

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441773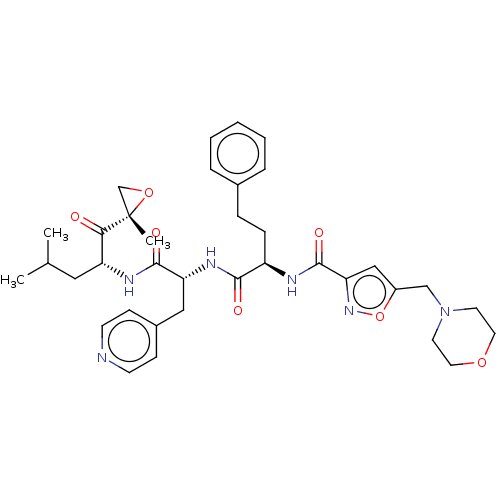 (US10640533, Identification number CX13-103 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.920 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441775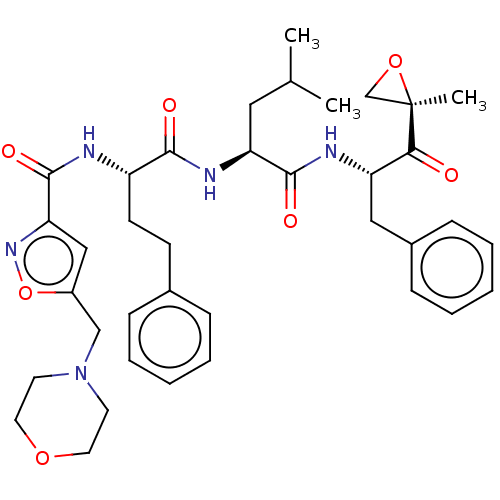 (US10640533, Identification number CX13-105 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 0.970 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441773 (US10640533, Identification number CX13-103 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.09 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441775 (US10640533, Identification number CX13-105 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.34 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50031442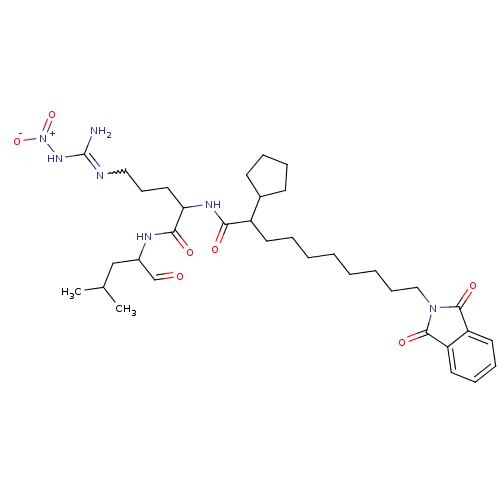 (1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | Article | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain | Bioorg Med Chem Lett 6: 287-290 (1996) Article DOI: 10.1016/0960-894X(96)00014-5 BindingDB Entry DOI: 10.7270/Q2JQ1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441779 (US10640533, Identification number CX13-133 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.58 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441789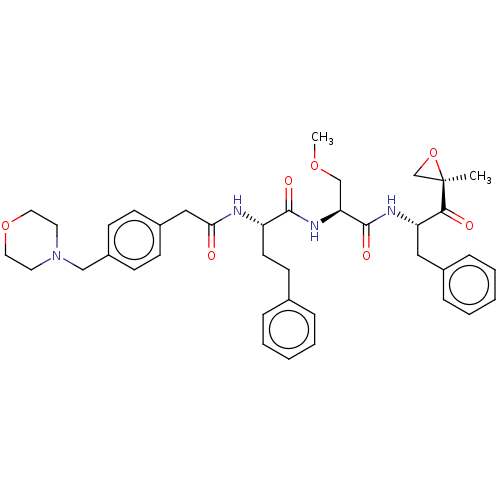 (US10640533, Identification number CX13-608 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3.73 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441789 (US10640533, Identification number CX13-608 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.26 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441784 (US10640533, Identification number CX13-600 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.28 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441779 (US10640533, Identification number CX13-133 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.65 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441776 (US10640533, Identification number CX13-107 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.74 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441776 (US10640533, Identification number CX13-107 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50031440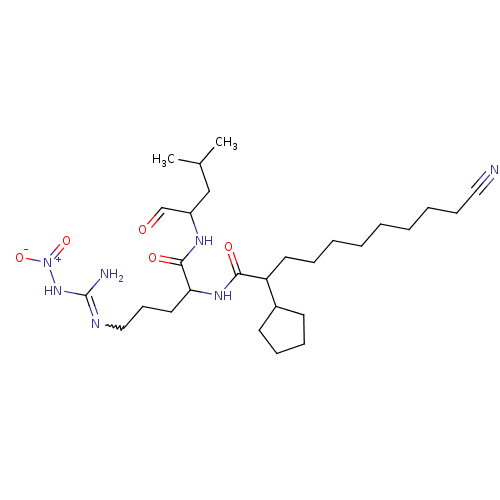 (1N-[4-amino(nitroimino)methylamino-1-(1-formyl-3-m...