 Found 114 hits of ic50 for UniProtKB: P20309
Found 114 hits of ic50 for UniProtKB: P20309 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280821

(2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES C[Si]1(C)CN(CCN2CCC(Cc3c[nH]c4ccc(F)cc34)CC2)S(=O)(=O)c2ccccc12 Show InChI InChI=1S/C25H32FN3O2SSi/c1-33(2)18-29(32(30,31)24-5-3-4-6-25(24)33)14-13-28-11-9-19(10-12-28)15-20-17-27-23-8-7-21(26)16-22(20)23/h3-8,16-17,19,27H,9-15,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280823
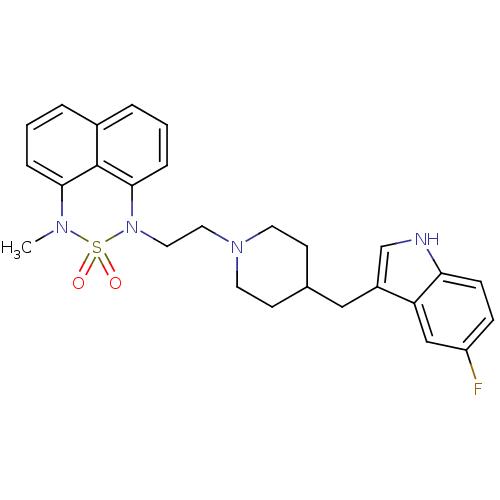
(1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES CN1c2cccc3cccc(N(CCN4CCC(Cc5c[nH]c6ccc(F)cc56)CC4)S1(=O)=O)c23 Show InChI InChI=1S/C27H29FN4O2S/c1-30-25-6-2-4-20-5-3-7-26(27(20)25)32(35(30,33)34)15-14-31-12-10-19(11-13-31)16-21-18-29-24-9-8-22(28)17-23(21)24/h2-9,17-19,29H,10-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280818
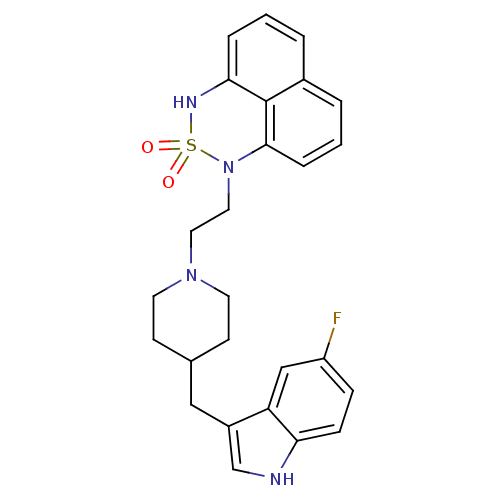
(1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCN4c5cccc6cccc(NS4(=O)=O)c56)CC3)c2c1 Show InChI InChI=1S/C26H27FN4O2S/c27-21-7-8-23-22(16-21)20(17-28-23)15-18-9-11-30(12-10-18)13-14-31-25-6-2-4-19-3-1-5-24(26(19)25)29-34(31,32)33/h1-8,16-18,28-29H,9-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280829
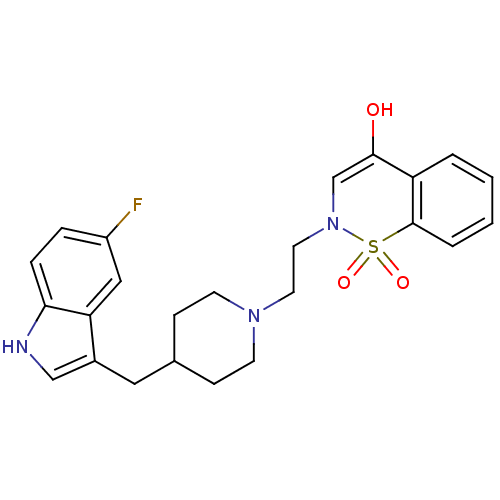
(2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES OC1=CN(CCN2CCC(Cc3c[nH]c4ccc(F)cc34)CC2)S(=O)(=O)c2ccccc12 |t:1| Show InChI InChI=1S/C24H26FN3O3S/c25-19-5-6-22-21(14-19)18(15-26-22)13-17-7-9-27(10-8-17)11-12-28-16-23(29)20-3-1-2-4-24(20)32(28,30)31/h1-6,14-17,26,29H,7-13H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280825

(1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Cn1c(=O)n(CCN2CCC(Cc3c[nH]c4ccc(F)cc34)CC2)c2ccccc2c1=O Show InChI InChI=1S/C25H27FN4O2/c1-28-24(31)20-4-2-3-5-23(20)30(25(28)32)13-12-29-10-8-17(9-11-29)14-18-16-27-22-7-6-19(26)15-21(18)22/h2-7,15-17,27H,8-14H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280822

(4-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCN4C(=O)CSc5ccccc45)CC3)c2c1 Show InChI InChI=1S/C24H26FN3OS/c25-19-5-6-21-20(14-19)18(15-26-21)13-17-7-9-27(10-8-17)11-12-28-22-3-1-2-4-23(22)30-16-24(28)29/h1-6,14-15,17,26H,7-13,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280820

