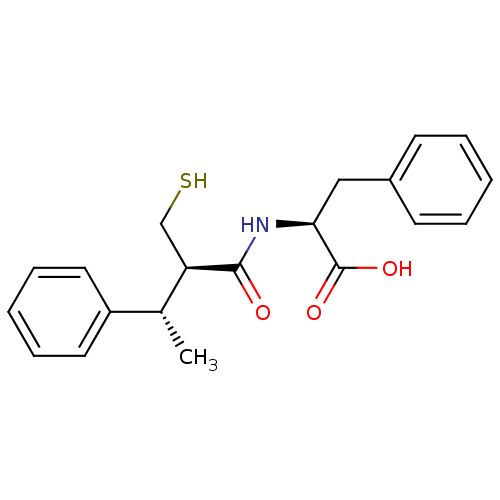 (CHEMBL2051772) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407297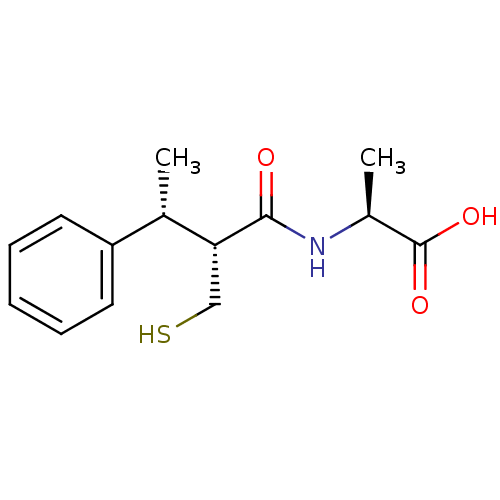 (CHEMBL2052008) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471709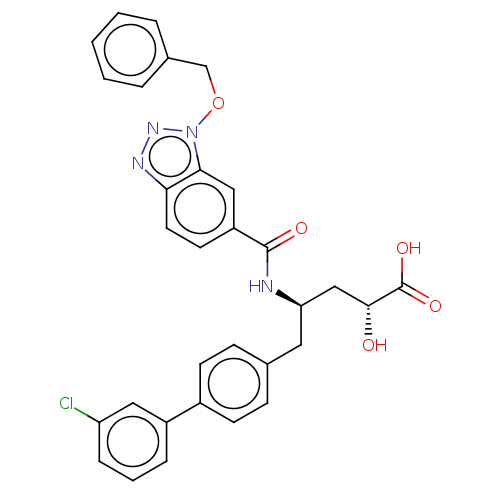 (US10829438, Example 1I | US11174219, Example 1I) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 35.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Theravance Biopharma R&D IP, LLC US Patent | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | US Patent US10829438 (2020) BindingDB Entry DOI: 10.7270/Q29C71H0 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme/Ribosomal RNA small subunit methyltransferase NEP1 (Human-Homo sapiens (Human)) | BDBM471709 (US10829438, Example 1I | US11174219, Example 1I) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | US Patent | 35.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
TBA | Assay Description The assays were performed in 384-well white opaque plates at 37° C. using the fluorogenic peptide substrates at a concentration of 10 μM in Assa... | Citation and Details BindingDB Entry DOI: 10.7270/Q20V8H0G | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50407297 (CHEMBL2052008) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50001591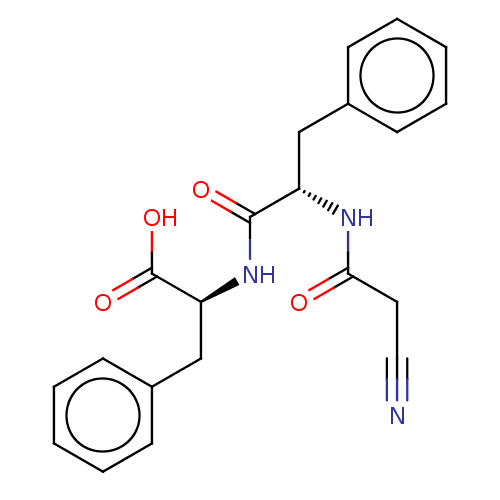 ((S)-2-[(S)-2-(2-Cyano-acetylamino)-1-oxo-3-phenyl-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 6.