 Found 989 hits of ic50 data for polymerid = 1180
Found 989 hits of ic50 data for polymerid = 1180 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274028

(CHEMBL499477 | N-(3,5-dichloropyridin-4-yl)-1-(3-(...)Show SMILES Cc1noc(n1)C(=C\c1cccc(c1)-n1cc(C(=O)Nc2c(Cl)cncc2Cl)c(=O)c2cccnc12)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H22Cl2N6O5S/c1-18-37-32(45-39-18)24(20-8-10-22(11-9-20)46(2,43)44)14-19-5-3-6-21(13-19)40-17-25(29(41)23-7-4-12-36-30(23)40)31(42)38-28-26(33)15-35-16-27(28)34/h3-17H,1-2H3,(H,35,38,42)/b24-14+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50092632
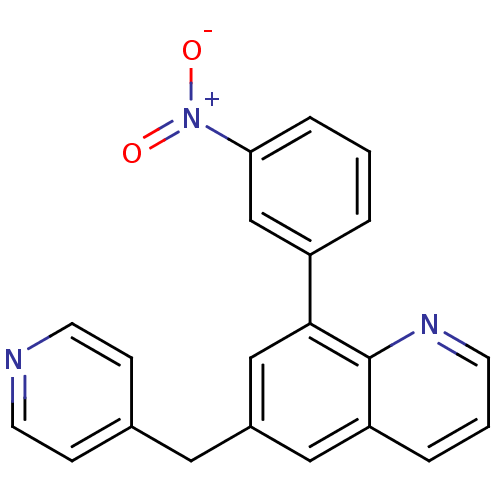
(8-(3-Nitro-phenyl)-6-pyridin-4-ylmethyl-quinoline ...)Show SMILES [O-][N+](=O)c1cccc(c1)-c1cc(Cc2ccncc2)cc2cccnc12 Show InChI InChI=1S/C21H15N3O2/c25-24(26)19-5-1-3-17(14-19)20-13-16(11-15-6-9-22-10-7-15)12-18-4-2-8-23-21(18)20/h1-10,12-14H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE4A expressed in Sf9 cells |
J Med Chem 43: 3820-3 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GG9 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183805
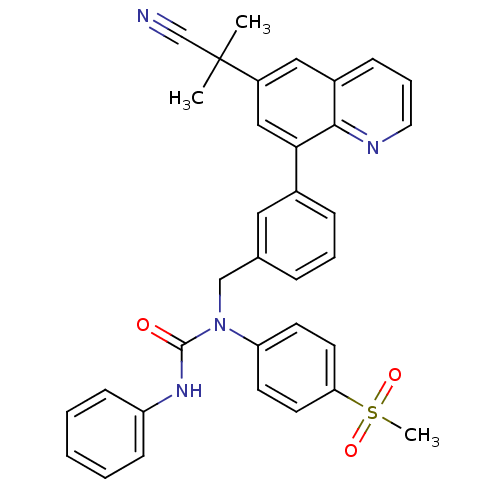
(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)Nc3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H30N4O3S/c1-34(2,23-35)27-20-26-11-8-18-36-32(26)31(21-27)25-10-7-9-24(19-25)22-38(33(39)37-28-12-5-4-6-13-28)29-14-16-30(17-15-29)42(3,40)41/h4-21H,22H2,1-3H3,(H,37,39) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183794
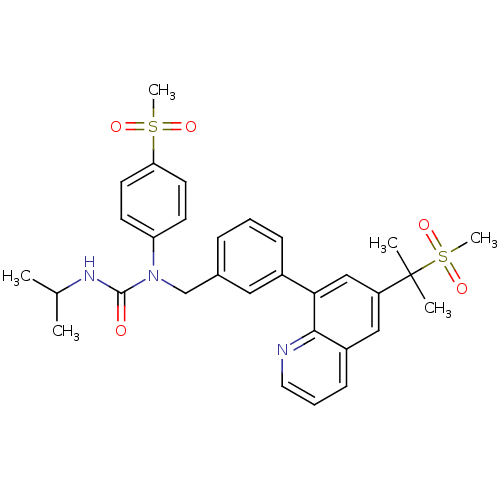
(3-isopropyl-1-(4-(methylsulfonyl)phenyl)-1-((3-(6-...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)S(C)(=O)=O)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H35N3O5S2/c1-21(2)33-30(35)34(26-12-14-27(15-13-26)40(5,36)37)20-22-9-7-10-23(17-22)28-19-25(31(3,4)41(6,38)39)18-24-11-8-16-32-29(24)28/h7-19,21H,20H2,1-6H3,(H,33,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM130938
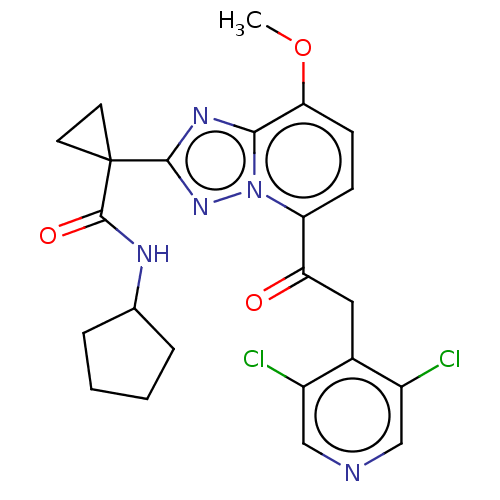
(US8829190, 116)Show SMILES COc1ccc(C(=O)Cc2c(Cl)cncc2Cl)n2nc(nc12)C1(CC1)C(=O)NC1CCCC1 Show InChI InChI=1S/C23H23Cl2N5O3/c1-33-19-7-6-17(18(31)10-14-15(24)11-26-12-16(14)25)30-20(19)28-21(29-30)23(8-9-23)22(32)27-13-4-2-3-5-13/h6-7,11-13H,2-5,8-10H2,1H3,(H,27,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Leo Pharma A/S
US Patent
| Assay Description
Human recombinant PDE4 (Gene bank accession no NM006203) was incubated for 1 hour with the test compound at concentrations up to 10 μM, with cAM... |
US Patent US8829190 (2014)
BindingDB Entry DOI: 10.7270/Q2Z89B3Z |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM336125

(US9738676, Comparative Compound A)Show SMILES CC[C@H]1OC(=O)[C@H](C)[C@@H](O)[C@H](C)[C@@H](O[C@@H]2O[C@H](C)C[C@@H]([C@H]2O)N(C)C(=O)N2CCOCC2)[C@@](C)(C[C@@H](C)C(=O)[C@H](C)[C@H]2[C@H](SCCOc3cc(ccc3CO)C(=O)Nc3c(Cl)cncc3Cl)C(=O)O[C@]12C)OC |r| Show InChI InChI=1S/C51H72Cl2N4O15S/c1-11-37-51(8)38(43(47(64)72-51)73-19-18-68-36-21-31(12-13-32(36)25-58)45(62)55-39-33(52)23-54-24-34(39)53)28(4)40(59)26(2)22-50(7,66-10)44(29(5)41(60)30(6)46(63)70-37)71-48-42(61)35(20-27(3)69-48)56(9)49(65)57-14-16-67-17-15-57/h12-13,21,23-24,26-30,35,37-38,41-44,48,58,60-61H,11,14-20,22,25H2,1-10H3,(H,54,55,62)/t26-,27-,28-,29+,30-,35+,37-,38+,41+,42-,43+,44-,48+,50-,51-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
BASILEA PHARMACEUTICA AG
US Patent
| Assay Description
PDEs specifically hydrolyze cAMP and/or cGMP and release the product AMP and/or GMP. The potency of PDE inhibition by test compounds is determined wi... |
US Patent US9738676 (2017)
BindingDB Entry DOI: 10.7270/Q2Z60R65 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183792