(1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES COc1nc2cccc3cccc(n1CCN1CCC(Cc4c[nH]c5ccc(F)cc45)CC1)c23 Show InChI InChI=1S/C28H29FN4O/c1-34-28-31-25-6-2-4-20-5-3-7-26(27(20)25)33(28)15-14-32-12-10-19(11-13-32)16-21-18-30-24-9-8-22(29)17-23(21)24/h2-9,17-19,30H,10-16H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280827
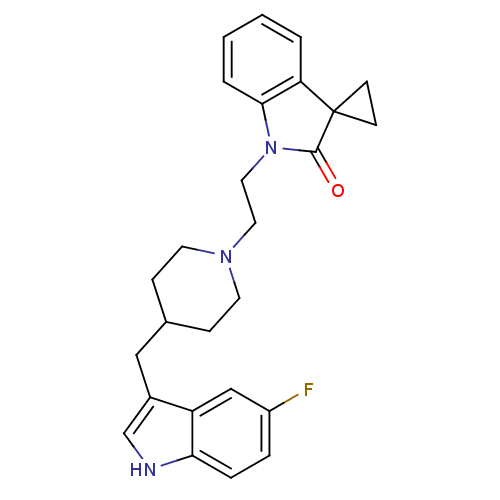
(1'-{2-[4-(5-fluoro-1H-3-indolylmethyl)hexahydro-1-...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCN4C(=O)C5(CC5)c5ccccc45)CC3)c2c1 Show InChI InChI=1S/C26H28FN3O/c27-20-5-6-23-21(16-20)19(17-28-23)15-18-7-11-29(12-8-18)13-14-30-24-4-2-1-3-22(24)26(9-10-26)25(30)31/h1-6,16-18,28H,7-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280817
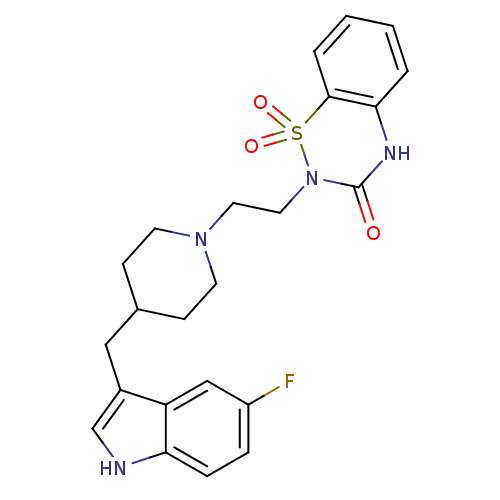
(2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCN4C(=O)Nc5ccccc5S4(=O)=O)CC3)c2c1 Show InChI InChI=1S/C23H25FN4O3S/c24-18-5-6-20-19(14-18)17(15-25-20)13-16-7-9-27(10-8-16)11-12-28-23(29)26-21-3-1-2-4-22(21)32(28,30)31/h1-6,14-16,25H,7-13H2,(H,26,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280831

(2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCN4C(=O)c5cccc6cccc(C4=O)c56)CC3)c2c1 Show InChI InChI=1S/C28H26FN3O2/c29-21-7-8-25-24(16-21)20(17-30-25)15-18-9-11-31(12-10-18)13-14-32-27(33)22-5-1-3-19-4-2-6-23(26(19)22)28(32)34/h1-8,16-18,30H,9-15H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280819

(1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCn4c5cccc6cccc([nH]c4=O)c56)CC3)c2c1 Show InChI InChI=1S/C27H27FN4O/c28-21-7-8-23-22(16-21)20(17-29-23)15-18-9-11-31(12-10-18)13-14-32-25-6-2-4-19-3-1-5-24(26(19)25)30-27(32)33/h1-8,16-18,29H,9-15H2,(H,30,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280824
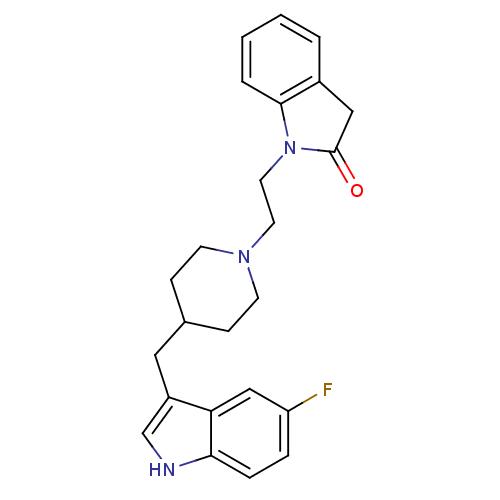
(1-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCN4C(=O)Cc5ccccc45)CC3)c2c1 Show InChI InChI=1S/C24H26FN3O/c25-20-5-6-22-21(15-20)19(16-26-22)13-17-7-9-27(10-8-17)11-12-28-23-4-2-1-3-18(23)14-24(28)29/h1-6,15-17,26H,7-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280826
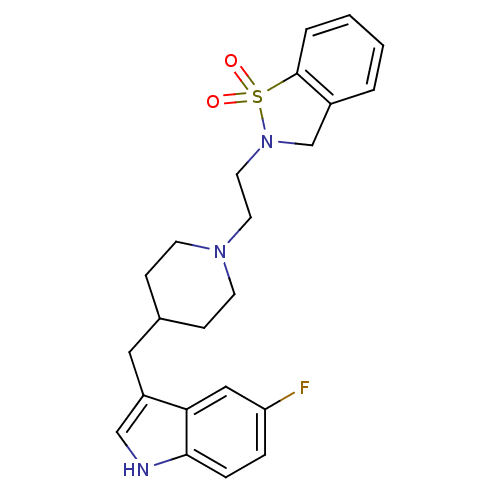
(2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCN4Cc5ccccc5S4(=O)=O)CC3)c2c1 Show InChI InChI=1S/C23H26FN3O2S/c24-20-5-6-22-21(14-20)19(15-25-22)13-17-7-9-26(10-8-17)11-12-27-16-18-3-1-2-4-23(18)30(27,28)29/h1-6,14-15,17,25H,7-13,16H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280816

(CHEMBL293729 | N-{2-[4-(5-Fluoro-1H-indol-3-ylmeth...)Show SMILES CN(CCN1CCC(Cc2c[nH]c3ccc(F)cc23)CC1)S(=O)(=O)c1ccccc1 Show InChI InChI=1S/C23H28FN3O2S/c1-26(30(28,29)21-5-3-2-4-6-21)13-14-27-11-9-18(10-12-27)15-19-17-25-23-8-7-20(24)16-22(19)23/h2-8,16-18,25H,9-15H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280815

(CHEMBL56377 | N-{2-[4-(5-Fluoro-1H-indol-3-ylmethy...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCNS(=O)(=O)c4ccccc4)CC3)c2c1 Show InChI InChI=1S/C22H26FN3O2S/c23-19-6-7-22-21(15-19)18(16-24-22)14-17-8-11-26(12-9-17)13-10-25-29(27,28)20-4-2-1-3-5-20/h1-7,15-17,24-25H,8-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280830

(3-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES Fc1ccc2[nH]cc(CC3CCN(CCn4c(=O)[nH]c5ccccc5c4=O)CC3)c2c1 Show InChI InChI=1S/C24H25FN4O2/c25-18-5-6-21-20(14-18)17(15-26-21)13-16-7-9-28(10-8-16)11-12-29-23(30)19-3-1-2-4-22(19)27-24(29)31/h1-6,14-16,26H,7-13H2,(H,27,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280832

(2-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES CC1(C)CN(CCN2CCC(Cc3c[nH]c4ccc(F)cc34)CC2)C(=O)c2ccccc12 Show InChI InChI=1S/C27H32FN3O/c1-27(2)18-31(26(32)22-5-3-4-6-24(22)27)14-13-30-11-9-19(10-12-30)15-20-17-29-25-8-7-21(28)16-23(20)25/h3-8,16-17,19,29H,9-15,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50280828

(3-{2-[4-(5-Fluoro-1H-indol-3-ylmethyl)-piperidin-1...)Show SMILES C[Si]1(C)CN(CCN2CCC(Cc3c[nH]c4ccc(F)cc34)CC2)C(=O)c2ccccc12 Show InChI InChI=1S/C26H32FN3OSi/c1-32(2)18-30(26(31)22-5-3-4-6-25(22)32)14-13-29-11-9-19(10-12-29)15-20-17-28-24-8-7-21(27)16-23(20)24/h3-8,16-17,19,28H,9-15,18H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Tested for its binding affinity against muscarinic receptor; showed no appreciable affinity at concentration specified |
Bioorg Med Chem Lett 3: 1913-1918 (1993)
Article DOI: 10.1016/S0960-894X(01)80986-0
BindingDB Entry DOI: 10.7270/Q27944M4 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449933
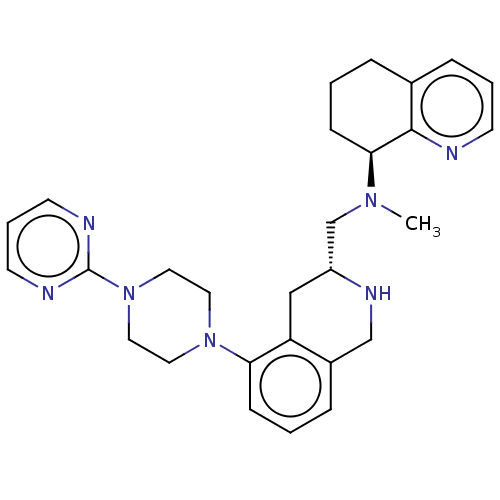
(CHEMBL4167145)Show SMILES CN(C[C@H]1Cc2c(CN1)cccc2N1CCN(CC1)c1ncccn1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C28H35N7/c1-33(26-10-2-6-21-8-4-11-29-27(21)26)20-23-18-24-22(19-32-23)7-3-9-25(24)34-14-16-35(17-15-34)28-30-12-5-13-31-28/h3-5,7-9,11-13,23,26,32H,2,6,10,14-20H2,1H3/t23-,26+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 887 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449936
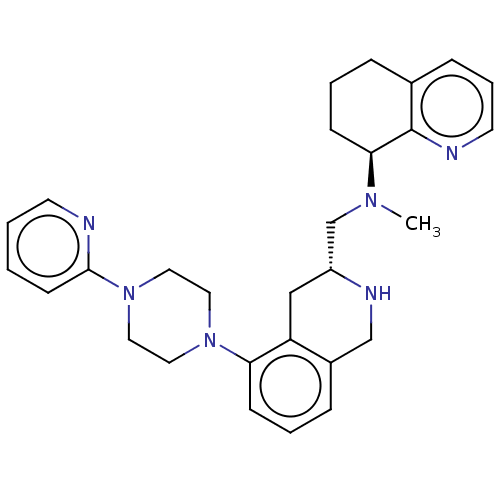
(CHEMBL4169875)Show SMILES CN(C[C@H]1Cc2c(CN1)cccc2N1CCN(CC1)c1ccccn1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C29H36N6/c1-33(27-11-4-7-22-9-6-14-31-29(22)27)21-24-19-25-23(20-32-24)8-5-10-26(25)34-15-17-35(18-16-34)28-12-2-3-13-30-28/h2-3,5-6,8-10,12-14,24,27,32H,4,7,11,15-21H2,1H3/t24-,27+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
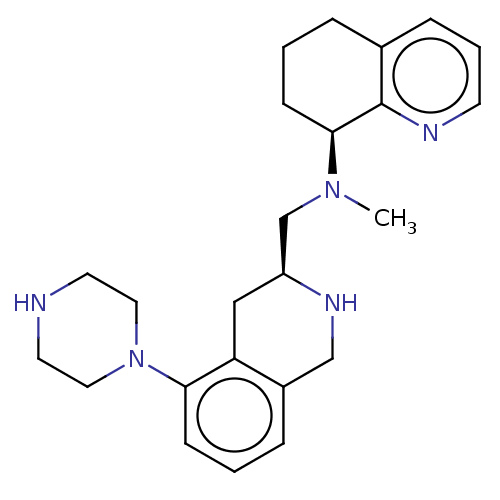
(Homo sapiens (Human)) | BDBM50449945
(CHEMBL4162011)Show SMILES CN(C[C@@H]1Cc2c(CN1)cccc2N1CCNCC1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C24H33N5/c1-28(23-9-2-5-18-7-4-10-26-24(18)23)17-20-15-21-19(16-27-20)6-3-8-22(21)29-13-11-25-12-14-29/h3-4,6-8,10,20,23,25,27H,2,5,9,11-17H2,1H3/t20-,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50286297

(CHEMBL4165634)Show SMILES Cc1ccnc(CN(CCCCN)C[C@H]2Cc3ccccc3CN2)c1 |r| Show InChI InChI=1S/C21H30N4/c1-17-8-10-23-20(12-17)15-25(11-5-4-9-22)16-21-13-18-6-2-3-7-19(18)14-24-21/h2-3,6-8,10,12,21,24H,4-5,9,11,13-16,22H2,1H3/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-stimulated Ca2+ flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 17-22 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00381
BindingDB Entry DOI: 10.7270/Q2NG4T6G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
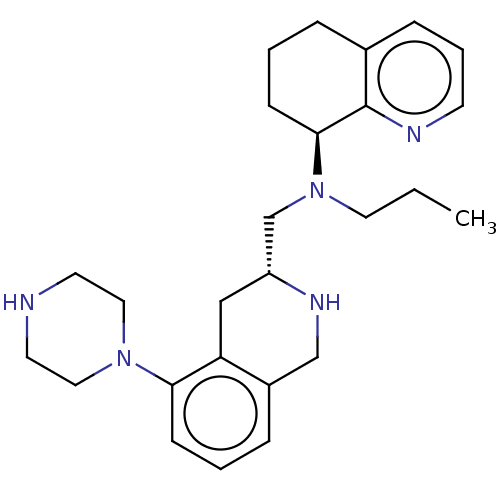
(Homo sapiens (Human)) | BDBM50449946
(CHEMBL4177130)Show SMILES CCCN(C[C@H]1Cc2c(CN1)cccc2N1CCNCC1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C26H37N5/c1-2-14-31(25-10-3-6-20-8-5-11-28-26(20)25)19-22-17-23-21(18-29-22)7-4-9-24(23)30-15-12-27-13-16-30/h4-5,7-9,11,22,25,27,29H,2-3,6,10,12-19H2,1H3/t22-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50310000

(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophenyl...)Show SMILES Cc1cc(Br)ccc1CNC(=O)c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccccc1Cl Show InChI InChI=1S/C27H21Br2ClN6O/c1-17-12-21(29)11-8-19(17)13-32-27(37)25-22(14-35-16-31-15-33-35)26(18-6-9-20(28)10-7-18)36(34-25)24-5-3-2-4-23(24)30/h2-12,15-16H,13-14H2,1H3,(H,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50286404

(CHEMBL4171868)Show SMILES NCCCCN(C[C@H]1Cc2ccccc2CN1)Cc1nccc2ccccc12 |r| Show InChI InChI=1S/C24H30N4/c25-12-5-6-14-28(17-22-15-20-8-1-2-9-21(20)16-27-22)18-24-23-10-4-3-7-19(23)11-13-26-24/h1-4,7-11,13,22,27H,5-6,12,14-18,25H2/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.35E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-stimulated Ca2+ flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 17-22 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00381
BindingDB Entry DOI: 10.7270/Q2NG4T6G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50310000

(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophenyl...)Show SMILES Cc1cc(Br)ccc1CNC(=O)c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccccc1Cl Show InChI InChI=1S/C27H21Br2ClN6O/c1-17-12-21(29)11-8-19(17)13-32-27(37)25-22(14-35-16-31-15-33-35)26(18-6-9-20(28)10-7-18)36(34-25)24-5-3-2-4-23(24)30/h2-12,15-16H,13-14H2,1H3,(H,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449940

(CHEMBL4176403)Show SMILES COCCN(C[C@H]1Cc2c(CN1)cccc2N1CCNCC1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C26H37N5O/c1-32-16-15-31(25-9-2-5-20-7-4-10-28-26(20)25)19-22-17-23-21(18-29-22)6-3-8-24(23)30-13-11-27-12-14-30/h3-4,6-8,10,22,25,27,29H,2,5,9,11-19H2,1H3/t22-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
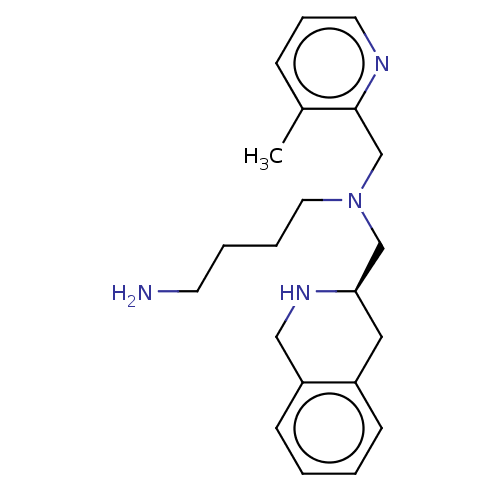
(Homo sapiens (Human)) | BDBM50286398
(CHEMBL4172866)Show InChI InChI=1S/C21H30N4/c1-17-7-6-11-23-21(17)16-25(12-5-4-10-22)15-20-13-18-8-2-3-9-19(18)14-24-20/h2-3,6-9,11,20,24H,4-5,10,12-16,22H2,1H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-stimulated Ca2+ flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 17-22 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00381
BindingDB Entry DOI: 10.7270/Q2NG4T6G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50286309

(CHEMBL4164685)Show SMILES C[C@H](N(CCCCN)C[C@H]1Cc2ccccc2CN1)c1ncccc1C |r| Show InChI InChI=1S/C22H32N4/c1-17-8-7-12-24-22(17)18(2)26(13-6-5-11-23)16-21-14-19-9-3-4-10-20(19)15-25-21/h3-4,7-10,12,18,21,25H,5-6,11,13-16,23H2,1-2H3/t18-,21+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-stimulated Ca2+ flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 17-22 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00381
BindingDB Entry DOI: 10.7270/Q2NG4T6G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50310000

(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophenyl...)Show SMILES Cc1cc(Br)ccc1CNC(=O)c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccccc1Cl Show InChI InChI=1S/C27H21Br2ClN6O/c1-17-12-21(29)11-8-19(17)13-32-27(37)25-22(14-35-16-31-15-33-35)26(18-6-9-20(28)10-7-18)36(34-25)24-5-3-2-4-23(24)30/h2-12,15-16H,13-14H2,1H3,(H,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449944

(CHEMBL4172669)Show SMILES CN(C[C@H]1Cc2c(CN1)cccc2N1CCNCC1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C24H33N5/c1-28(23-9-2-5-18-7-4-10-26-24(18)23)17-20-15-21-19(16-27-20)6-3-8-22(21)29-13-11-25-12-14-29/h3-4,6-8,10,20,23,25,27H,2,5,9,11-17H2,1H3/t20-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.18E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449950
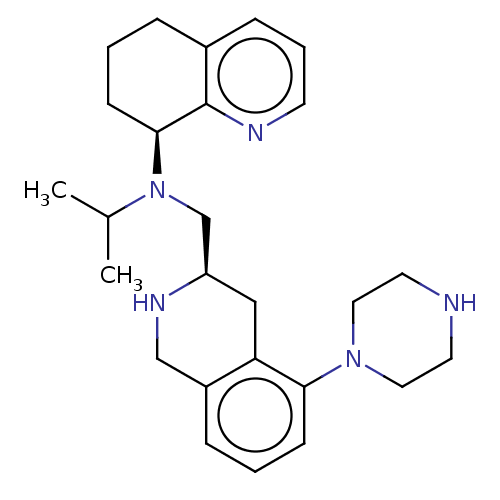
(CHEMBL4173777)Show SMILES CC(C)N(C[C@H]1Cc2c(CN1)cccc2N1CCNCC1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C26H37N5/c1-19(2)31(25-10-3-6-20-8-5-11-28-26(20)25)18-22-16-23-21(17-29-22)7-4-9-24(23)30-14-12-27-13-15-30/h4-5,7-9,11,19,22,25,27,29H,3,6,10,12-18H2,1-2H3/t22-,25+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.34E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
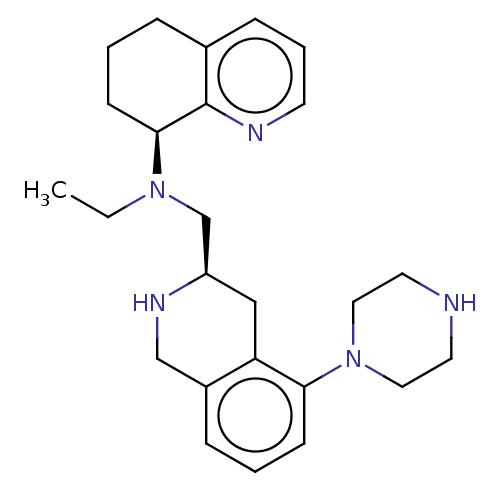
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449938
(CHEMBL4169280)Show SMILES CCN(C[C@H]1Cc2c(CN1)cccc2N1CCNCC1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C25H35N5/c1-2-29(24-10-3-6-19-8-5-11-27-25(19)24)18-21-16-22-20(17-28-21)7-4-9-23(22)30-14-12-26-13-15-30/h4-5,7-9,11,21,24,26,28H,2-3,6,10,12-18H2,1H3/t21-,24+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
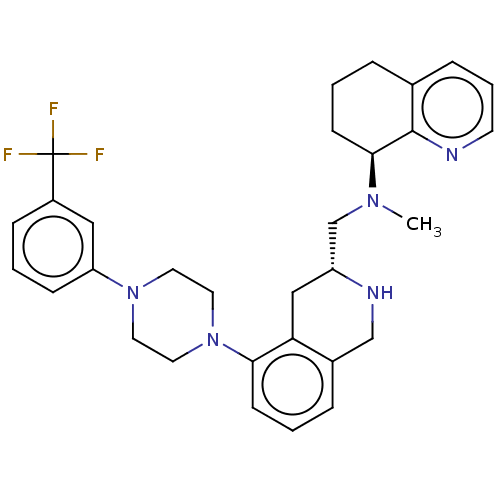
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449941
(CHEMBL4161949)Show SMILES CN(C[C@H]1Cc2c(CN1)cccc2N1CCN(CC1)c1cccc(c1)C(F)(F)F)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C31H36F3N5/c1-37(29-12-2-6-22-8-5-13-35-30(22)29)21-25-19-27-23(20-36-25)7-3-11-28(27)39-16-14-38(15-17-39)26-10-4-9-24(18-26)31(32,33)34/h3-5,7-11,13,18,25,29,36H,2,6,12,14-17,19-21H2,1H3/t25-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
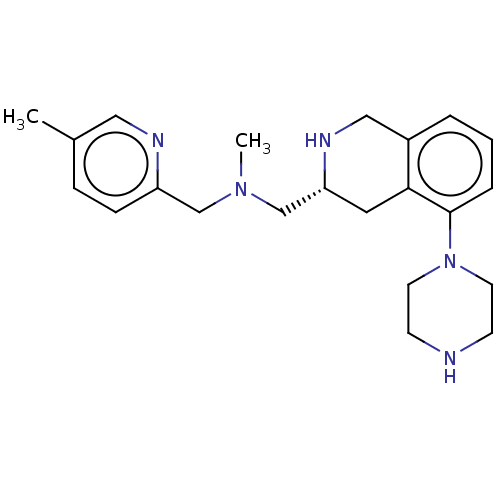
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449955
(CHEMBL4166081)Show SMILES CN(C[C@H]1Cc2c(CN1)cccc2N1CCNCC1)Cc1ccc(C)cn1 |r| Show InChI InChI=1S/C22H31N5/c1-17-6-7-19(24-13-17)15-26(2)16-20-12-21-18(14-25-20)4-3-5-22(21)27-10-8-23-9-11-27/h3-7,13,20,23,25H,8-12,14-16H2,1-2H3/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 9.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50009073

(4-(5-cyclopentyloxycarbonylamino-1-methyl-1H-indol...)Show SMILES COc1cc(ccc1Cc1cn(C)c2ccc(NC(=O)OC3CCCC3)cc12)C(=O)NS(=O)(=O)c1ccccc1C Show InChI InChI=1S/C31H33N3O6S/c1-20-8-4-7-11-29(20)41(37,38)33-30(35)22-13-12-21(28(17-22)39-3)16-23-19-34(2)27-15-14-24(18-26(23)27)32-31(36)40-25-9-5-6-10-25/h4,7-8,11-15,17-19,25H,5-6,9-10,16H2,1-3H3,(H,32,36)(H,33,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against muscarinic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50284689

(7-Chloro-2-((E)-2-{3-[4-(2-methyl-imidazo[4,5-c]py...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3ccc4ccc(Cl)cc4n3)c2)cc1 Show InChI InChI=1S/C31H23ClN4O/c1-21-34-30-19-33-16-15-31(30)36(21)27-11-13-28(14-12-27)37-20-23-4-2-3-22(17-23)5-9-26-10-7-24-6-8-25(32)18-29(24)35-26/h2-19H,20H2,1H3/b9-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against muscarinic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50284690

(1-(4-{3-[(E)-2-(5-Fluoro-benzothiazol-2-yl)-vinyl]...)Show SMILES Cc1nc2cnccc2n1-c1ccc(OCc2cccc(\C=C\c3nc4cc(F)ccc4s3)c2)cc1 Show InChI InChI=1S/C29H21FN4OS/c1-19-32-26-17-31-14-13-27(26)34(19)23-7-9-24(10-8-23)35-18-21-4-2-3-20(15-21)5-12-29-33-25-16-22(30)6-11-28(25)36-29/h2-17H,18H2,1H3/b12-5+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
| Article
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory concentration against muscarinic receptor |
Bioorg Med Chem Lett 5: 1377-1382 (1995)
Article DOI: 10.1016/0960-894X(95)00227-K
BindingDB Entry DOI: 10.7270/Q2M045D5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50286303
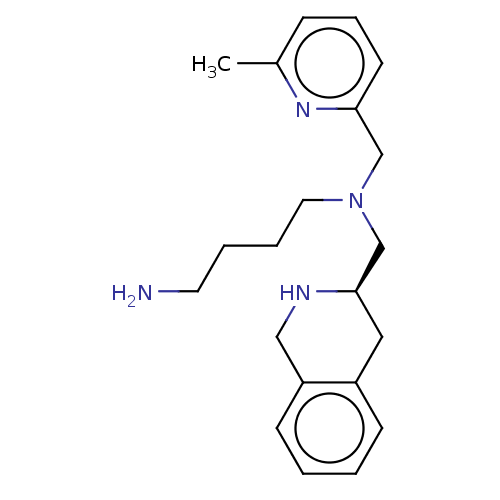
(CHEMBL4168512)Show SMILES Cc1cccc(CN(CCCCN)C[C@H]2Cc3ccccc3CN2)n1 |r| Show InChI InChI=1S/C21H30N4/c1-17-7-6-10-20(24-17)15-25(12-5-4-11-22)16-21-13-18-8-2-3-9-19(18)14-23-21/h2-3,6-10,21,23H,4-5,11-16,22H2,1H3/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-stimulated Ca2+ flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 17-22 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00381
BindingDB Entry DOI: 10.7270/Q2NG4T6G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449947
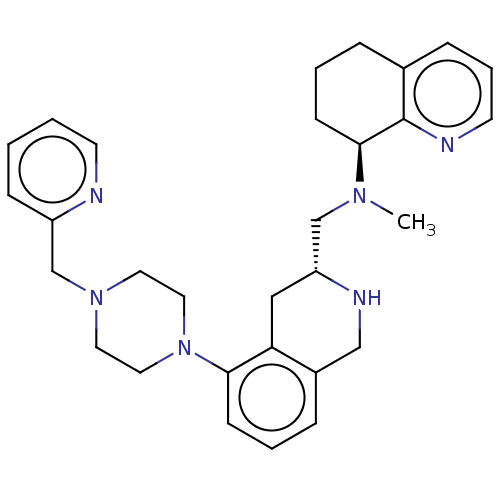
(CHEMBL4159234)Show SMILES CN(C[C@H]1Cc2c(CN1)cccc2N1CCN(Cc2ccccn2)CC1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C30H38N6/c1-34(29-12-4-7-23-9-6-14-32-30(23)29)21-26-19-27-24(20-33-26)8-5-11-28(27)36-17-15-35(16-18-36)22-25-10-2-3-13-31-25/h2-3,5-6,8-11,13-14,26,29,33H,4,7,12,15-22H2,1H3/t26-,29+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.08E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50024992

(3-Isopropoxy-4,5,6,7-tetrahydro-isoxazolo[4,5-c]py...)Show InChI InChI=1S/C9H14N2O2/c1-6(2)12-9-7-5-10-4-3-8(7)13-11-9/h6,10H,3-5H2,1-2H3/p+1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
In vitro antagonistic activity against peripheral Muscarinic acetylcholine receptor in guinea pig ileum as inhibition of acetylcholine induced muscle... |
J Med Chem 29: 1004-9 (1986)
BindingDB Entry DOI: 10.7270/Q2XP73XW |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50449953
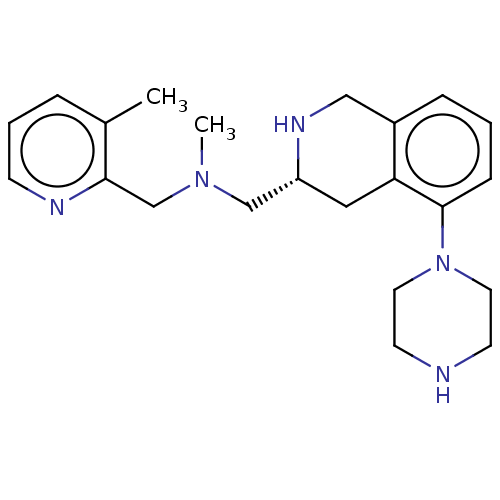
(CHEMBL4169504)Show SMILES CN(C[C@H]1Cc2c(CN1)cccc2N1CCNCC1)Cc1ncccc1C |r| Show InChI InChI=1S/C22H31N5/c1-17-5-4-8-24-21(17)16-26(2)15-19-13-20-18(14-25-19)6-3-7-22(20)27-11-9-23-10-12-27/h3-8,19,23,25H,9-16H2,1-2H3/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium release preincubated for 25 mins followe... |
J Med Chem 61: 7168-7188 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00450
BindingDB Entry DOI: 10.7270/Q2GH9MJ5 |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50286405
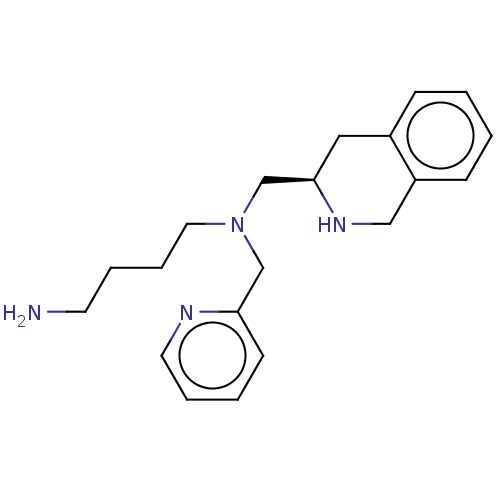
(CHEMBL4175319)Show InChI InChI=1S/C20H28N4/c21-10-4-6-12-24(15-19-9-3-5-11-22-19)16-20-13-17-7-1-2-8-18(17)14-23-20/h1-3,5,7-9,11,20,23H,4,6,10,12-16,21H2/t20-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-stimulated Ca2+ flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 17-22 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00381
BindingDB Entry DOI: 10.7270/Q2NG4T6G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50286421
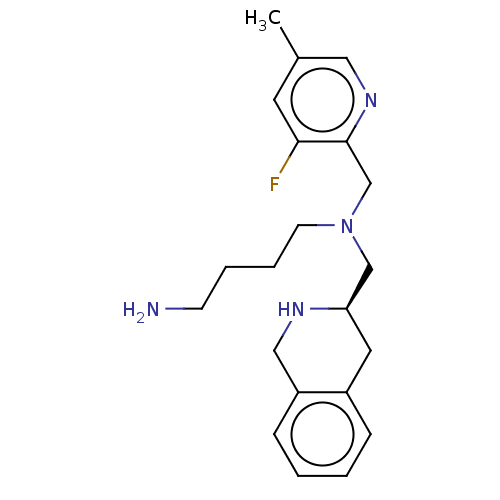
(CHEMBL4172727)Show SMILES Cc1cnc(CN(CCCCN)C[C@H]2Cc3ccccc3CN2)c(F)c1 |r| Show InChI InChI=1S/C21H29FN4/c1-16-10-20(22)21(25-12-16)15-26(9-5-4-8-23)14-19-11-17-6-2-3-7-18(17)13-24-19/h2-3,6-7,10,12,19,24H,4-5,8-9,11,13-15,23H2,1H3/t19-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-stimulated Ca2+ flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 17-22 (2018)
Article DOI: 10.1021/acsmedchemlett.7b00381
BindingDB Entry DOI: 10.7270/Q2NG4T6G |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50327366
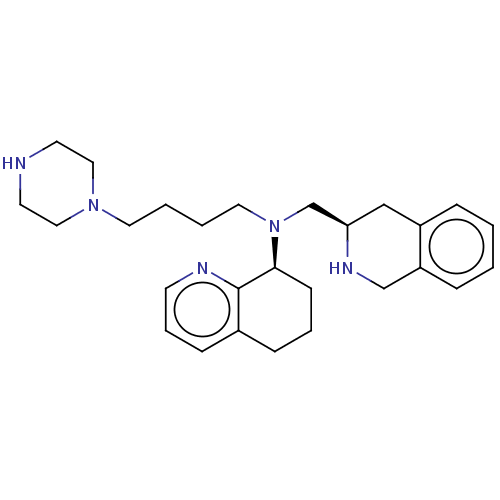
(CHEMBL4159150)Show SMILES C(CCN1CCNCC1)CN(C[C@H]1Cc2ccccc2CN1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C23H24FN3O3/c1-27(2)12-13-29-14-15-30-23-21(10-6-17-4-8-19(28-3)9-5-17)25-22-16-18(24)7-11-20(22)26-23/h4-5,7-9,11,16H,12-15H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50310000

(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophenyl...)Show SMILES Cc1cc(Br)ccc1CNC(=O)c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccccc1Cl Show InChI InChI=1S/C27H21Br2ClN6O/c1-17-12-21(29)11-8-19(17)13-32-27(37)25-22(14-35-16-31-15-33-35)26(18-6-9-20(28)10-7-18)36(34-25)24-5-3-2-4-23(24)30/h2-12,15-16H,13-14H2,1H3,(H,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50443541
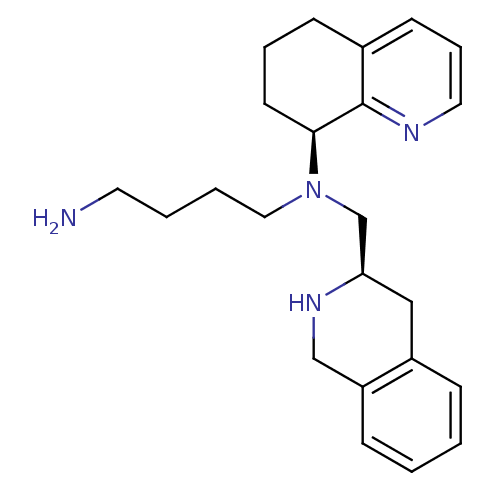
(CHEMBL3091687)Show SMILES NCCCCN(C[C@H]1Cc2ccccc2CN1)[C@H]1CCCc2cccnc12 |r| Show InChI InChI=1S/C23H32N4/c24-12-3-4-14-27(22-11-5-9-18-10-6-13-25-23(18)22)17-21-15-19-7-1-2-8-20(19)16-26-21/h1-2,6-8,10,13,21-22,26H,3-5,9,11-12,14-17,24H2/t21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50310000

(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophenyl...)Show SMILES Cc1cc(Br)ccc1CNC(=O)c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccccc1Cl Show InChI InChI=1S/C27H21Br2ClN6O/c1-17-12-21(29)11-8-19(17)13-32-27(37)25-22(14-35-16-31-15-33-35)26(18-6-9-20(28)10-7-18)36(34-25)24-5-3-2-4-23(24)30/h2-12,15-16H,13-14H2,1H3,(H,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50327365
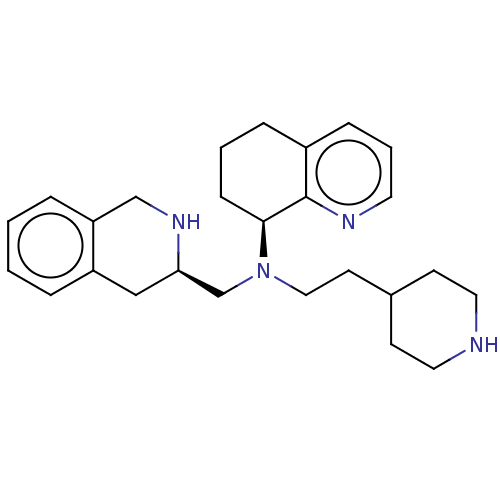
(CHEMBL4164702)Show SMILES C(CN(C[C@H]1Cc2ccccc2CN1)[C@H]1CCCc2cccnc12)C1CCNCC1 |r| Show InChI InChI=1S/C22H22N4O/c1-26-13-5-8-18(26)15-27-22-20(11-9-16-6-3-2-4-7-16)24-21-14-17(23)10-12-19(21)25-22/h2-4,6-7,10,12,14,18H,5,8,13,15,23H2,1H3/t18-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |
Muscarinic acetylcholine receptor M1/M2/M3/M4/M5
(Homo sapiens (Human)) | BDBM50310000

(4-((1H-1,2,4-Triazol-1-yl)methyl)-5-(4-bromophenyl...)Show SMILES Cc1cc(Br)ccc1CNC(=O)c1nn(c(c1Cn1cncn1)-c1ccc(Br)cc1)-c1ccccc1Cl Show InChI InChI=1S/C27H21Br2ClN6O/c1-17-12-21(29)11-8-19(17)13-32-27(37)25-22(14-35-16-31-15-33-35)26(18-6-9-20(28)10-7-18)36(34-25)24-5-3-2-4-23(24)30/h2-12,15-16H,13-14H2,1H3,(H,32,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Emory University
Curated by ChEMBL
| Assay Description
Antagonist activity at mAChR in human CCRF-CEM cells assessed as inhibition of acetylcholine-induced calcium flux pretreated for 25 mins followed by ... |
ACS Med Chem Lett 9: 446-451 (2018)
Article DOI: 10.1021/acsmedchemlett.8b00030
BindingDB Entry DOI: 10.7270/Q2Z89FZD |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data