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Baxter Diagnostics Inc. Curated by ChEMBL | Assay Description Inhibition of hydrolysis of N-[3-(2-furyl)acryloyll-Phe-Gly-Gly7 by angiotensin I converting enzyme | J Med Chem 35: 4175-9 (1992) BindingDB Entry DOI: 10.7270/Q2416W1P | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50017129 ((S)-1-((S)-2-((R)-1-ethoxy-1-oxo-4-phenylbutan-2-y...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibition against angiotensin converting enzyme (ACE) | Bioorg Med Chem Lett 4: 2673-2676 (1994) Article DOI: 10.1016/S0960-894X(01)80694-6 BindingDB Entry DOI: 10.7270/Q2X34XXT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50367254 (ENALAPRILAT) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PDB PubMed | n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Rorer Central Research Curated by ChEMBL | Assay Description In vitro inhibition of Angiotensin I converting enzyme in Hog plasma | J Med Chem 33: 1606-15 (1990) BindingDB Entry DOI: 10.7270/Q2CF9QPK | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027142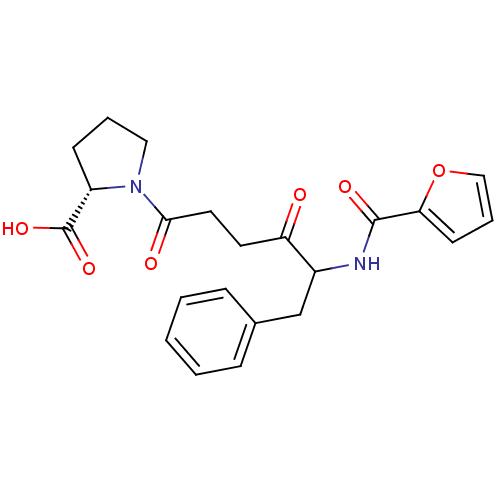 (1-{5-[(Furan-2-carbonyl)-amino]-4-oxo-6-phenyl-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | J Med Chem 24: 964-9 (1982) BindingDB Entry DOI: 10.7270/Q2959J4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027132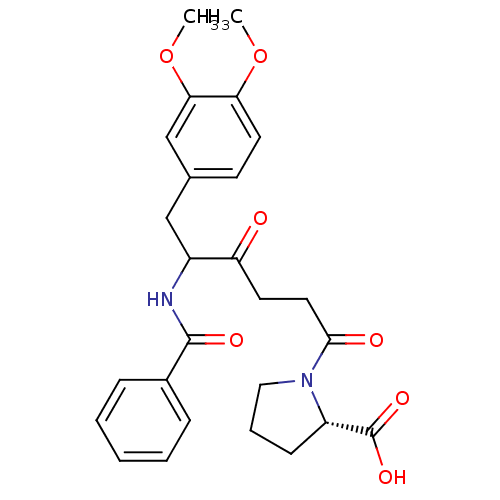 (1-[5-Benzoylamino-6-(3,4-dimethoxy-phenyl)-4-oxo-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | J Med Chem 24: 964-9 (1982) BindingDB Entry DOI: 