(1-((3-(6-(2-cyanopropan-2-yl)quinolin-8-yl)phenyl)...)Show SMILES CC(C)NC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H32N4O3S/c1-21(2)34-30(36)35(26-11-13-27(14-12-26)39(5,37)38)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3,(H,34,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174030
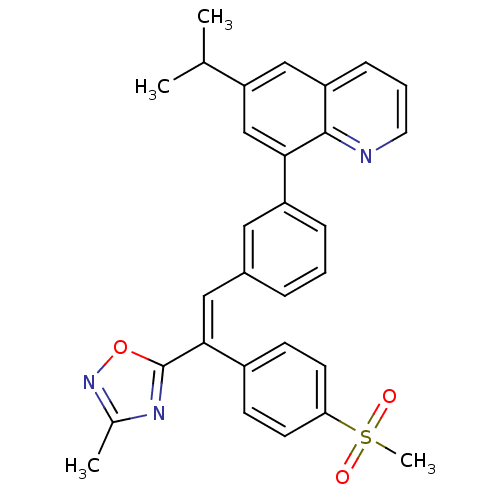
((E)-6-isopropyl-8-(3-(2-(3-methyl-1,2,4-oxadiazol-...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nc(C)no3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H27N3O3S/c1-19(2)25-17-24-9-6-14-31-29(24)27(18-25)23-8-5-7-21(15-23)16-28(30-32-20(3)33-36-30)22-10-12-26(13-11-22)37(4,34)35/h5-19H,1-4H3/b28-16+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274031
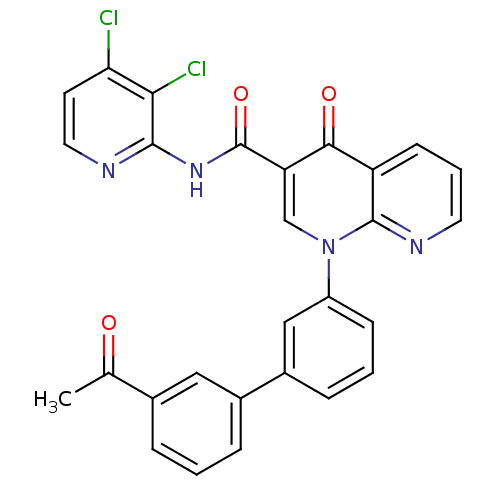
(1-(3'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(=O)c1cccc(c1)-c1cccc(c1)-n1cc(C(=O)Nc2nccc(Cl)c2Cl)c(=O)c2cccnc12 Show InChI InChI=1S/C28H18Cl2N4O3/c1-16(35)17-5-2-6-18(13-17)19-7-3-8-20(14-19)34-15-22(25(36)21-9-4-11-32-27(21)34)28(37)33-26-24(30)23(29)10-12-31-26/h2-15H,1H3,(H,31,33,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM130937

(US8829190, 115)Show SMILES COc1ccc(C(=O)Cc2c(Cl)cncc2Cl)n2nc(nc12)C1(CC1)C(=O)NCC(C)(C)C Show InChI InChI=1S/C23H25Cl2N5O3/c1-22(2,3)12-27-21(32)23(7-8-23)20-28-19-18(33-4)6-5-16(30(19)29-20)17(31)9-13-14(24)10-26-11-15(13)25/h5-6,10-11H,7-9,12H2,1-4H3,(H,27,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Leo Pharma A/S
US Patent
| Assay Description
Human recombinant PDE4 (Gene bank accession no NM006203) was incubated for 1 hour with the test compound at concentrations up to 10 μM, with cAM... |
US Patent US8829190 (2014)
BindingDB Entry DOI: 10.7270/Q2Z89B3Z |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM130939
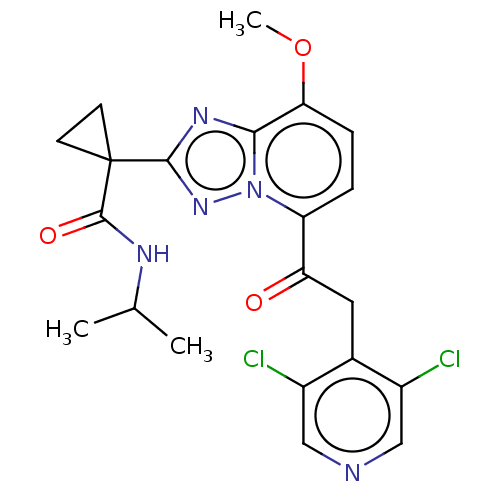
(US8829190, 117)Show SMILES COc1ccc(C(=O)Cc2c(Cl)cncc2Cl)n2nc(nc12)C1(CC1)C(=O)NC(C)C Show InChI InChI=1S/C21H21Cl2N5O3/c1-11(2)25-20(30)21(6-7-21)19-26-18-17(31-3)5-4-15(28(18)27-19)16(29)8-12-13(22)9-24-10-14(12)23/h4-5,9-11H,6-8H2,1-3H3,(H,25,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Leo Pharma A/S
US Patent
| Assay Description
Human recombinant PDE4 (Gene bank accession no NM006203) was incubated for 1 hour with the test compound at concentrations up to 10 μM, with cAM... |
US Patent US8829190 (2014)
BindingDB Entry DOI: 10.7270/Q2Z89B3Z |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174028
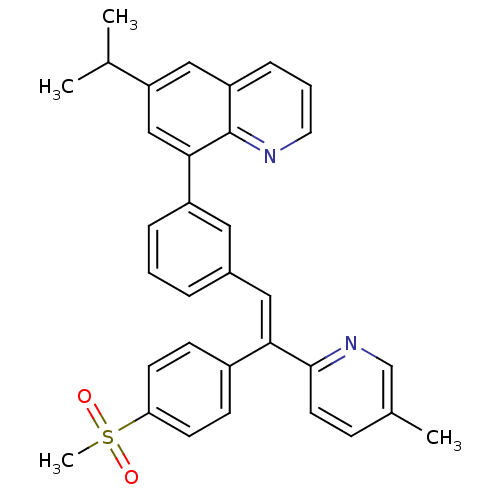
((E)-6-isopropyl-8-(3-(2-(5-methylpyridin-2-yl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(/c3ccc(cc3)S(C)(=O)=O)c3ccc(C)cn3)c2)c2ncccc2c1 Show InChI InChI=1S/C33H30N2O2S/c1-22(2)28-19-27-9-6-16-34-33(27)31(20-28)26-8-5-7-24(17-26)18-30(32-15-10-23(3)21-35-32)25-11-13-29(14-12-25)38(4,36)37/h5-22H,1-4H3/b30-18+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183808

(CHEMBL383225 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES CC(C)(C#N)c1cc(-c2cccc(CN(C(=O)c3ccccc3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C34H29N3O3S/c1-34(2,23-35)28-20-27-13-8-18-36-32(27)31(21-28)26-12-7-9-24(19-26)22-37(33(38)25-10-5-4-6-11-25)29-14-16-30(17-15-29)41(3,39)40/h4-21H,22H2,1-3H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50092630
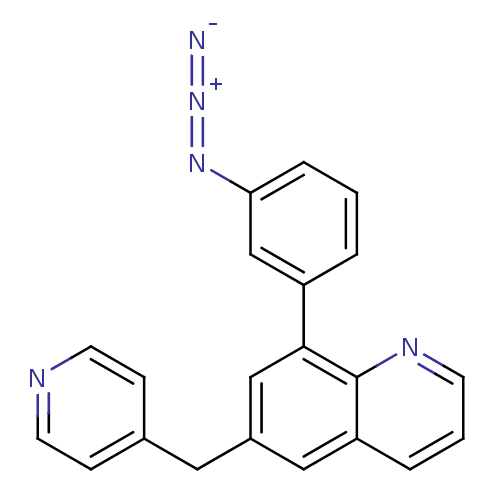
(8-(3-Azido-phenyl)-6-pyridin-4-ylmethyl-quinoline ...)Show SMILES [N-]=[N+]=Nc1cccc(c1)-c1cc(Cc2ccncc2)cc2cccnc12 Show InChI InChI=1S/C21H15N5/c22-26-25-19-5-1-3-17(14-19)20-13-16(11-15-6-9-23-10-7-15)12-18-4-2-8-24-21(18)20/h1-10,12-14H,11H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE4A expressed in Sf9 cells |
J Med Chem 43: 3820-3 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GG9 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM14774

(3-(cyclopropylmethoxy)-N-(3,5-dichloropyridin-4-yl...)Show SMILES FC(F)Oc1ccc(cc1OCC1CC1)C(=O)Nc1c(Cl)cncc1Cl Show InChI InChI=1S/C17H14Cl2F2N2O3/c18-11-6-22-7-12(19)15(11)23-16(24)10-3-4-13(26-17(20)21)14(5-10)25-8-9-1-2-9/h3-7,9,17H,1-2,8H2,(H,22,23,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of human recombinant PDE4A using [3H]cAMP as substrate preincubated with enzyme for 10 mins followed by substrate addition and measured af... |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.112492
BindingDB Entry DOI: 10.7270/Q2R21511 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM120866
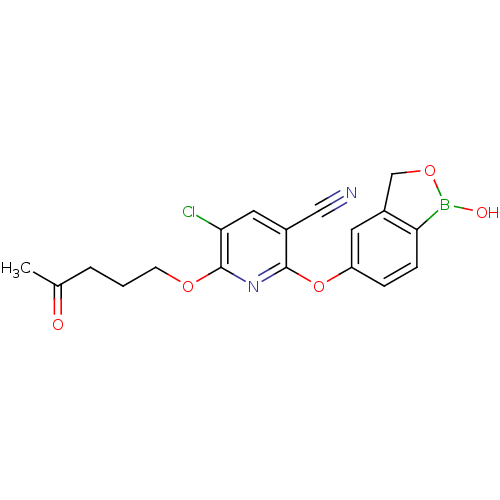
(US8716478, D253 | US9499570, D253)Show SMILES CC(=O)CCCOc1nc(Oc2ccc3B(O)OCc3c2)c(cc1Cl)C#N Show InChI InChI=1S/C18H16BClN2O5/c1-11(23)3-2-6-25-18-16(20)8-12(9-21)17(22-18)27-14-4-5-15-13(7-14)10-26-19(15)24/h4-5,7-8,24H,2-3,6,10H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | 7.5 | 37 |
Anacor Pharmaceuticals, Inc.
US Patent
| Assay Description
Inhibition of the human PDE4 enzyme, using semi-purified enzyme from human U937 cells. The PDE4 enzyme was partially purified from human U937 myeloid... |
US Patent US8716478 (2014)
BindingDB Entry DOI: 10.7270/Q2Z60MQ7 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM130933
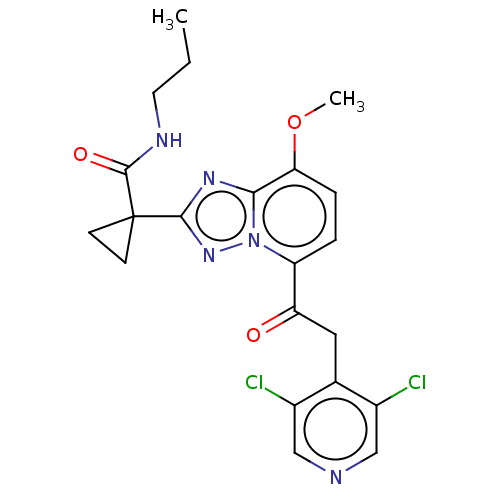
(US8829190, 111)Show SMILES CCCNC(=O)C1(CC1)c1nc2c(OC)ccc(C(=O)Cc3c(Cl)cncc3Cl)n2n1 Show InChI InChI=1S/C21H21Cl2N5O3/c1-3-8-25-20(30)21(6-7-21)19-26-18-17(31-2)5-4-15(28(18)27-19)16(29)9-12-13(22)10-24-11-14(12)23/h4-5,10-11H,3,6-9H2,1-2H3,(H,25,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Leo Pharma A/S
US Patent
| Assay Description
Human recombinant PDE4 (Gene bank accession no NM006203) was incubated for 1 hour with the test compound at concentrations up to 10 μM, with cAM... |
US Patent US8829190 (2014)
BindingDB Entry DOI: 10.7270/Q2Z89B3Z |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50113362
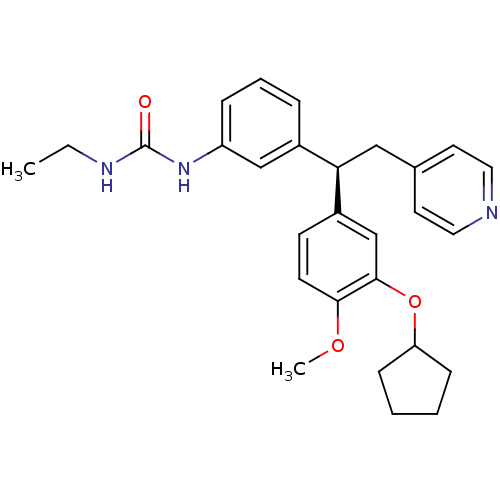
(1-{3-[1-((R)-3-Cyclopentyloxy-4-methoxy-phenyl)-2-...)Show SMILES CCNC(=O)Nc1cccc(c1)[C@@H](Cc1ccncc1)c1ccc(OC)c(OC2CCCC2)c1 Show InChI InChI=1S/C28H33N3O3/c1-3-30-28(32)31-23-8-6-7-21(18-23)25(17-20-13-15-29-16-14-20)22-11-12-26(33-2)27(19-22)34-24-9-4-5-10-24/h6-8,11-16,18-19,24-25H,3-5,9-10,17H2,1-2H3,(H2,30,31,32)/t25-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant phosphodiesterase 4A |
Bioorg Med Chem Lett 12: 1451-6 (2002)
BindingDB Entry DOI: 10.7270/Q22J6B5D |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50125066
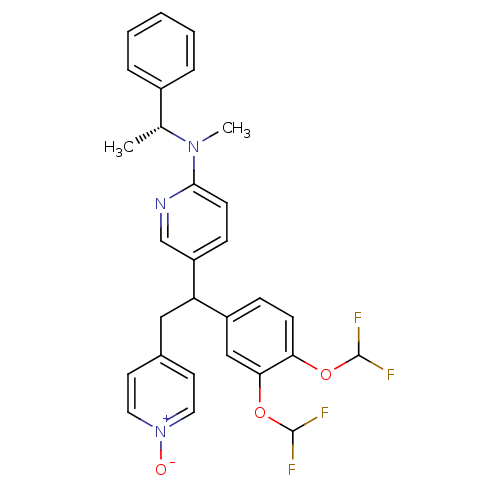
(CHEMBL165889 | {5-[1-(3,4-Bis-difluoromethoxy-phen...)Show SMILES C[C@@H](N(C)c1ccc(cn1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC(F)F)c1)c1ccccc1 Show InChI InChI=1S/C29H27F4N3O3/c1-19(21-6-4-3-5-7-21)35(2)27-11-9-23(18-34-27)24(16-20-12-14-36(37)15-13-20)22-8-10-25(38-28(30)31)26(17-22)39-29(32)33/h3-15,17-19,24,28-29H,16H2,1-2H3/t19-,24?/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against human PDE4A expressed isoform using construct representing the common region of spliced variants as GST-fusion protein in... |
Bioorg Med Chem Lett 13: 741-4 (2003)
BindingDB Entry DOI: 10.7270/Q2PG1R3G |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174013
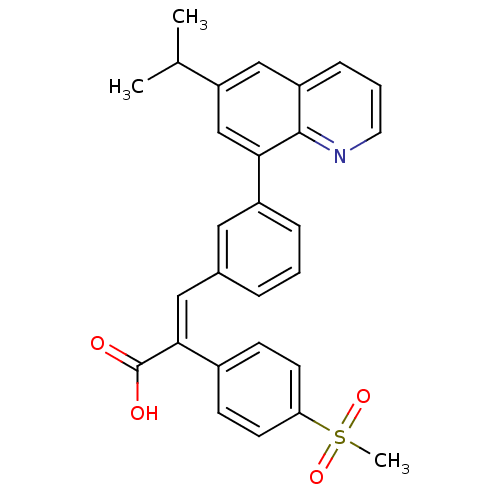
((E)-3-(3-(6-isopropylquinolin-8-yl)phenyl)-2-(4-(m...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\C(O)=O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C28H25NO4S/c1-18(2)23-16-22-8-5-13-29-27(22)25(17-23)21-7-4-6-19(14-21)15-26(28(30)31)20-9-11-24(12-10-20)34(3,32)33/h4-18H,1-3H3,(H,30,31)/b26-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183803

