

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10742 (1-amino-2,3-dihydro-1H-inden-4-yl N,N-dimethylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10796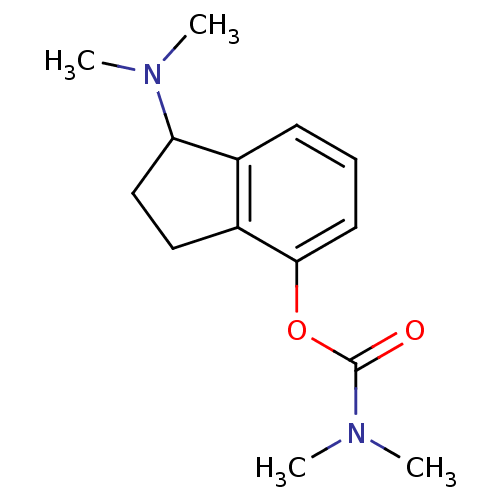 (1-(dimethylamino)-2,3-dihydro-1H-inden-4-yl N,N-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10743 (1-amino-2,3-dihydro-1H-inden-4-yl N-ethyl-N-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10726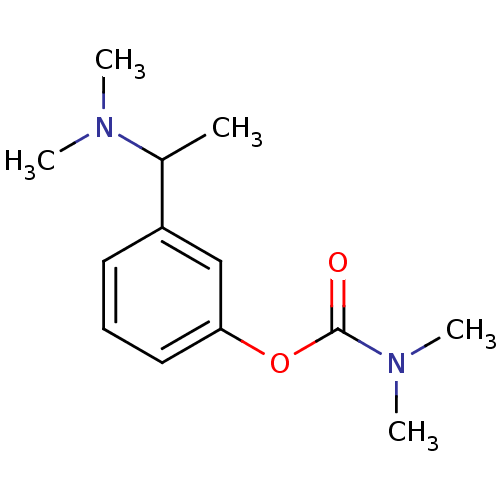 (3-[1-(dimethylamino)ethyl]phenyl N,N-dimethylcarba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29400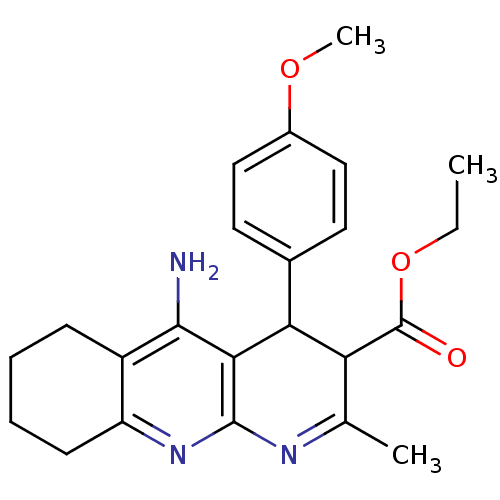 (CHEMBL374184 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29402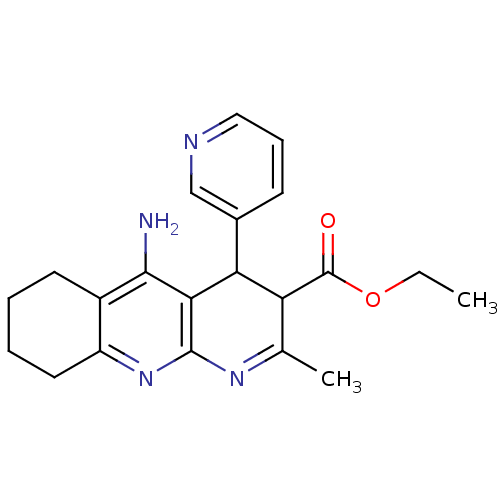 (tacrine-dihydropyridine hybrid (tacripyrine), 13) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29391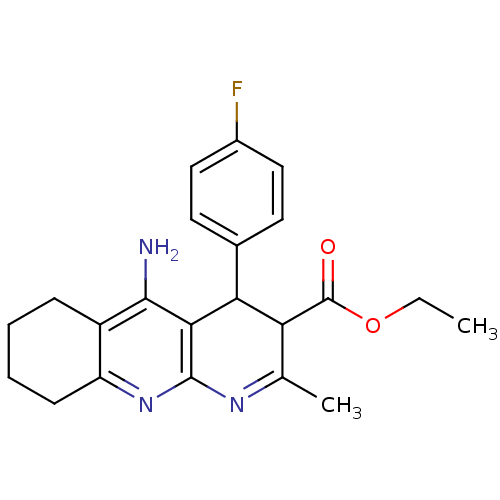 (CHEMBL219172 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10783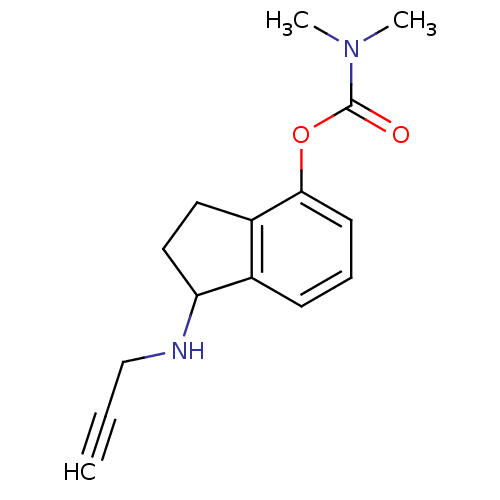 (1-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 53 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29399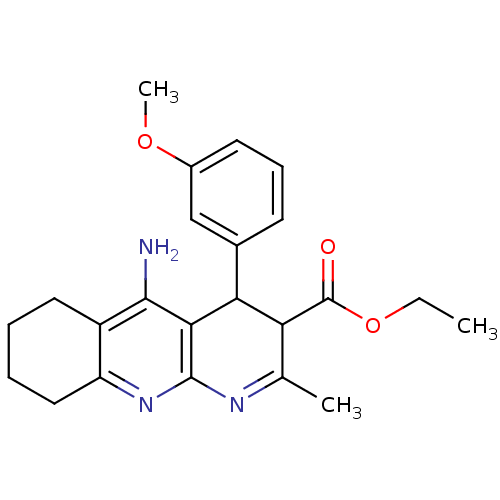 (CHEMBL218940 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29390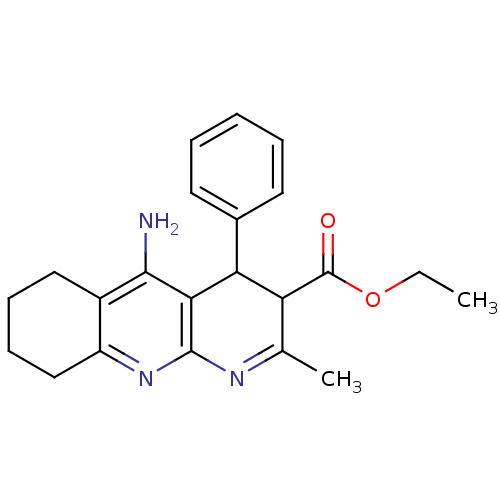 (CHEMBL218939 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29397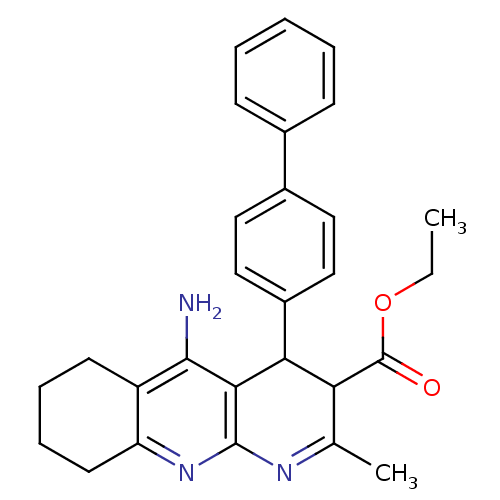 (tacrine-dihydropyridine hybrid (tacripyrine), 8) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29396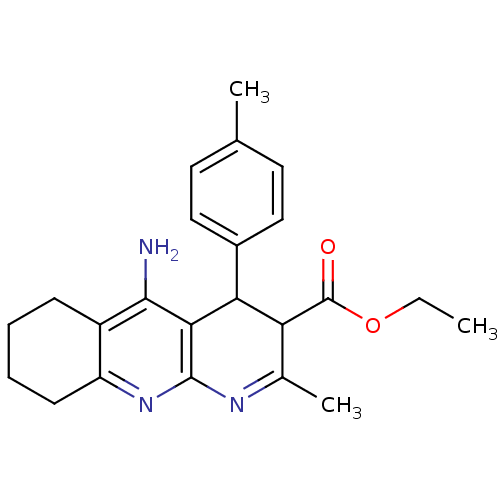 (CHEMBL219406 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29401 (tacrine-dihydropyridine hybrid (tacripyrine), 12) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 103 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29394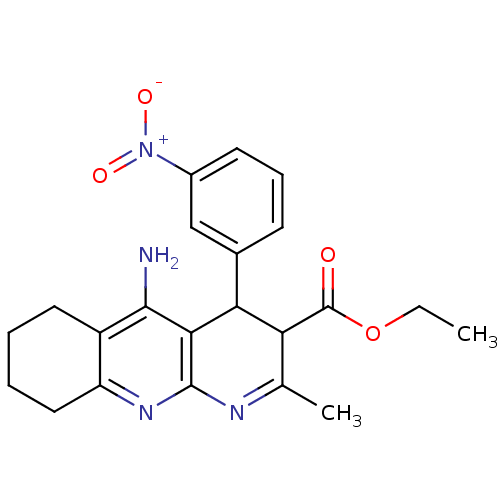 (CHEMBL220294 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29398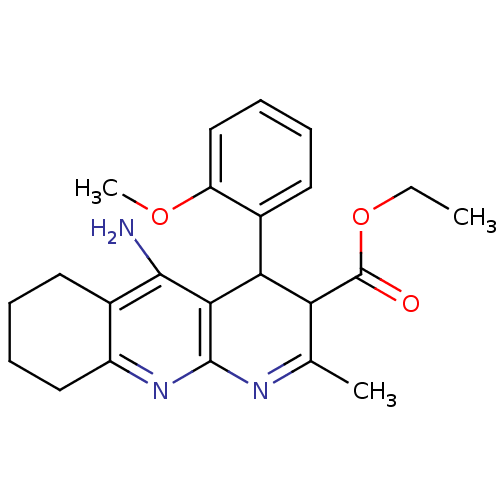 (CHEMBL436302 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10812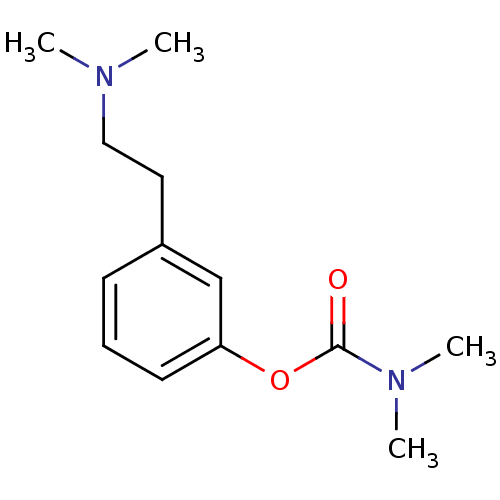 (3-[2-(dimethylamino)ethyl]phenyl N,N-dimethylcarba...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10832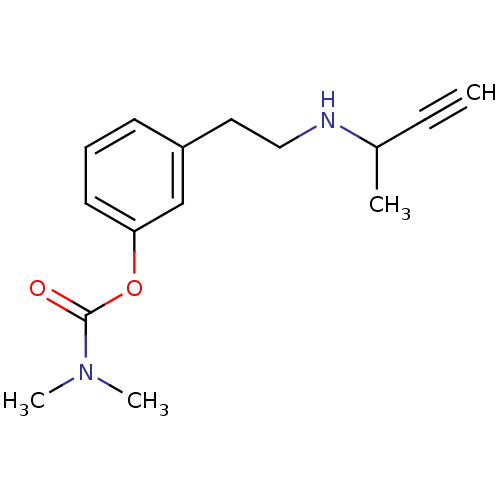 (3-[2-(but-3-yn-2-ylamino)ethyl]phenyl N,N-dimethyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM8961 (1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29392 (tacrine-dihydropyridine hybrid (tacripyrine), 3) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10813 (3-[2-(prop-2-yn-1-ylamino)ethyl]phenyl N,N-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29403 (tacrine-dihydropyridine hybrid (tacripyrine), 14) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10797 (3-(2-aminoethyl)phenyl N,N-dimethylcarbamate | Phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10810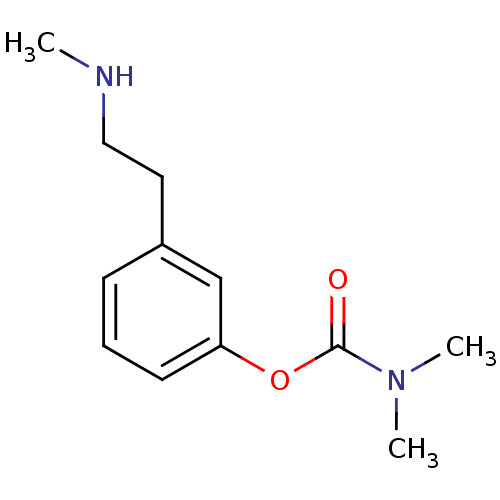 (3-[2-(methylamino)ethyl]phenyl N,N-dimethylcarbama...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 280 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10732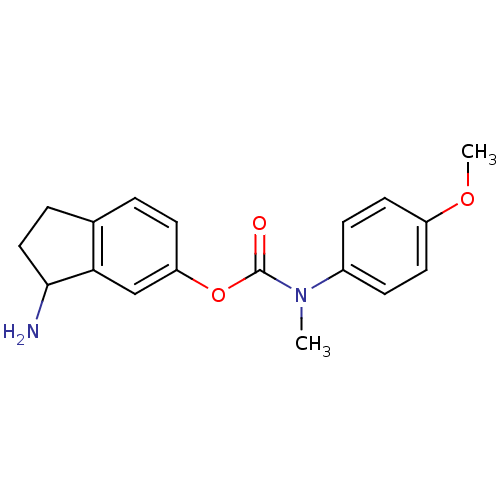 (3-amino-2,3-dihydro-1H-inden-5-yl N-(4-methoxyphen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29393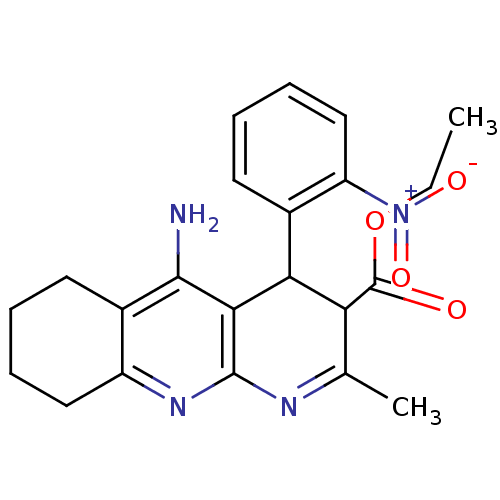 (CHEMBL219400 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 304 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10739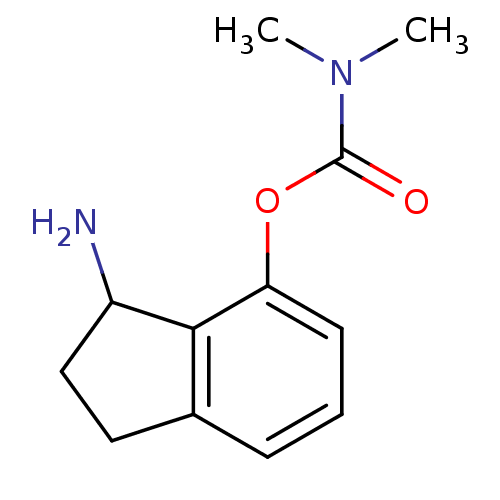 (3-amino-2,3-dihydro-1H-inden-4-yl N,N-dimethylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 460 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10741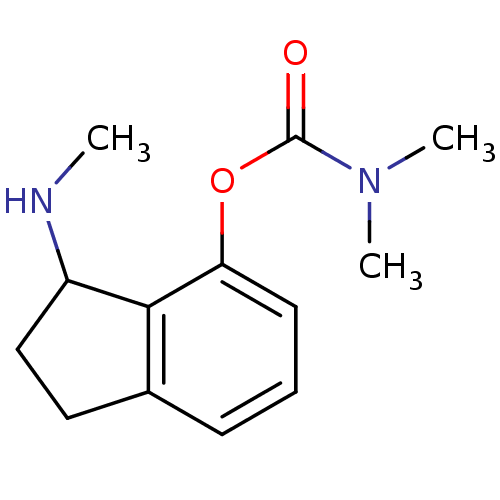 (3-(methylamino)-2,3-dihydro-1H-inden-4-yl N,N-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10730 (3-amino-2,3-dihydro-1H-inden-5-yl N-hexyl-N-methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 530 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10818 (3-[2-(prop-2-yn-1-ylamino)propyl]phenyl N,N-dimeth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 540 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10771 (3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-5-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 550 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Electrophorus electricus (Electric eel)) | BDBM29395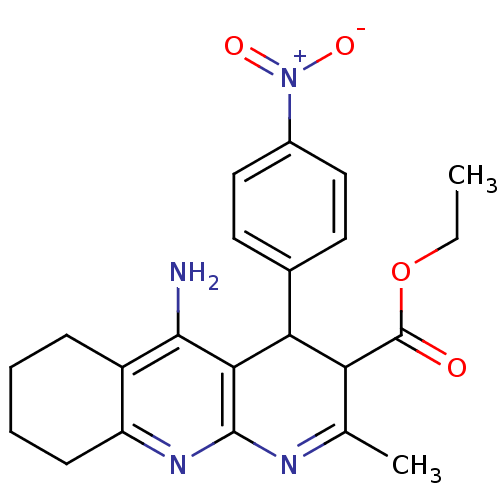 (CHEMBL219405 | tacrine-dihydropyridine hybrid (tac...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
CSIC | Assay Description The inhibitory activity of the compounds towards AChE was determined following the spectrophotometric method of Rappaport using purified AChE from Ee... | J Med Chem 52: 2724-32 (2009) Article DOI: 10.1021/jm801292b BindingDB Entry DOI: 10.7270/Q2C827NZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10727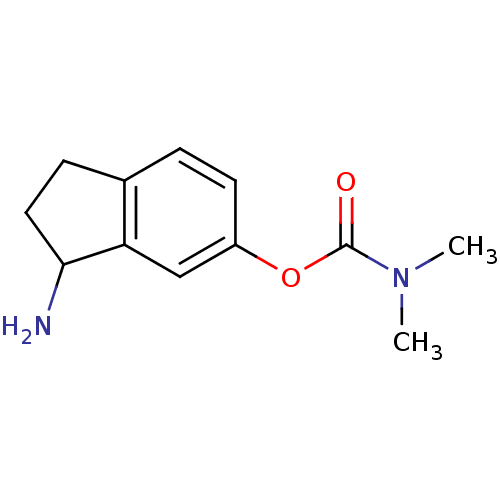 (3-amino-2,3-dihydro-1H-inden-5-yl N,N-dimethylcarb...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10816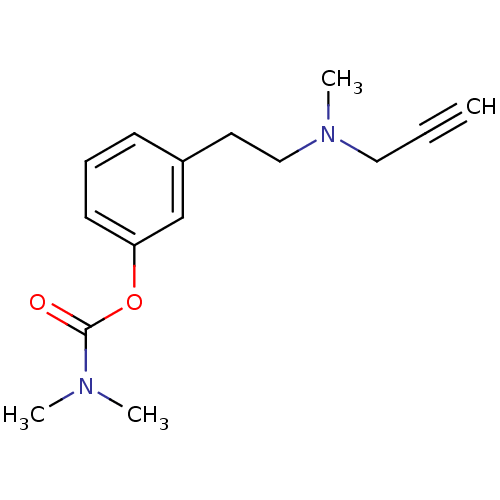 (3-{2-[methyl(prop-2-yn-1-yl)amino]ethyl}phenyl N,N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10762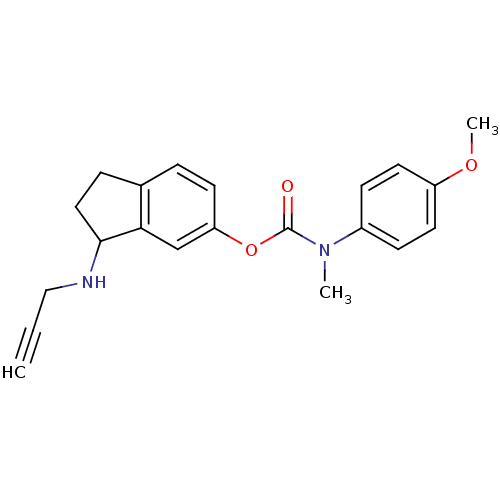 (3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-5-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 860 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10620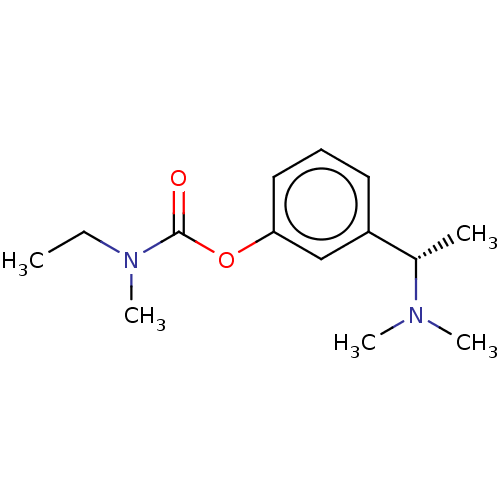 ((S)-3-(1-(dimethylamino)ethyl)phenyl ethyl(methyl)...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid UniChem Patents Similars | DrugBank Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10735 (3-(methylamino)-2,3-dihydro-1H-inden-5-yl N,N-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.07E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10744 (8-amino-5,6,7,8-tetrahydronaphthalen-2-yl N,N-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10734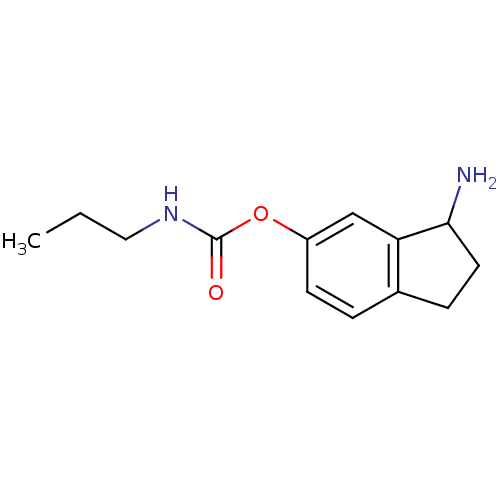 (3-amino-2,3-dihydro-1H-inden-5-yl N-propylcarbamat...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.48E+3 | n/a | n/a | n/a | n/a | 7.4 | 37 |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10823 (3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10770 (3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-5-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10789 (3-(but-3-yn-2-ylamino)-2,3-dihydro-1H-inden-5-yl N...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10748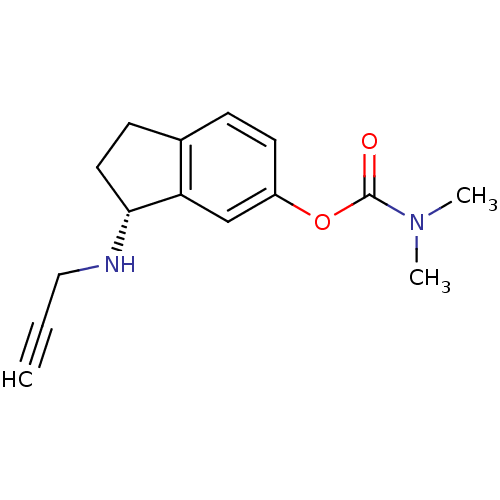 ((3R)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10827 (3-[(2R)-2-[methyl(prop-2-yn-1-yl)amino]propyl]phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10784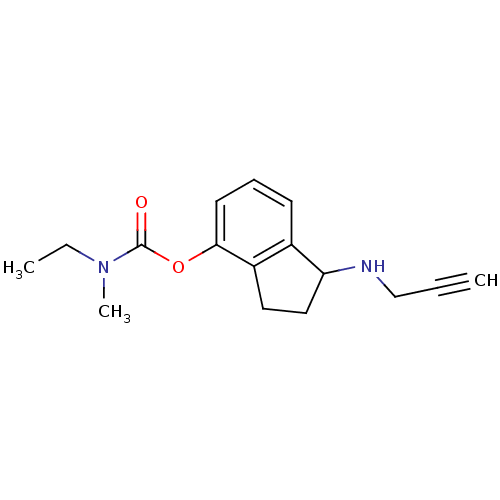 (1-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10795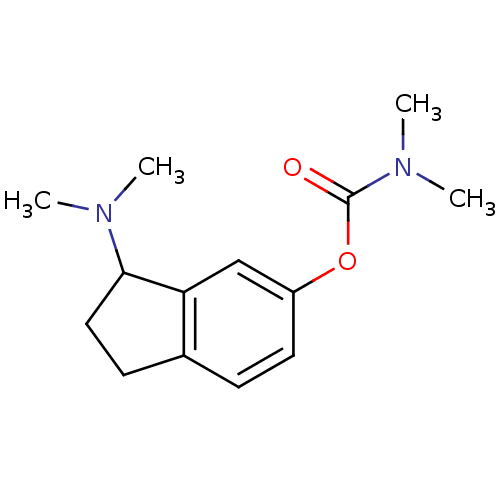 (3-(dimethylamino)-2,3-dihydro-1H-inden-5-yl N,N-di...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10749 ((3S)-3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.25E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10764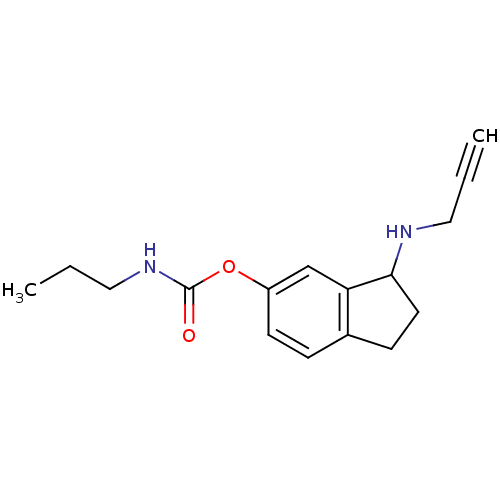 (3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-5-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10777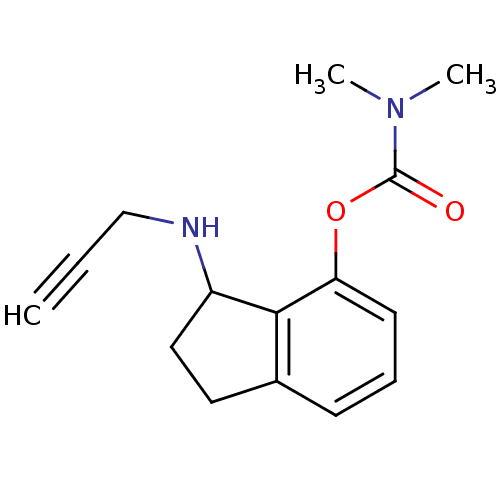 (3-(prop-2-yn-1-ylamino)-2,3-dihydro-1H-inden-4-yl ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10826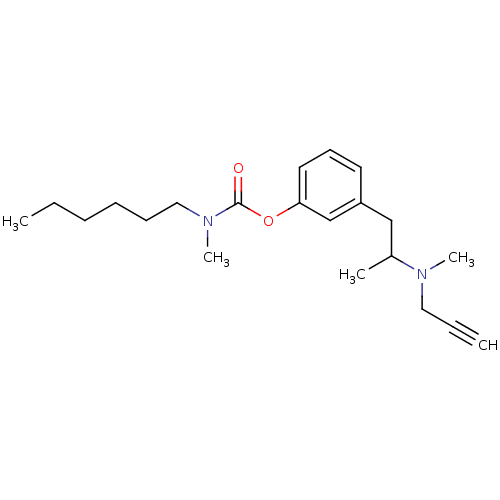 (3-{2-[methyl(prop-2-yn-1-yl)amino]propyl}phenyl N-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetylcholinesterase (Homo sapiens (Human)) | BDBM10746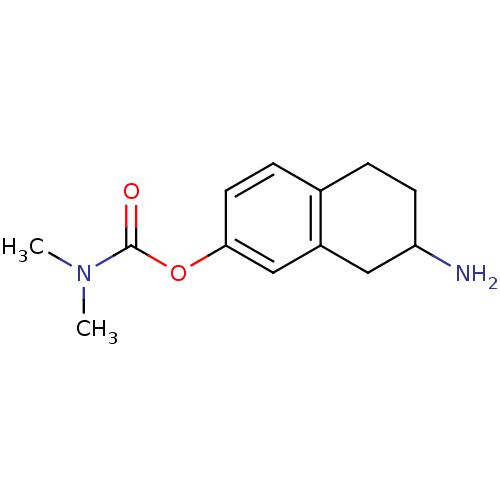 (7-amino-5,6,7,8-tetrahydronaphthalen-2-yl N,N-dime...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Teva Pharmaceutical Industries | Assay Description The cholinesterase assays were performed using colorimetric method reported by Ellman. The absorbance changes at 412 nm were recorded for 5 min with ... | J Med Chem 45: 5260-79 (2002) Article DOI: 10.1021/jm020120c BindingDB Entry DOI: 10.7270/Q2GQ6W07 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 108 total ) | Next | Last >> |