10.7270/Q2959J4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027344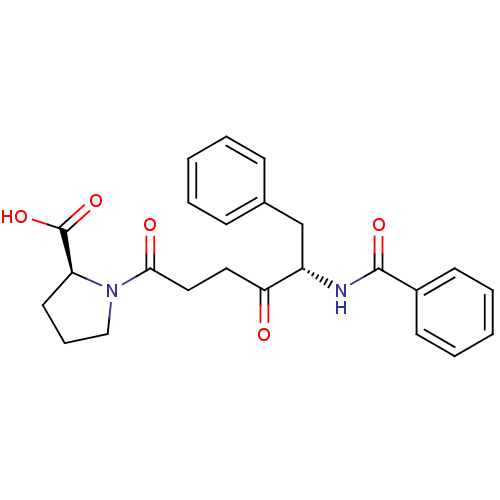 ((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | J Med Chem 24: 964-9 (1982) BindingDB Entry DOI: 10.7270/Q2959J4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027344 ((S)-1-((S)-5-benzamido-4-oxo-6-phenylhexanoyl)pyrr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Angiotensin I converting enzyme | J Med Chem 25: 996-9 (1982) BindingDB Entry DOI: 10.7270/Q2154G1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041062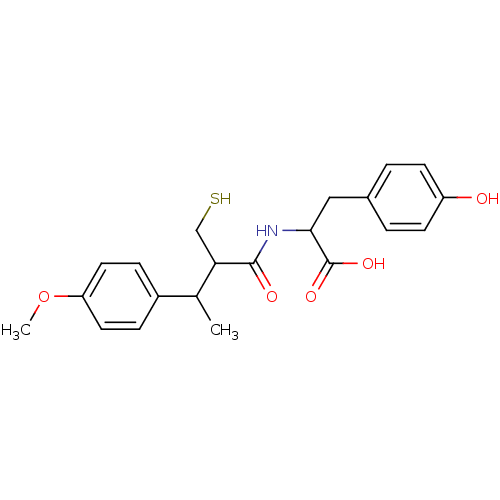 (3-(4-Hydroxy-phenyl)-2-[2-mercaptomethyl-3-(4-meth...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041063 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-pentano...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041060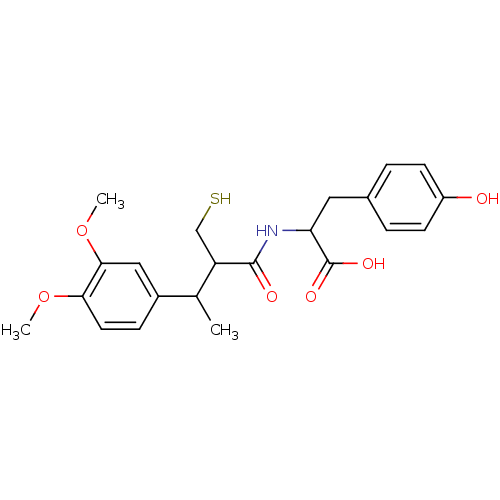 (2-[3-(3,4-Dimethoxy-phenyl)-2-mercaptomethyl-butyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041054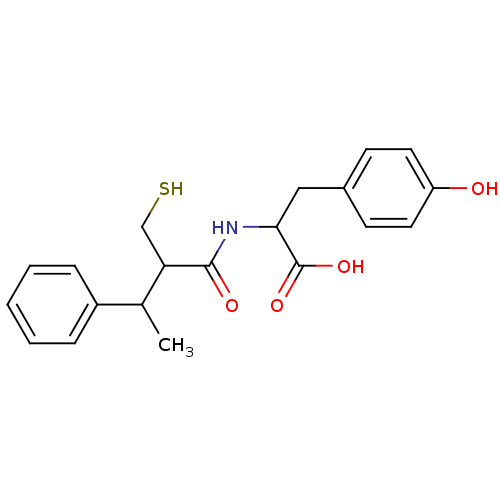 (3-(4-Hydroxy-phenyl)-2-(2-mercaptomethyl-3-phenyl-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027476 (1-[2-(3-Benzoylamino-2-oxo-4-phenyl-butoxy)-acetyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Angiotensin I converting enzyme | J Med Chem 25: 996-9 (1982) BindingDB Entry DOI: 10.7270/Q2154G1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041057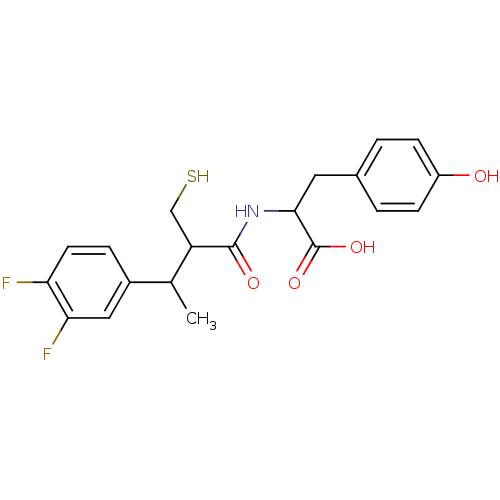 (2-[3-(3,4-Difluoro-phenyl)-2-mercaptomethyl-butyry...