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain | Bioorg Med Chem Lett 6: 287-290 (1996) Article DOI: 10.1016/0960-894X(96)00014-5 BindingDB Entry DOI: 10.7270/Q2JQ1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441788 (US10640533, Identification number CX13-606 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.10 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441780 (US10640533, Identification number CX13-135 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.56 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441787 (US10640533, Identification number CX13-605 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 6.81 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441784 (US10640533, Identification number CX13-600 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 7.41 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50288611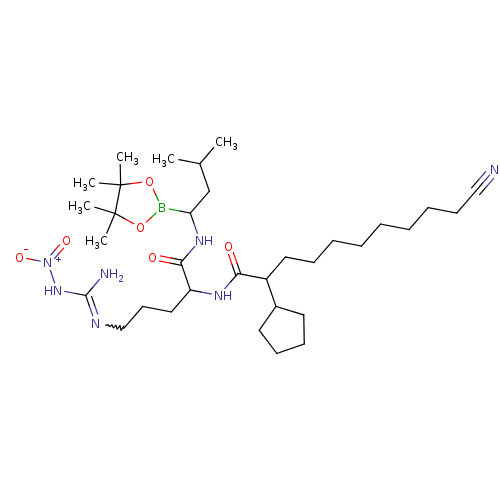 (Alpha-ketocarbonyl boronic ester derivative | CHEM...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain | Bioorg Med Chem Lett 6: 287-290 (1996) Article DOI: 10.1016/0960-894X(96)00014-5 BindingDB Entry DOI: 10.7270/Q2JQ1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441790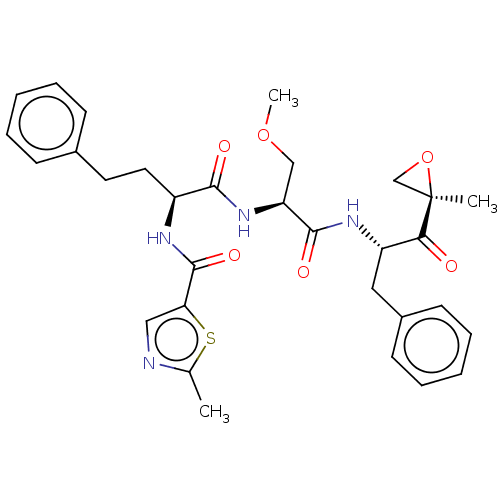 (US10640533, Identification number CX13-705 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 9.75 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441786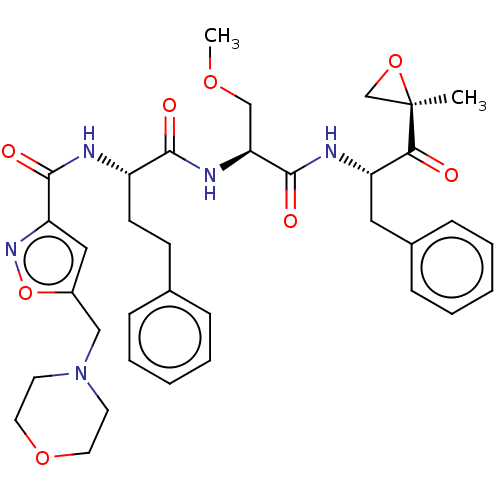 (US10640533, Identification number CX13-603 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 11.1 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441787 (US10640533, Identification number CX13-605 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.2 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441781 (US10640533, Identification