(CHEMBL206968 | N-((3-(6-(2-cyanopropan-2-yl)quinol...)Show SMILES Cc1cc(no1)C(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C32H28N4O4S/c1-21-15-29(35-40-21)31(37)36(26-10-12-27(13-11-26)41(4,38)39)19-22-7-5-8-23(16-22)28-18-25(32(2,3)20-33)17-24-9-6-14-34-30(24)28/h5-18H,19H2,1-4H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50353703
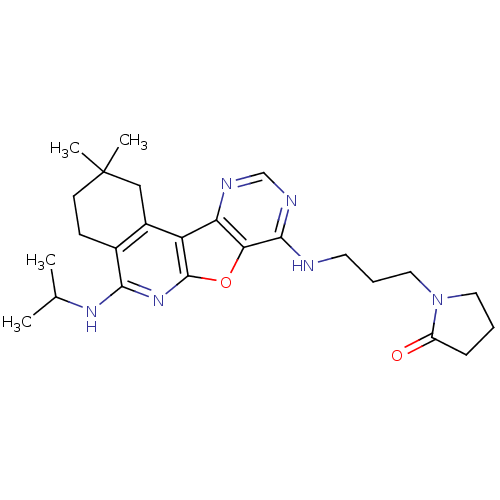
(CHEMBL1830646)Show SMILES CC(C)Nc1nc2oc3c(NCCCN4CCCC4=O)ncnc3c2c2CC(C)(C)CCc12 Show InChI InChI=1S/C25H34N6O2/c1-15(2)29-22-16-8-9-25(3,4)13-17(16)19-20-21(33-24(19)30-22)23(28-14-27-20)26-10-6-12-31-11-5-7-18(31)32/h14-15H,5-13H2,1-4H3,(H,29,30)(H,26,27,28) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4A4 assessed as inhibition of [3H]cAMP hydrolysis to [3H]AMP after 15 mins by scintillation proximity assay |
Eur J Med Chem 46: 4946-56 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.054
BindingDB Entry DOI: 10.7270/Q2VM4CPM |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174018
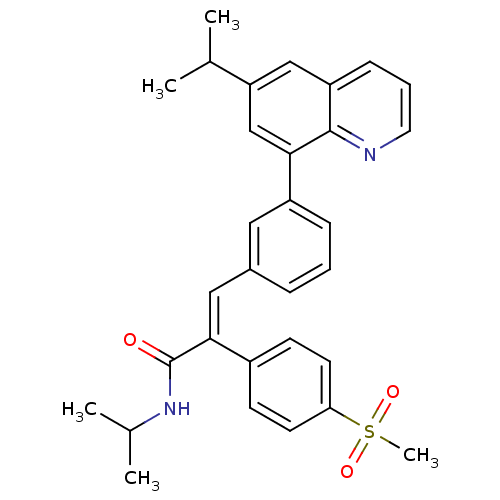
((E)-N-isopropyl-3-(3-(6-isopropylquinolin-8-yl)phe...)Show SMILES CC(C)NC(=O)C(=C\c1cccc(c1)-c1cc(cc2cccnc12)C(C)C)\c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H32N2O3S/c1-20(2)26-18-25-10-7-15-32-30(25)28(19-26)24-9-6-8-22(16-24)17-29(31(34)33-21(3)4)23-11-13-27(14-12-23)37(5,35)36/h6-21H,1-5H3,(H,33,34)/b29-17+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50183791
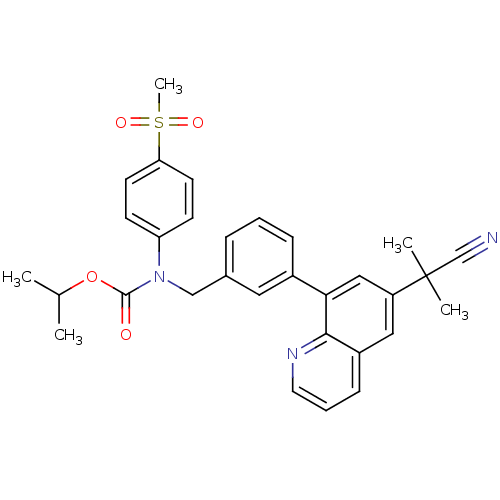
(CHEMBL209295 | isopropyl (3-(6-(2-cyanopropan-2-yl...)Show SMILES CC(C)OC(=O)N(Cc1cccc(c1)-c1cc(cc2cccnc12)C(C)(C)C#N)c1ccc(cc1)S(C)(=O)=O Show InChI InChI=1S/C31H31N3O4S/c1-21(2)38-30(35)34(26-11-13-27(14-12-26)39(5,36)37)19-22-8-6-9-23(16-22)28-18-25(31(3,4)20-32)17-24-10-7-15-33-29(24)28/h6-18,21H,19H2,1-5H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of human GST-PDE4A |
Bioorg Med Chem Lett 16: 2608-12 (2006)
Article DOI: 10.1016/j.bmcl.2006.02.043
BindingDB Entry DOI: 10.7270/Q2F18Z9N |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274188
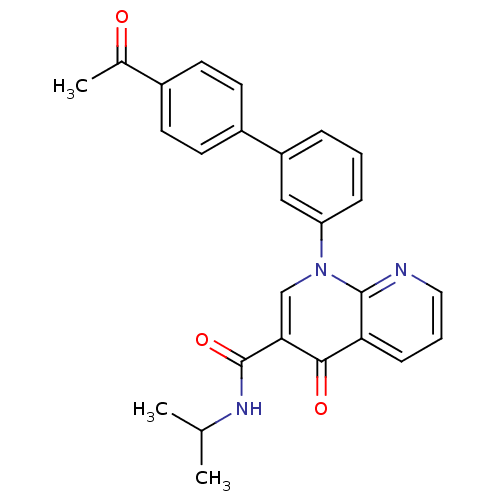
(1-(4'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(c2)-c2ccc(cc2)C(C)=O)c2ncccc2c1=O Show InChI InChI=1S/C26H23N3O3/c1-16(2)28-26(32)23-15-29(25-22(24(23)31)8-5-13-27-25)21-7-4-6-20(14-21)19-11-9-18(10-12-19)17(3)30/h4-16H,1-3H3,(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.340 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347344
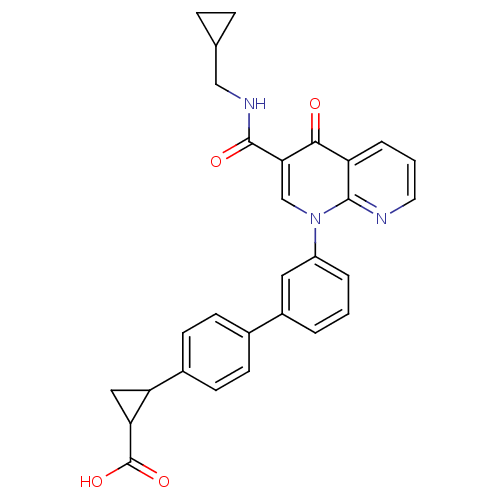
(CHEMBL1801156)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C29H25N3O4/c33-26-22-5-2-12-30-27(22)32(16-25(26)28(34)31-15-17-6-7-17)21-4-1-3-20(13-21)18-8-10-19(11-9-18)23-14-24(23)29(35)36/h1-5,8-13,16-17,23-24H,6-7,14-15H2,(H,31,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.350 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274789

(1-(3'-Acetyl-biphenyl-3-yl)-4-oxo-1,4-dihydro-[1,8...)Show SMILES CC(C)NC(=O)c1cn(-c2cccc(c2)-c2cccc(c2)C(C)=O)c2ncccc2c1=O Show InChI InChI=1S/C26H23N3O3/c1-16(2)28-26(32)23-15-29(25-22(24(23)31)11-6-12-27-25)21-10-5-9-20(14-21)19-8-4-7-18(13-19)17(3)30/h4-16H,1-3H3,(H,28,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50017340
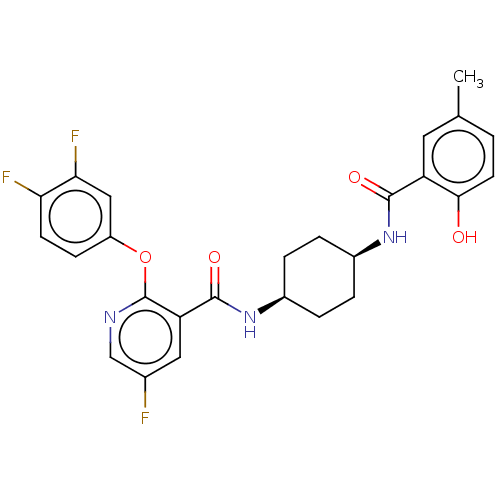
(CHEMBL3113734)Show SMILES Cc1ccc(O)c(c1)C(=O)N[C@H]1CC[C@H](CC1)NC(=O)c1cc(F)cnc1Oc1ccc(F)c(F)c1 |r,wU:14.18,11.11,(24.27,-11.72,;24.28,-13.26,;25.62,-14.03,;25.62,-15.57,;24.29,-16.34,;24.28,-17.88,;22.96,-15.57,;22.95,-14.04,;21.63,-16.34,;21.63,-17.88,;20.29,-15.57,;18.96,-16.34,;18.96,-17.88,;17.63,-18.65,;16.29,-17.87,;16.28,-16.34,;17.62,-15.57,;14.96,-18.65,;13.62,-17.88,;13.62,-16.34,;12.29,-18.66,;10.95,-17.9,;9.63,-18.67,;8.29,-17.9,;9.62,-20.21,;10.96,-20.98,;12.3,-20.21,;13.63,-20.98,;13.63,-22.52,;12.3,-23.29,;12.3,-24.83,;13.64,-25.6,;13.64,-27.14,;14.97,-24.81,;16.31,-25.58,;14.96,-23.28,)| Show InChI InChI=1S/C26H24F3N3O4/c1-14-2-9-23(33)19(10-14)24(34)31-16-3-5-17(6-4-16)32-25(35)20-11-15(27)13-30-26(20)36-18-7-8-21(28)22(29)12-18/h2,7-13,16-17,33H,3-6H2,1H3,(H,31,34)(H,32,35)/t16-,17+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of PDE4A (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.9b02170
BindingDB Entry DOI: 10.7270/Q2W95DVV |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174031
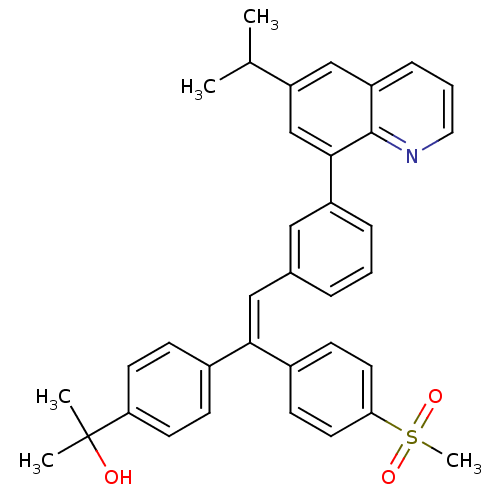
((Z)-2-(4-(2-(3-(6-isopropylquinolin-8-yl)phenyl)-1...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3ccc(cc3)C(C)(C)O)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C36H35NO3S/c1-24(2)30-22-29-10-7-19-37-35(29)34(23-30)28-9-6-8-25(20-28)21-33(26-11-15-31(16-12-26)36(3,4)38)27-13-17-32(18-14-27)41(5,39)40/h6-24,38H,1-5H3/b33-21- | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174020
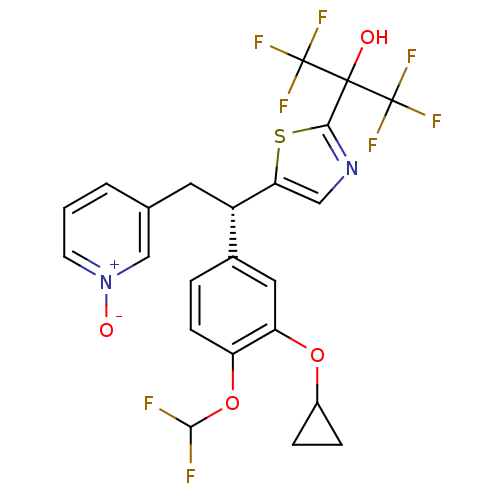
((S)-(+)-3-{2-[(3-Cyclopropyloxy-4-difluromethoxy)-...)Show SMILES OC(c1ncc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)18-10-32-19(38-18)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50092626
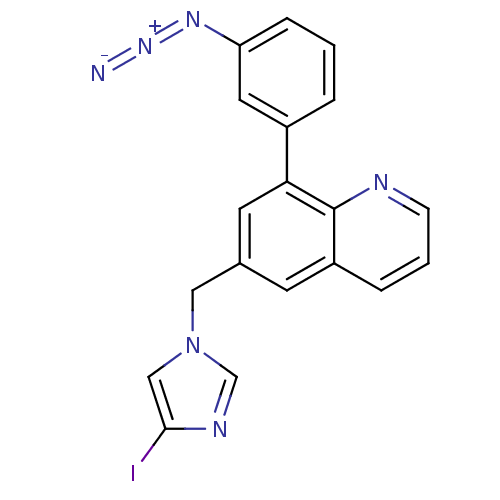
(8-(3-Azido-phenyl)-6-(4-iodo-imidazol-1-ylmethyl)-...)Show SMILES Ic1cn(Cc2cc(-c3cccc(c3)N=[N+]=[N-])c3ncccc3c2)cn1 Show InChI InChI=1S/C19H13IN6/c20-18-11-26(12-23-18)10-13-7-15-4-2-6-22-19(15)17(8-13)14-3-1-5-16(9-14)24-25-21/h1-9,11-12H,10H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of recombinant human PDE4A expressed in Sf9 cells |
J Med Chem 43: 3820-3 (2000)
BindingDB Entry DOI: 10.7270/Q23T9GG9 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM130936
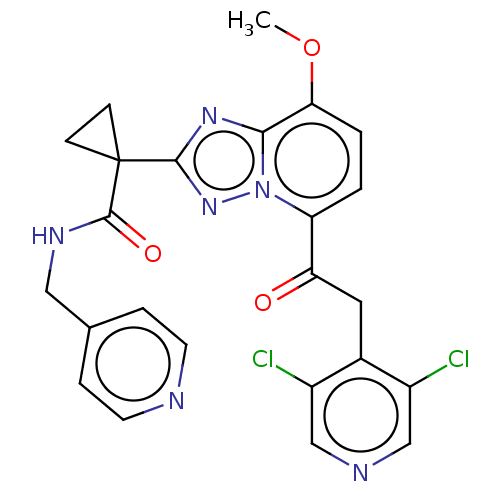
(US8829190, 114)Show SMILES COc1ccc(C(=O)Cc2c(Cl)cncc2Cl)n2nc(nc12)C1(CC1)C(=O)NCc1ccncc1 Show InChI InChI=1S/C24H20Cl2N6O3/c1-35-20-3-2-18(19(33)10-15-16(25)12-28-13-17(15)26)32-21(20)30-22(31-32)24(6-7-24)23(34)29-11-14-4-8-27-9-5-14/h2-5,8-9,12-13H,6-7,10-11H2,1H3,(H,29,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Leo Pharma A/S
US Patent
| Assay Description
Human recombinant PDE4 (Gene bank accession no NM006203) was incubated for 1 hour with the test compound at concentrations up to 10 μM, with cAM... |
US Patent US8829190 (2014)
BindingDB Entry DOI: 10.7270/Q2Z89B3Z |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM14359

((+)-1 | (S)-(+)-3-{2-[(3-Cyclopropyloxy-4-diflurom...)Show SMILES OC(c1cnc(s1)[C@@H](Cc1ccc[n+]([O-])c1)c1ccc(OC(F)F)c(OC2CC2)c1)(C(F)(F)F)C(F)(F)F Show InChI InChI=1S/C23H18F8N2O4S/c24-20(25)37-16-6-3-13(9-17(16)36-14-4-5-14)15(8-12-2-1-7-33(35)11-12)19-32-10-18(38-19)21(34,22(26,27)28)23(29,30)31/h1-3,6-7,9-11,14-15,20,34H,4-5,8H2/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | 7.5 | 22 |
University of North Carolina at Chapel Hill
| Assay Description
PDE4 catalytic activity was monitored by measuring the hydrolysis of [3H]-cAMP to [3H]-AMP using PDE-SPA kit (Amersham International). [3H]-AMP was c... |
J Med Chem 49: 1867-73 (2006)
Article DOI: 10.1021/jm051273d
BindingDB Entry DOI: 10.7270/Q2MK6B48 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50274027

(1-(4-chlorophenyl)-N-(3,5-dichloropyridin-4-yl)-4-...)Show SMILES Clc1ccc(cc1)-n1cc(C(=O)Nc2c(Cl)cncc2Cl)c(=O)c2cccnc12 Show InChI InChI=1S/C20H11Cl3N4O2/c21-11-3-5-12(6-4-11)27-10-14(18(28)13-2-1-7-25-19(13)27)20(29)26-17-15(22)8-24-9-16(17)23/h1-10H,(H,24,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Center for Therapeutic Research
Curated by ChEMBL
| Assay Description
Intrinsic inhibition of GST-fused human PDE4A expressed in SF9 cells |
Bioorg Med Chem Lett 18: 5554-8 (2008)
Article DOI: 10.1016/j.bmcl.2008.09.009
BindingDB Entry DOI: 10.7270/Q24X57M1 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347349
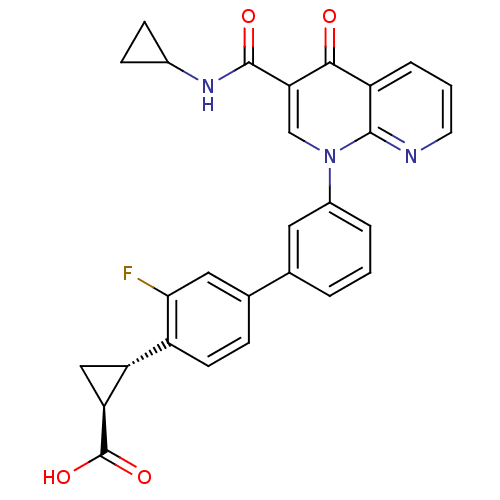
(CHEMBL1801161)Show SMILES OC(=O)[C@H]1C[C@@H]1c1ccc(cc1F)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 |r| Show InChI InChI=1S/C28H22FN3O4/c29-24-12-16(6-9-19(24)21-13-22(21)28(35)36)15-3-1-4-18(11-15)32-14-23(27(34)31-17-7-8-17)25(33)20-5-2-10-30-26(20)32/h1-6,9-12,14,17,21-22H,7-8,13H2,(H,31,34)(H,35,36)/t21-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174025
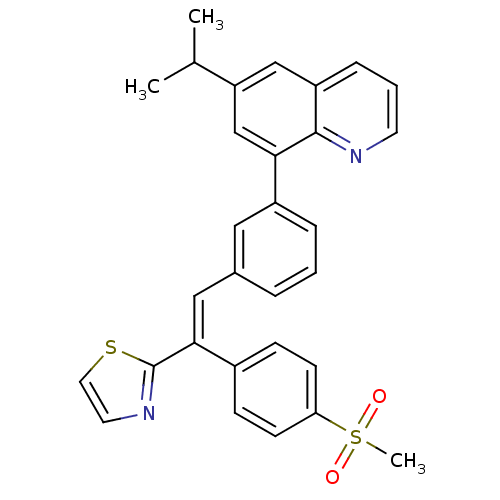
(6-isopropyl-8-(3-(2-(4-(methylsulfonyl)phenyl)-2-(...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nccs3)c3ccc(cc3)S(C)(=O)=O)c2)c2ncccc2c1 Show InChI InChI=1S/C30H26N2O2S2/c1-20(2)25-18-24-8-5-13-31-29(24)27(19-25)23-7-4-6-21(16-23)17-28(30-32-14-15-35-30)22-9-11-26(12-10-22)36(3,33)34/h4-20H,1-3H3/b28-17+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM285634
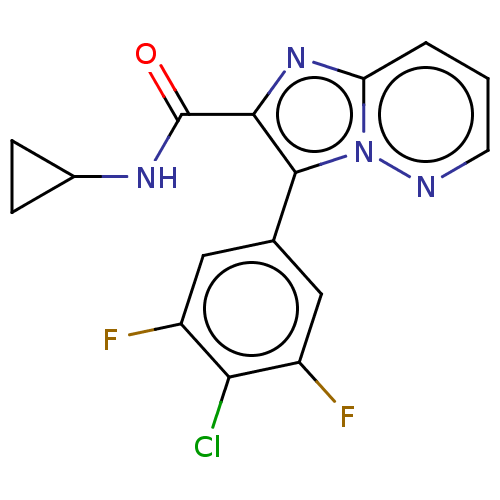
(3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...)Show SMILES Fc1cc(cc(F)c1Cl)-c1c(nc2cccnn12)C(=O)NC1CC1 Show InChI InChI=1S/C16H11ClF2N4O/c17-13-10(18)6-8(7-11(13)19)15-14(16(24)21-9-3-4-9)22-12-2-1-5-20-23(12)15/h1-2,5-7,9H,3-4H2,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
The PDE4A3, PDE4B1, PDE4C1 and PDE4D3 assays use the Scintillation Proximity Assay (SPA) technology to measure the inhibition of human recombinant PD... |
US Patent US9598421 (2017)
BindingDB Entry DOI: 10.7270/Q2K939KG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM285634

(3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...)Show SMILES Fc1cc(cc(F)c1Cl)-c1c(nc2cccnn12)C(=O)NC1CC1 Show InChI InChI=1S/C16H11ClF2N4O/c17-13-10(18)6-8(7-11(13)19)15-14(16(24)21-9-3-4-9)22-12-2-1-5-20-23(12)15/h1-2,5-7,9H,3-4H2,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM285634

(3-(4-chloro-3,5-difluoro-phenyl)-N-cyclopropyl-imi...)Show SMILES Fc1cc(cc(F)c1Cl)-c1c(nc2cccnn12)C(=O)NC1CC1 Show InChI InChI=1S/C16H11ClF2N4O/c17-13-10(18)6-8(7-11(13)19)15-14(16(24)21-9-3-4-9)22-12-2-1-5-20-23(12)15/h1-2,5-7,9H,3-4H2,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | <0.488 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
US Patent
| Assay Description
Human PDE4A3 coding sequence (amino acids 2 to 825 from the sequence with accession number NP_001104779) was cloned into the baculovirus expression v... |
US Patent US10669279 (2020)
BindingDB Entry DOI: 10.7270/Q2765JC9 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50174023
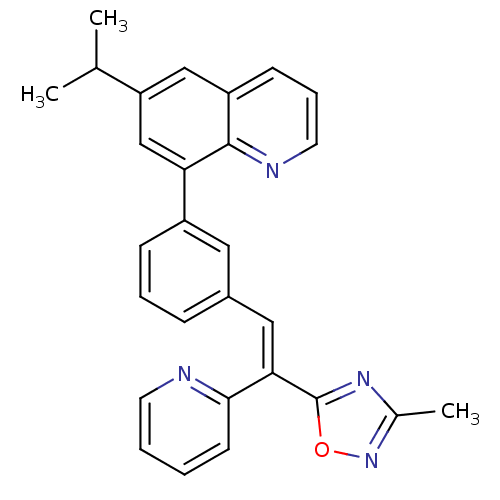
((E)-6-isopropyl-8-(3-(2-(3-methyl-1,2,4-oxadiazol-...)Show SMILES CC(C)c1cc(-c2cccc(\C=C(\c3nc(C)no3)c3ccccn3)c2)c2ncccc2c1 Show InChI InChI=1S/C28H24N4O/c1-18(2)23-16-22-10-7-13-30-27(22)24(17-23)21-9-6-8-20(14-21)15-25(26-11-4-5-12-29-26)28-31-19(3)32-33-28/h4-18H,1-3H3/b25-15+ | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory activity against PDE4A |
Bioorg Med Chem Lett 15: 5241-6 (2005)
Article DOI: 10.1016/j.bmcl.2005.08.036
BindingDB Entry DOI: 10.7270/Q2B56KHG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM130932
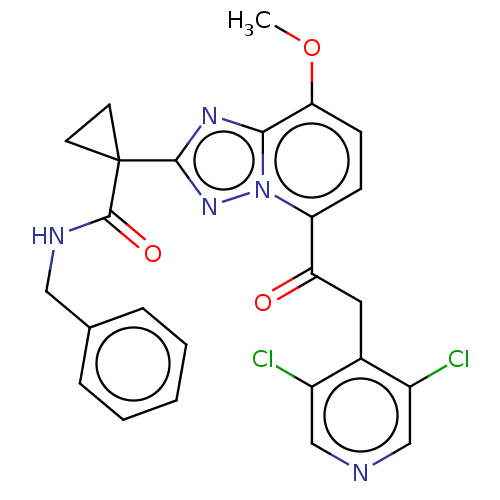
(US8829190, 110)Show SMILES COc1ccc(C(=O)Cc2c(Cl)cncc2Cl)n2nc(nc12)C1(CC1)C(=O)NCc1ccccc1 Show InChI InChI=1S/C25H21Cl2N5O3/c1-35-21-8-7-19(20(33)11-16-17(26)13-28-14-18(16)27)32-22(21)30-23(31-32)25(9-10-25)24(34)29-12-15-5-3-2-4-6-15/h2-8,13-14H,9-12H2,1H3,(H,29,34) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Leo Pharma A/S
US Patent
| Assay Description
Human recombinant PDE4 (Gene bank accession no NM006203) was incubated for 1 hour with the test compound at concentrations up to 10 μM, with cAM... |
US Patent US8829190 (2014)
BindingDB Entry DOI: 10.7270/Q2Z89B3Z |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50353700

(CHEMBL1830643)Show SMILES CC(C)Nc1nc2oc3c(NCCN4CCOCC4)ncnc3c2c2CC(C)(C)CCc12 Show InChI InChI=1S/C24H34N6O2/c1-15(2)28-21-16-5-6-24(3,4)13-17(16)18-19-20(32-23(18)29-21)22(27-14-26-19)25-7-8-30-9-11-31-12-10-30/h14-15H,5-13H2,1-4H3,(H,28,29)(H,25,26,27) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Rhône-Poulenc Rorer
Curated by ChEMBL
| Assay Description
Inhibition of human PDE4A4 assessed as inhibition of [3H]cAMP hydrolysis to [3H]AMP after 15 mins by scintillation proximity assay |
Eur J Med Chem 46: 4946-56 (2011)
Article DOI: 10.1016/j.ejmech.2011.07.054
BindingDB Entry DOI: 10.7270/Q2VM4CPM |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50128685

(2-{5-[1-(3-Cyclobutoxy-4-difluoromethoxy-phenyl)-2...)Show SMILES CC(C)(O)c1ncc(s1)C(Cc1cc[n+]([O-])cc1)c1ccc(OC(F)F)c(OC2CCC2)c1 Show InChI InChI=1S/C24H26F2N2O4S/c1-24(2,29)22-27-14-21(33-22)18(12-15-8-10-28(30)11-9-15)16-6-7-19(32-23(25)26)20(13-16)31-17-4-3-5-17/h6-11,13-14,17-18,23,29H,3-5,12H2,1-2H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
In vitro inhibitory activity was determined against human Phosphodiesterase 4 isoform using a construct representing the common region of spliced var... |
J Med Chem 46: 2413-26 (2003)
Article DOI: 10.1021/jm0204542
BindingDB Entry DOI: 10.7270/Q24X5756 |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50113417
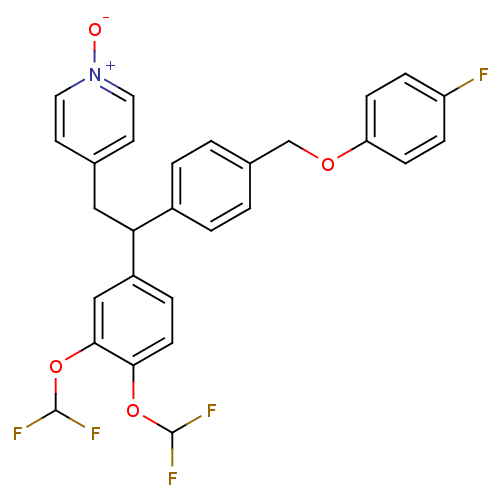
(4-{2-(3,4-Bis-difluoromethoxy-phenyl)-2-[4-(4-fluo...)Show SMILES [O-][n+]1ccc(CC(c2ccc(COc3ccc(F)cc3)cc2)c2ccc(OC(F)F)c(OC(F)F)c2)cc1 Show InChI InChI=1S/C28H22F5NO4/c29-22-6-8-23(9-7-22)36-17-19-1-3-20(4-2-19)24(15-18-11-13-34(35)14-12-18)21-5-10-25(37-27(30)31)26(16-21)38-28(32)33/h1-14,16,24,27-28H,15,17H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against human PDE4A isoform using a construct representing the common region of spliced variants expressed as GST-fusion pro... |
Bioorg Med Chem Lett 12: 1457-61 (2002)
BindingDB Entry DOI: 10.7270/Q2KP82PM |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347343
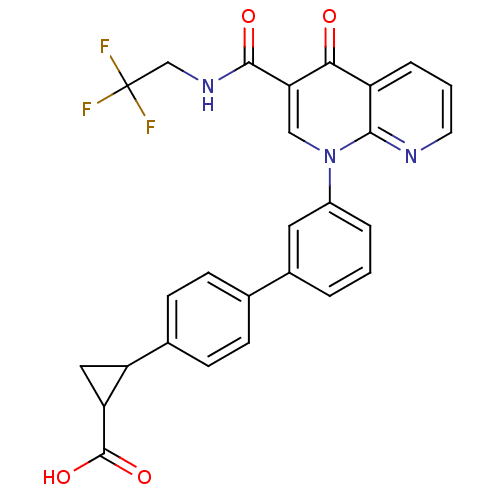
(CHEMBL1801155)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NCC(F)(F)F)c(=O)c2cccnc12 Show InChI InChI=1S/C27H20F3N3O4/c28-27(29,30)14-32-25(35)22-13-33(24-19(23(22)34)5-2-10-31-24)18-4-1-3-17(11-18)15-6-8-16(9-7-15)20-12-21(20)26(36)37/h1-11,13,20-21H,12,14H2,(H,32,35)(H,36,37) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.510 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347345
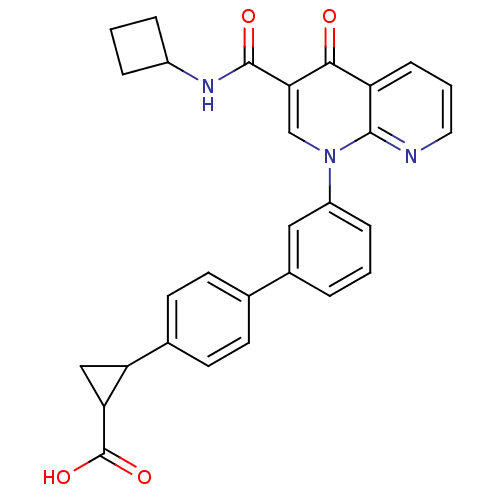
(CHEMBL1801157)Show SMILES OC(=O)C1CC1c1ccc(cc1)-c1cccc(c1)-n1cc(C(=O)NC2CCC2)c(=O)c2cccnc12 Show InChI InChI=1S/C29H25N3O4/c33-26-22-8-3-13-30-27(22)32(16-25(26)28(34)31-20-5-2-6-20)21-7-1-4-19(14-21)17-9-11-18(12-10-17)23-15-24(23)29(35)36/h1,3-4,7-14,16,20,23-24H,2,5-6,15H2,(H,31,34)(H,35,36) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.520 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347348
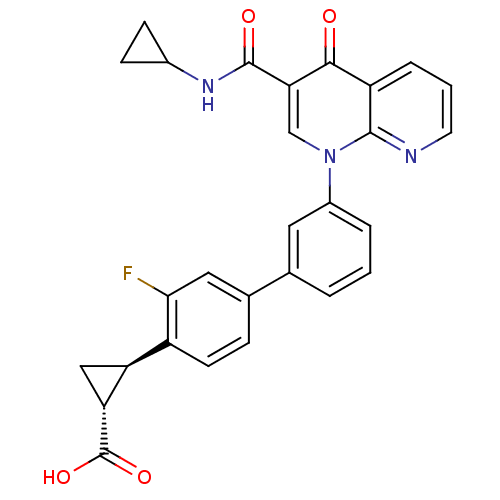
(CHEMBL1801160)Show SMILES OC(=O)[C@@H]1C[C@H]1c1ccc(cc1F)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 |r| Show InChI InChI=1S/C28H22FN3O4/c29-24-12-16(6-9-19(24)21-13-22(21)28(35)36)15-3-1-4-18(11-15)32-14-23(27(34)31-17-7-8-17)25(33)20-5-2-10-30-26(20)32/h1-6,9-12,14,17,21-22H,7-8,13H2,(H,31,34)(H,35,36)/t21-,22+/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50232731
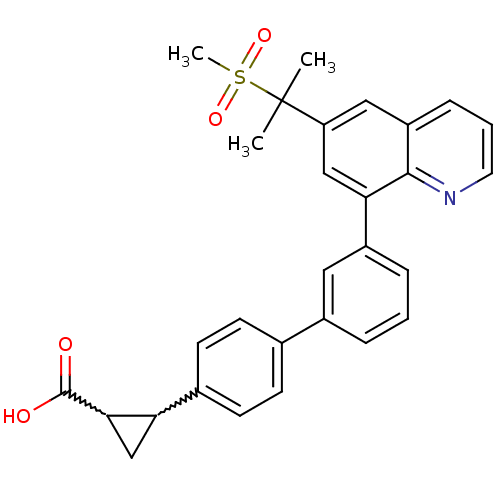
(2-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(cc2)C2CC2C(O)=O)c2ncccc2c1)S(C)(=O)=O |w:18.19,20.23| Show InChI InChI=1S/C29H27NO4S/c1-29(2,35(3,33)34)23-15-22-8-5-13-30-27(22)25(16-23)21-7-4-6-20(14-21)18-9-11-19(12-10-18)24-17-26(24)28(31)32/h4-16,24,26H,17H2,1-3H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50347335
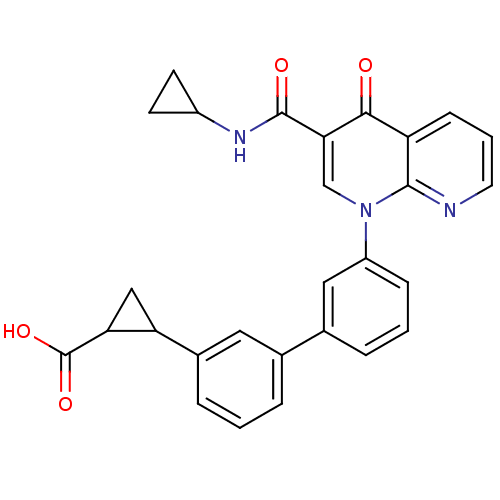
(CHEMBL1801068)Show SMILES OC(=O)C1CC1c1cccc(c1)-c1cccc(c1)-n1cc(C(=O)NC2CC2)c(=O)c2cccnc12 Show InChI InChI=1S/C28H23N3O4/c32-25-21-8-3-11-29-26(21)31(15-24(25)27(33)30-19-9-10-19)20-7-2-5-17(13-20)16-4-1-6-18(12-16)22-14-23(22)28(34)35/h1-8,11-13,15,19,22-23H,9-10,14H2,(H,30,33)(H,34,35) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |
cAMP-specific 3',5'-cyclic phosphodiesterase 4A
(Homo sapiens (Human)) | BDBM50232731

(2-{3'-[6-(1-methanesulfonyl-1-methyl-ethyl)-quinol...)Show SMILES CC(C)(c1cc(-c2cccc(c2)-c2ccc(cc2)C2CC2C(O)=O)c2ncccc2c1)S(C)(=O)=O |w:18.19,20.23| Show InChI InChI=1S/C29H27NO4S/c1-29(2,35(3,33)34)23-15-22-8-5-13-30-27(22)25(16-23)21-7-4-6-20(14-21)18-9-11-19(12-10-18)24-17-26(24)28(31)32/h4-16,24,26H,17H2,1-3H3,(H,31,32) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research
Curated by ChEMBL
| Assay Description
Inhibition of PDE4A |
Bioorg Med Chem Lett 20: 6387-93 (2010)
Article DOI: 10.1016/j.bmcl.2010.09.087
BindingDB Entry DOI: 10.7270/Q2QJ7HPG |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data