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027147 (1-[5-Benzoylamino-6-(4-hydroxy-phenyl)-4-oxo-hexan...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | J Med Chem 24: 964-9 (1982) BindingDB Entry DOI: 10.7270/Q2959J4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041053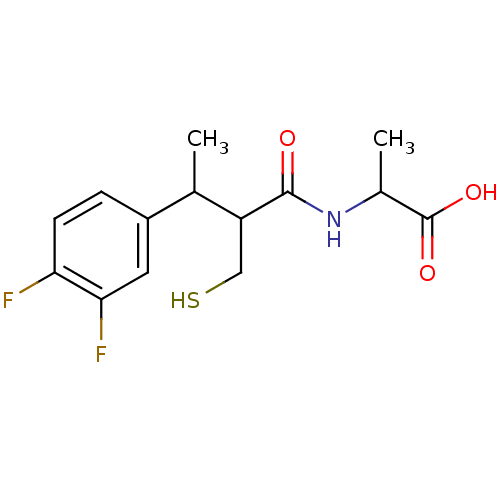 (2-[3-(3,4-Difluoro-phenyl)-2-mercaptomethyl-butyry...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041052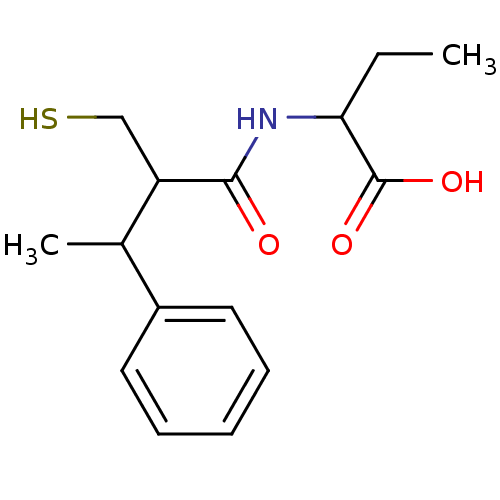 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-butyric...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027144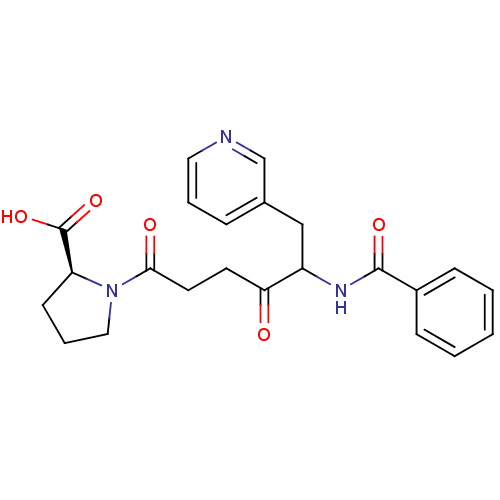 (1-(5-Benzoylamino-4-oxo-6-pyridin-3-yl-hexanoyl)-p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 5.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | J Med Chem 24: 964-9 (1982) BindingDB Entry DOI: 10.7270/Q2959J4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041064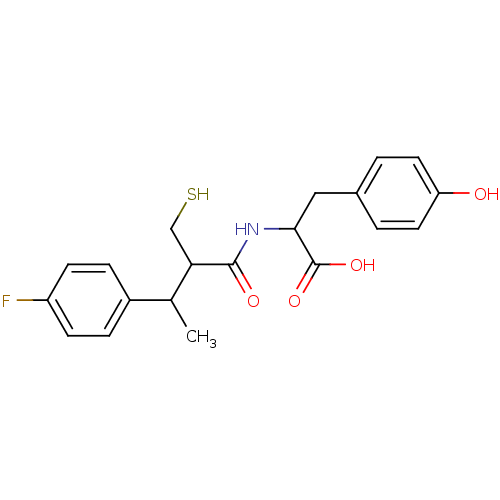 (2-[3-(4-Fluoro-phenyl)-2-mercaptomethyl-butyrylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041061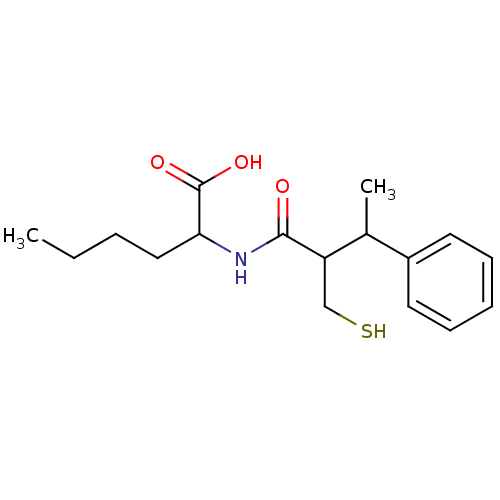 (2-(2-Mercaptomethyl-3-phenyl-butyrylamino)-hexanoi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041068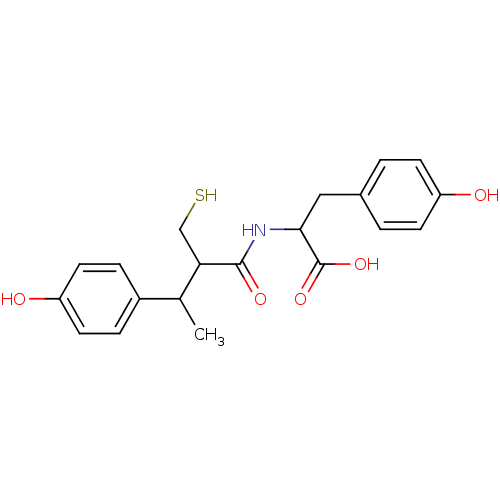 (3-(4-Hydroxy-phenyl)-2-[3-(4-hydroxy-phenyl)-2-mer...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | J Med Chem 24: 964-9 (1982) BindingDB Entry DOI: 10.7270/Q2959J4Z | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041058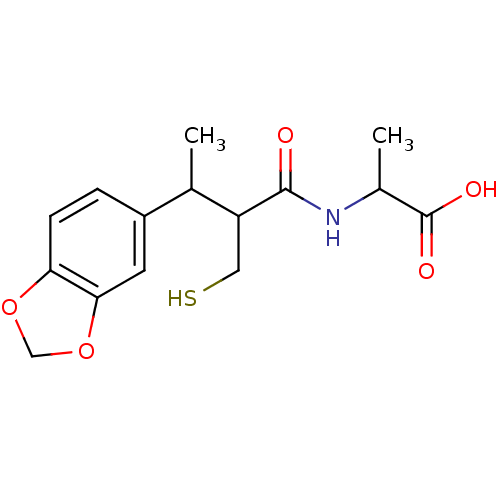 (2-(3-Benzo[1,3]dioxol-5-yl-2-mercaptomethyl-butyry...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 9.5 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027146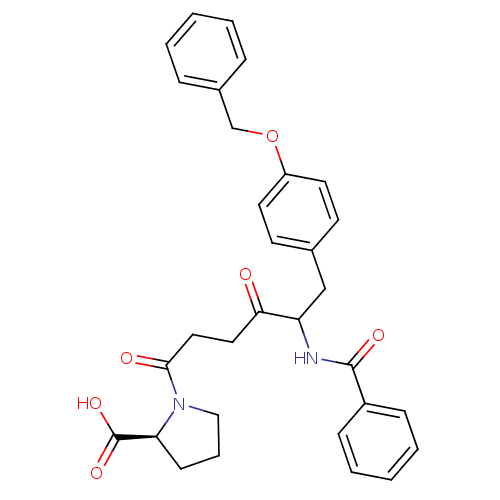 (1-[5-Benzoylamino-6-(4-benzyloxy-phenyl)-4-oxo-hex...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required to inhibit the activity of Angiotensin I converting enzyme by 50% | J Med Chem 24: 964-9 (1982) BindingDB Entry DOI: 10.7270/Q2959J4Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041067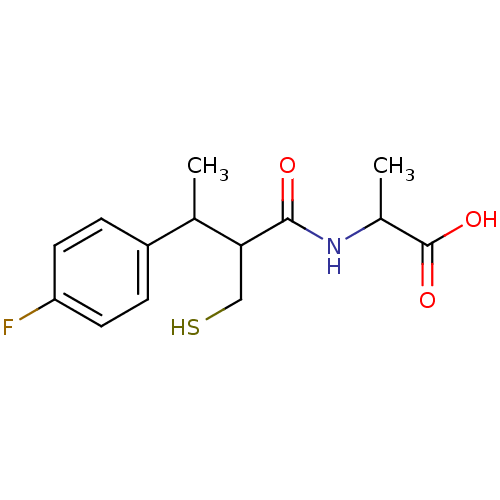 (2-[3-(4-Fluoro-phenyl)-2-mercaptomethyl-butyrylami...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article | n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description In vitro inhibitory concentration against angiotensin-converting enzyme | Bioorg Med Chem Lett 4: 2715-2720 (1994) Article DOI: 10.1016/S0960-894X(01)80703-4 BindingDB Entry DOI: 10.7270/Q2SB467B | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM21642 ((2S)-1-[(2S)-2-methyl-3-sulfanylpropanoyl]pyrrolid...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Angiotensin I converting enzyme | J Med Chem 24: 104-9 (1981) BindingDB Entry DOI: 10.7270/Q2ZW1MGC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50027478 (1-[2-(3-Benzoylamino-2-oxo-4-phenyl-butylsulfanyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Concentration required for 50% inhibition of Angiotensin I converting enzyme | J Med Chem 25: 996-9 (1982) BindingDB Entry DOI: 10.7270/Q2154G1D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041055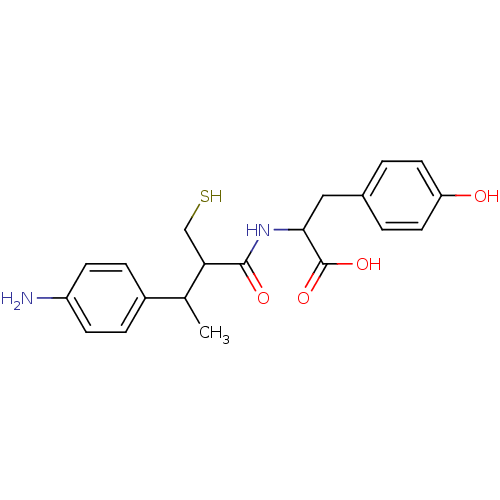 (2-[3-(4-Amino-phenyl)-2-mercaptomethyl-butyrylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50367254 (ENALAPRILAT) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Ranbaxy Laboratories Limited Curated by ChEMBL | Assay Description Inhibitory activity against human renin inhibition (at pH 7.4) | Bioorg Med Chem Lett 27: 2313-2318 (2017) Article DOI: 10.1016/j.bmcl.2017.04.036 BindingDB Entry DOI: 10.7270/Q2ZC858S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme 2 (Homo sapiens (Human)) | BDBM50286721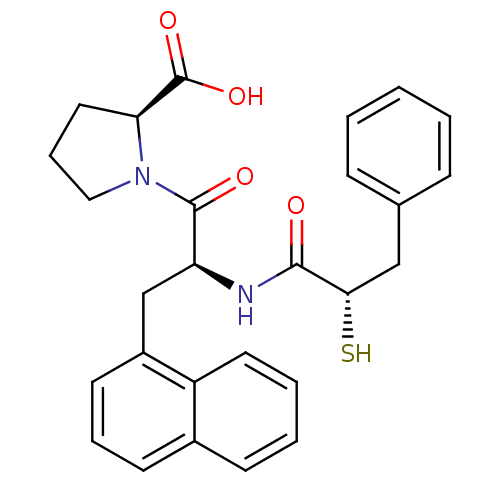 ((S)-1-[(S)-2-((S)-2-Mercapto-3-phenyl-propionylami...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Inhibition of angiotensin converting enzyme (ACE) | Bioorg Med Chem Lett 5: 735-738 (1995) Article DOI: 10.1016/0960-894X(95)00105-3 BindingDB Entry DOI: 10.7270/Q2611097 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Angiotensin-converting enzyme (Mus musculus) | BDBM50041051 (2-[3-(4-Hydroxy-3-methoxy-phenyl)-2-mercaptomethyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 14 | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Inhibitory potency against angiotensin converting enzyme by displacement of [3H]-trandolaprilate binding in mouse lung | J Med Chem 37: 1070-83 (1994) BindingDB Entry DOI: 10.7270/Q26W995T | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 159 total ) | Next | Last >> |