number CX13-137 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 13.5 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441786 (US10640533, Identification number CX13-603 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 14.2 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441790 (US10640533, Identification number CX13-705 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.3 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441785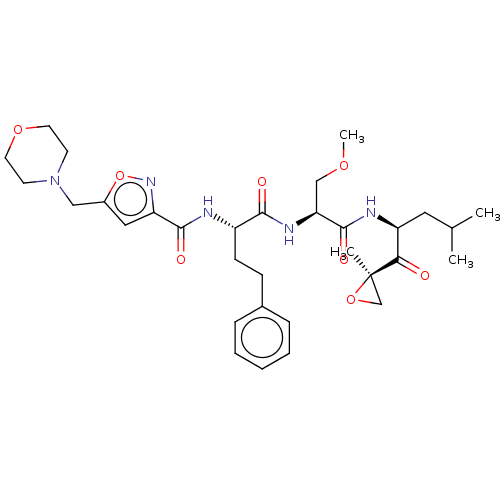 (US10640533, Identification number CX13-601 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50288609 (Alpha-ketocarbonyl derivative | CHEMBL317337) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain | Bioorg Med Chem Lett 6: 287-290 (1996) Article DOI: 10.1016/0960-894X(96)00014-5 BindingDB Entry DOI: 10.7270/Q2JQ1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50288609 (Alpha-ketocarbonyl derivative | CHEMBL317337) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain | Bioorg Med Chem Lett 6: 287-290 (1996) Article DOI: 10.1016/0960-894X(96)00014-5 BindingDB Entry DOI: 10.7270/Q2JQ1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441780 (US10640533, Identification number CX13-135 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 28.1 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441785 (US10640533, Identification number CX13-601 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 31.7 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441774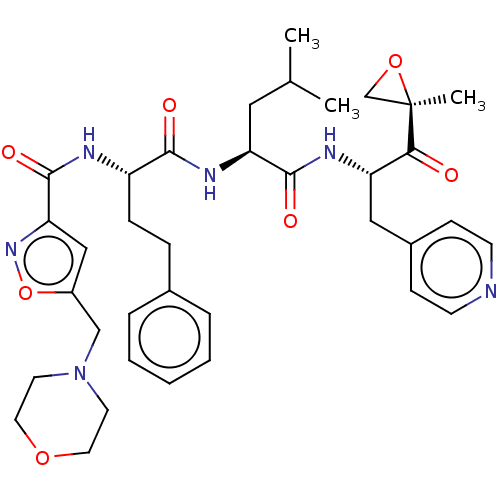 (US10640533, Identification number CX13-104 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33.3 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441774 (US10640533, Identification number CX13-104 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 33.6 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441788 (US10640533, Identification number CX13-606 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 59.3 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441781 (US10640533, Identification number CX13-137 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 60.4 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441778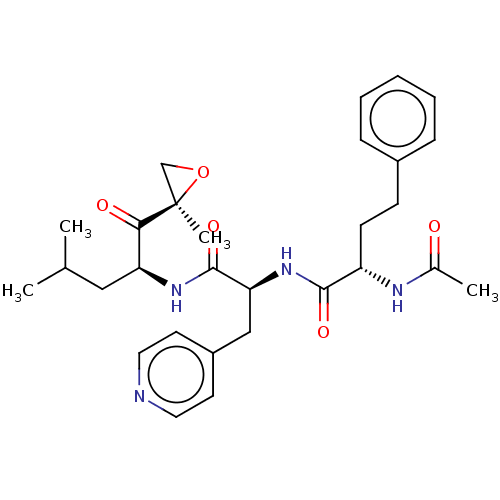 (US10640533, Identification number CX13-130 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 62.4 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484478 (CHEMBL1929019 | jm5b01461, Compound 47) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 65 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484484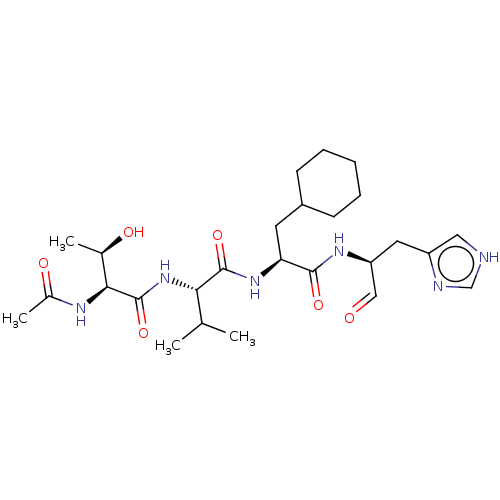 (CHEMBL1929023) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 98 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441783 (US10640533, Identification number CX13-501 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 113 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441782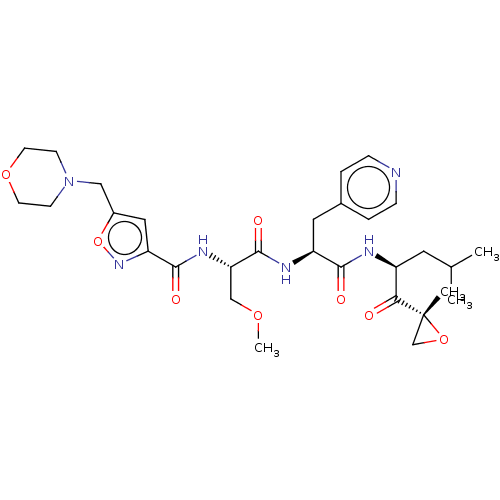 (US10640533, Identification number CX13-500 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 121 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50398607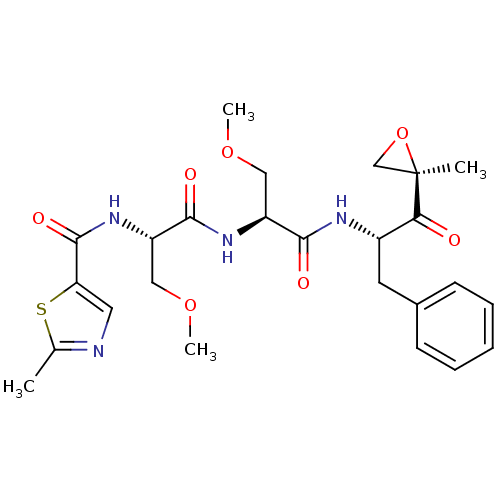 (ONX 0912 | OPROZOMIB | US10640533, Identification ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 124 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441782 (US10640533, Identification number CX13-500 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441778 (US10640533, Identification number CX13-130 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 248 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50398607 (ONX 0912 | OPROZOMIB | US10640533, Identification ...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | US Patent | n/a | n/a | 256 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484483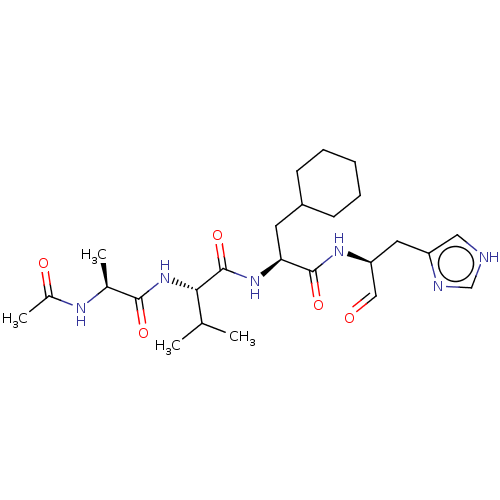 (CHEMBL1929020 | acs.jmedchem.1c00409_ST.132) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484491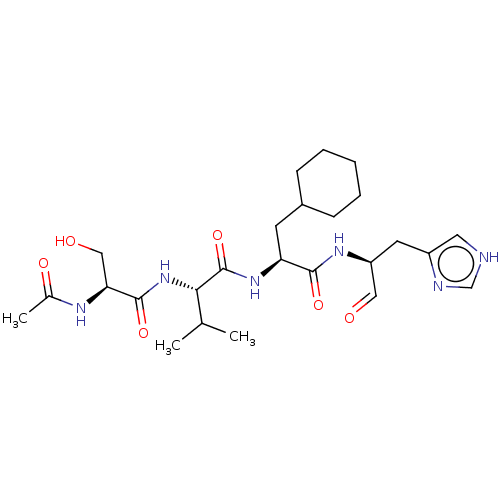 (CHEMBL1929022 | acs.jmedchem.1c00409_ST.144) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50484487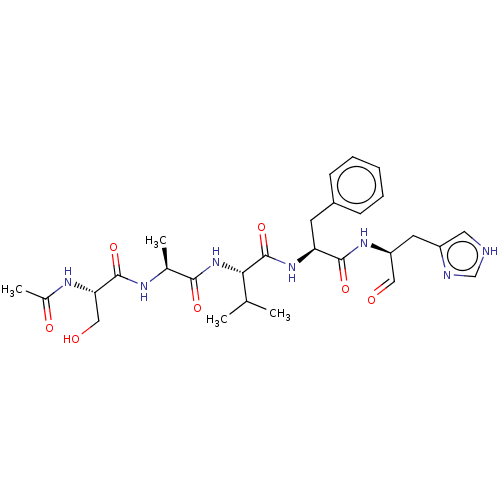 (CHEMBL1929018 | acs.jmedchem.1c00409_ST.150) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Kyoto Pharmaceutical University Curated by ChEMBL | Assay Description Inhibition of chymotrypsin-like protease R188I mutant using peptide substrate SO1 measured after 60 mins by HPLC analysis | J Med Chem 54: 7962-73 (2011) Article DOI: 10.1021/jm200870n BindingDB Entry DOI: 10.7270/Q29G5QNB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441783 (US10640533, Identification number CX13-501 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 392 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441777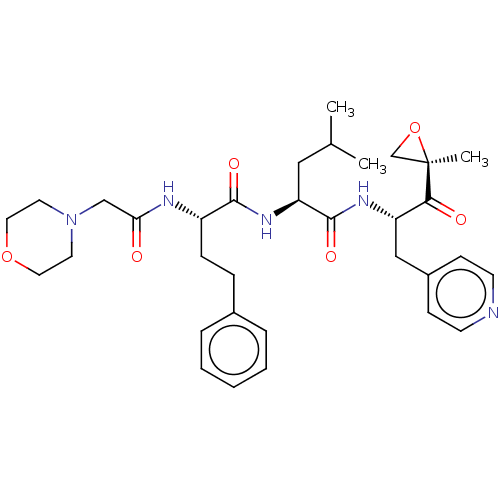 (US10640533, Identification number CX13-109 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM441777 (US10640533, Identification number CX13-109 | US106...) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | >500 | n/a | n/a | n/a | n/a | n/a | n/a |
CENTRAX INTERNATIONAL, INC. US Patent | Assay Description Inhibition of the chymotrypsin-like (CT-L), peptidylglutamyl peptide hydrolyzing activity (PGPH), and trypsin-like (T-L) activities of the 20S protea... | US Patent US10640533 (2020) BindingDB Entry DOI: 10.7270/Q28G8PQN | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50288610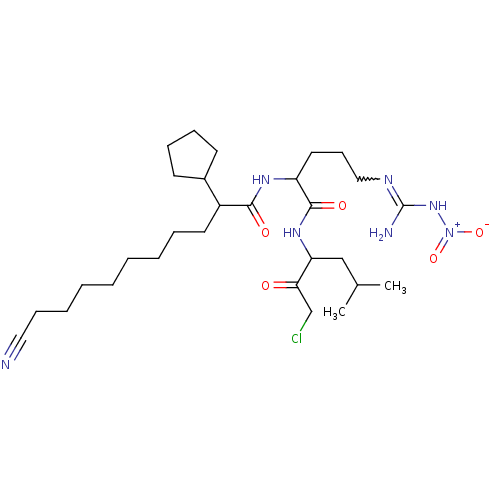 (Alpha-ketocarbonyl derivative | CHEMBL98937) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for thromboxane TXA2 synthase inhibitory activity using human platelet | Bioorg Med Chem Lett 6: 287-290 (1996) Article DOI: 10.1016/0960-894X(96)00014-5 BindingDB Entry DOI: 10.7270/Q2JQ1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Chymotrypsin-like protease CTRL-1 (Homo sapiens (Human)) | BDBM50288610 (Alpha-ketocarbonyl derivative | CHEMBL98937) | KEGG UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description Compound was tested for inhibition of chymotrypsin-like protease of proteasome from postmortem human liver and brain | Bioorg Med Chem Lett 6: 287-290 (1996) Article DOI: 10.1016/0960-894X(96)00014-5 BindingDB Entry DOI: 10.7270/Q2JQ1112 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |