 Found 557 hits of ic50 for UniProtKB: P04818
Found 557 hits of ic50 for UniProtKB: P04818 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional purine biosynthesis protein ATIC/Thymidylate synthase/Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM50277355
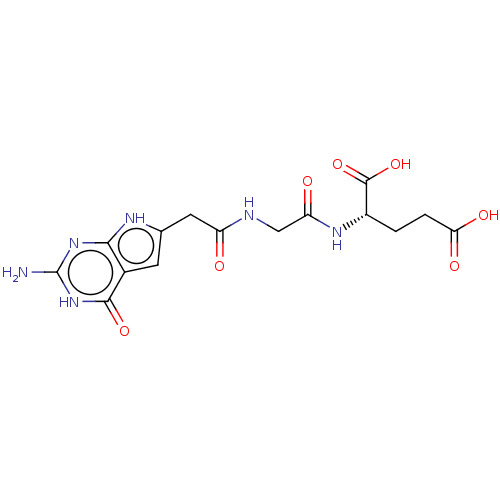
(CHEMBL4177183)Show SMILES Nc1nc2[nH]c(CC(=O)NCC(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C15H18N6O7/c16-15-20-12-7(13(26)21-15)3-6(18-12)4-9(22)17-5-10(23)19-8(14(27)28)1-2-11(24)25/h3,8H,1-2,4-5H2,(H,17,22)(H,19,23)(H,24,25)(H,27,28)(H4,16,18,20,21,26)/t8-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0780 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of TS/AICARFTase/GARFTase in human KB cells assessed as reduction in cell proliferation in folate free medium after 72 hrs in presence of ... |
Eur J Med Chem 139: 531-541 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.032
BindingDB Entry DOI: 10.7270/Q2WW7M6F |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC/Thymidylate synthase/Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM50277352
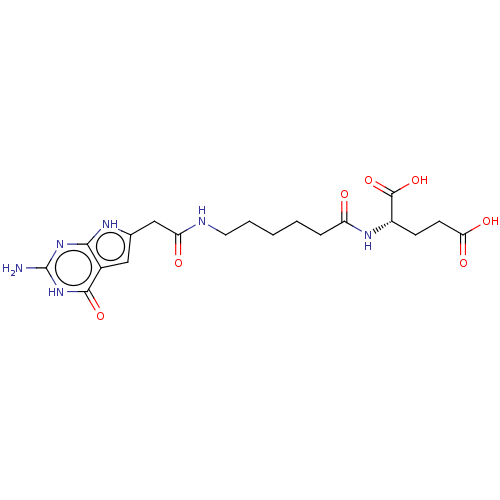
(CHEMBL4162649)Show SMILES Nc1nc2[nH]c(CC(=O)NCCCCCC(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C19H26N6O7/c20-19-24-16-11(17(30)25-19)8-10(22-16)9-14(27)21-7-3-1-2-4-13(26)23-12(18(31)32)5-6-15(28)29/h8,12H,1-7,9H2,(H,21,27)(H,23,26)(H,28,29)(H,31,32)(H4,20,22,24,25,30)/t12-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.140 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of TS/AICARFTase/GARFTase in human KB cells assessed as reduction in cell proliferation in folate free medium after 72 hrs in presence of ... |
Eur J Med Chem 139: 531-541 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.032
BindingDB Entry DOI: 10.7270/Q2WW7M6F |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50326712
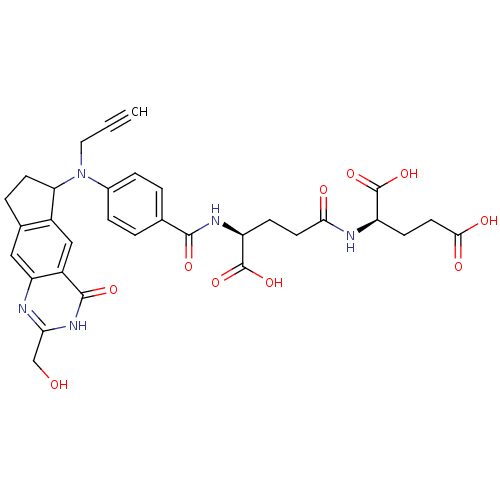
(2(R)-[4(S)-carboxy-4-[4-[N-(2-hydroxymethyl-4-oxo-...)Show SMILES OCc1nc2cc3CCC(N(CC#C)c4ccc(cc4)C(=O)N[C@@H](CCC(=O)N[C@H](CCC(O)=O)C(O)=O)C(O)=O)c3cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C32H33N5O10/c1-2-13-37(25-10-5-18-14-24-21(15-20(18)25)30(43)36-26(16-38)33-24)19-6-3-17(4-7-19)29(42)35-23(32(46)47)8-11-27(39)34-22(31(44)45)9-12-28(40)41/h1,3-4,6-7,14-15,22-23,25,38H,5,8-13,16H2,(H,34,39)(H,35,42)(H,40,41)(H,44,45)(H,46,47)(H,33,36,43)/t22-,23+,25?/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Institute of Theoretical Studies gGmbH
Curated by ChEMBL
| Assay Description
Inhibition of thymidin synthase |
J Med Chem 53: 6539-49 (2010)
Article DOI: 10.1021/jm901869w
BindingDB Entry DOI: 10.7270/Q24J0F9S |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615595
(CHEMBL5268759) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615600
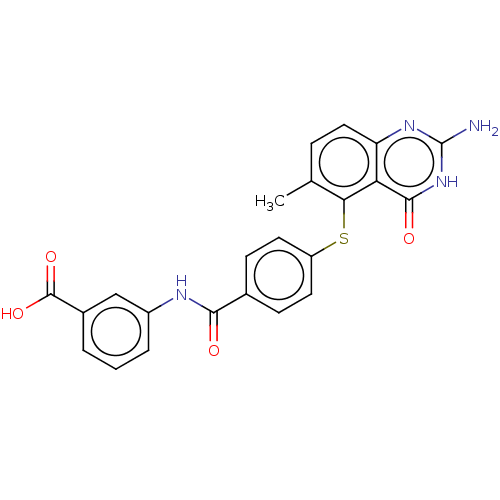
(CHEMBL5273148) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615599

(CHEMBL5271165) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615598
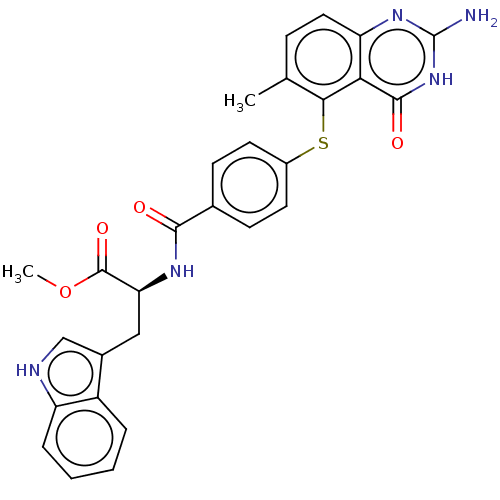
(CHEMBL5266971) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615597

(CHEMBL5282201) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615596
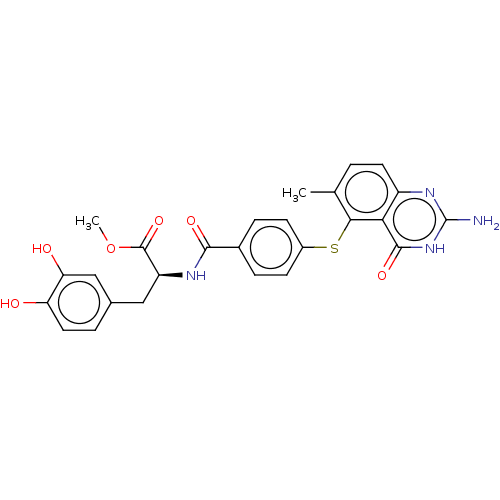
(CHEMBL5286548) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615594

(CHEMBL5288172) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615593
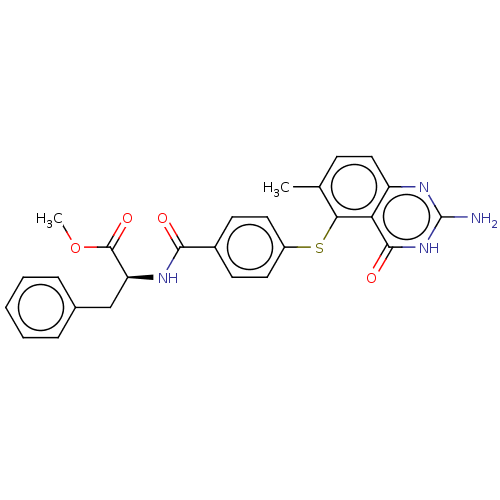
(CHEMBL5287291) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615592

(CHEMBL5283610) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615591
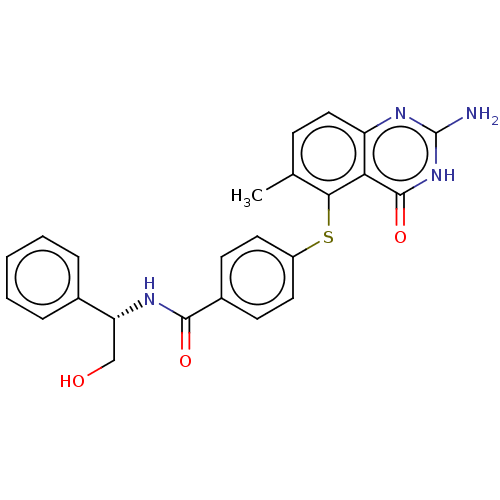
(CHEMBL5289689) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615583

(CHEMBL321678) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615584
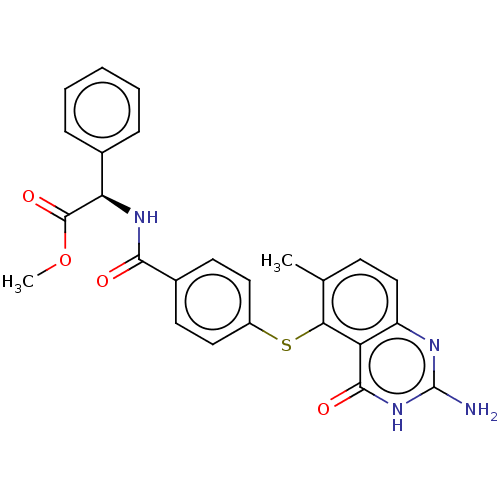
(CHEMBL5267424) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615585

(CHEMBL111859) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615586
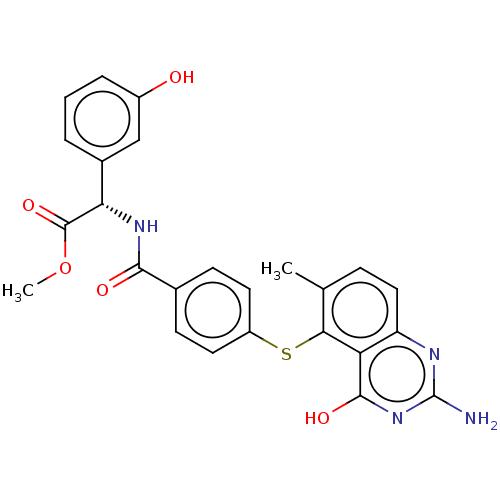
(CHEMBL113019) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615587

(CHEMBL109881) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615588
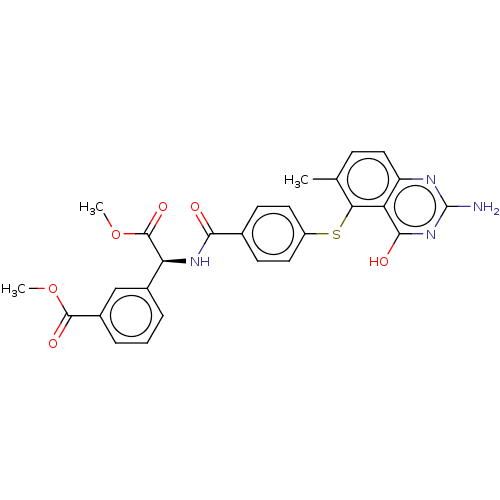
(CHEMBL320036) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615589
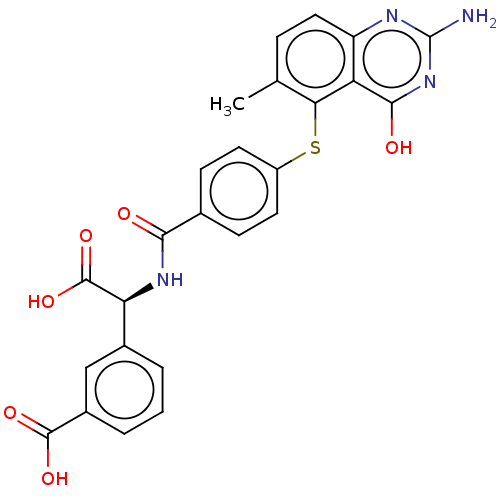
(CHEMBL264198) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50615590
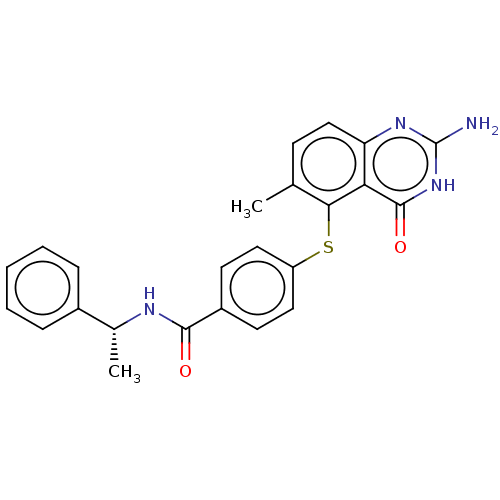
(CHEMBL5289121) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022231
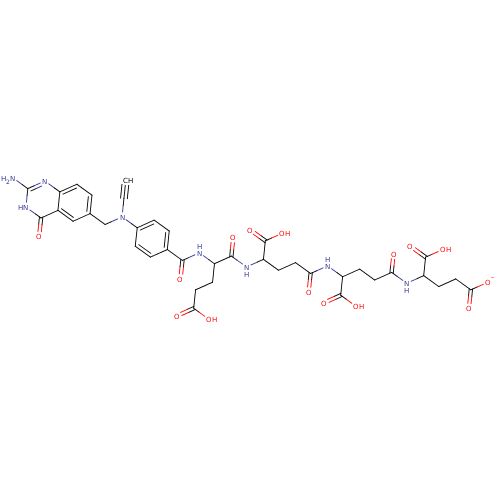
(4-{4-[4-(2-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylm...)Show SMILES Nc1nc2ccc(CN(C#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(=O)NC(CCC(=O)NC(CCC(=O)NC(CCC([O-])=O)C(O)=O)C(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C38H42N8O15/c1-2-46(18-19-3-8-23-22(17-19)33(54)45-38(39)44-23)21-6-4-20(5-7-21)32(53)42-24(11-15-30(49)50)34(55)43-27(37(60)61)10-14-29(48)40-25(35(56)57)9-13-28(47)41-26(36(58)59)12-16-31(51)52/h1,3-8,17,24-27H,9-16,18H2,(H,40,48)(H,41,47)(H,42,53)(H,43,55)(H,49,50)(H,51,52)(H,56,57)(H,58,59)(H,60,61)(H3,39,44,45,54)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.70 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidylate synthase of Streptococcus faecium |
J Med Chem 29: 1754-60 (1986)
BindingDB Entry DOI: 10.7270/Q2K074VD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022234
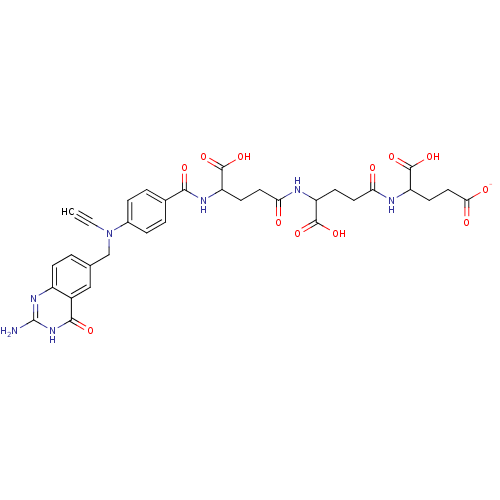
(4-[4-(4-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylmeth...)Show SMILES Nc1nc2ccc(CN(C#C)c3ccc(cc3)C(=O)NC(CCC(=O)NC(CCC(=O)NC(CCC([O-])=O)C(O)=O)C(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C33H35N7O12/c1-2-40(16-17-3-8-21-20(15-17)29(46)39-33(34)38-21)19-6-4-18(5-7-19)28(45)37-24(32(51)52)10-13-26(42)35-22(30(47)48)9-12-25(41)36-23(31(49)50)11-14-27(43)44/h1,3-8,15,22-24H,9-14,16H2,(H,35,42)(H,36,41)(H,37,45)(H,43,44)(H,47,48)(H,49,50)(H,51,52)(H3,34,38,39,46)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidylate synthase of Streptococcus faecium |
J Med Chem 29: 1754-60 (1986)
BindingDB Entry DOI: 10.7270/Q2K074VD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022235
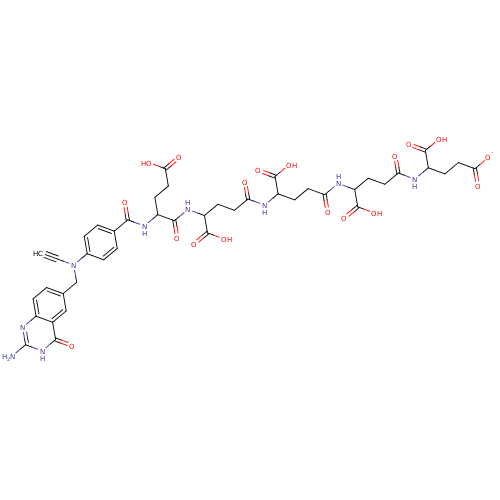
(4-(4-{4-[4-(2-{4-[(2-Amino-4-hydroxy-quinazolin-6-...)Show SMILES Nc1nc2ccc(CN(C#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(=O)NC(CCC(=O)NC(CCC(=O)NC(CCC(=O)NC(CCC([O-])=O)C(O)=O)C(O)=O)C(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C43H49N9O18/c1-2-52(20-21-3-8-25-24(19-21)37(61)51-43(44)50-25)23-6-4-22(5-7-23)36(60)48-26(12-17-34(56)57)38(62)49-30(42(69)70)11-16-33(55)46-28(40(65)66)9-14-31(53)45-27(39(63)64)10-15-32(54)47-29(41(67)68)13-18-35(58)59/h1,3-8,19,26-30H,9-18,20H2,(H,45,53)(H,46,55)(H,47,54)(H,48,60)(H,49,62)(H,56,57)(H,58,59)(H,63,64)(H,65,66)(H,67,68)(H,69,70)(H3,44,50,51,61)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidylate synthase of Streptococcus faecium |
J Med Chem 29: 1754-60 (1986)
BindingDB Entry DOI: 10.7270/Q2K074VD |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC/Thymidylate synthase/Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM50277353
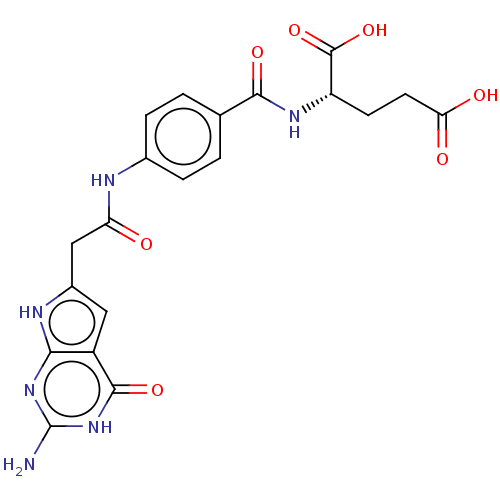
(CHEMBL4170578)Show SMILES Nc1nc2[nH]c(CC(=O)Nc3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H20N6O7/c21-20-25-16-12(18(31)26-20)7-11(23-16)8-14(27)22-10-3-1-9(2-4-10)17(30)24-13(19(32)33)5-6-15(28)29/h1-4,7,13H,5-6,8H2,(H,22,27)(H,24,30)(H,28,29)(H,32,33)(H4,21,23,25,26,31)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of TS/AICARFTase/GARFTase in human KB cells assessed as reduction in cell proliferation in folate free medium after 72 hrs in presence of ... |
Eur J Med Chem 139: 531-541 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.032
BindingDB Entry DOI: 10.7270/Q2WW7M6F |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50014498
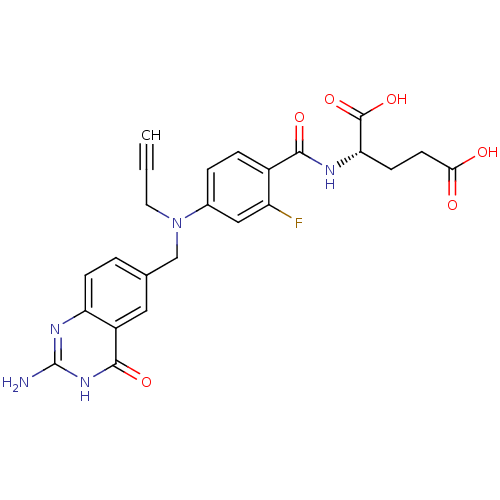
((S)-2-(4-(((2-amino-4-oxo-3,4-dihydroquinazolin-6-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H22FN5O6/c1-2-9-30(12-13-3-6-18-16(10-13)22(34)29-24(26)28-18)14-4-5-15(17(25)11-14)21(33)27-19(23(35)36)7-8-20(31)32/h1,3-6,10-11,19H,7-9,12H2,(H,27,33)(H,31,32)(H,35,36)(H3,26,28,29,34)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Meerut Institute of Engineering and Technology
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase |
Eur J Med Chem 45: 1560-71 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.065
BindingDB Entry DOI: 10.7270/Q2D50P61 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50027655

(CHEBI:5847 | Raltitrexed | Tomudex | US9422297, Ra...)Show SMILES CN(Cc1ccc2[nH]c(C)nc(=O)c2c1)c1ccc(s1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C21H22N4O6S/c1-11-22-14-4-3-12(9-13(14)19(28)23-11)10-25(2)17-7-6-16(32-17)20(29)24-15(21(30)31)5-8-18(26)27/h3-4,6-7,9,15H,5,8,10H2,1-2H3,(H,24,29)(H,26,27)(H,30,31)(H,22,23,28)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2020.113016
BindingDB Entry DOI: 10.7270/Q28W3JD6 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022233
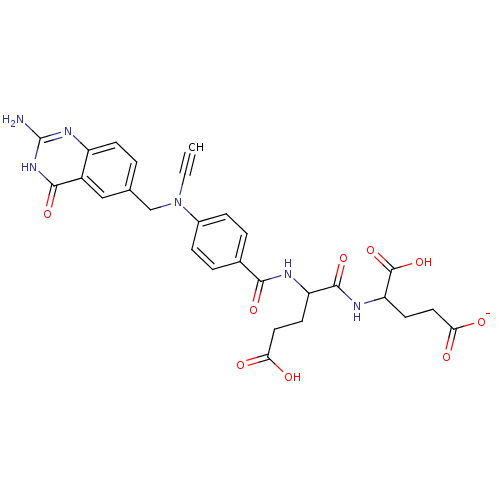
(4-(2-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylmethyl)...)Show SMILES Nc1nc2ccc(CN(C#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(=O)NC(CCC([O-])=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C28H28N6O9/c1-2-34(14-15-3-8-19-18(13-15)25(40)33-28(29)32-19)17-6-4-16(5-7-17)24(39)30-20(9-11-22(35)36)26(41)31-21(27(42)43)10-12-23(37)38/h1,3-8,13,20-21H,9-12,14H2,(H,30,39)(H,31,41)(H,35,36)(H,37,38)(H,42,43)(H3,29,32,33,40)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidylate synthase of Streptococcus faecium |
J Med Chem 29: 1754-60 (1986)
BindingDB Entry DOI: 10.7270/Q2K074VD |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC/Thymidylate synthase/Trifunctional purine biosynthetic protein adenosine-3
(Homo sapiens (Human)) | BDBM66082

((2S)-2-[[4-[(2,4-diaminopteridin-6-yl)methyl-methy...)Show SMILES CN(Cc1cnc2nc(N)nc(N)c2n1)c1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O Show InChI InChI=1S/C20H22N8O5/c1-28(9-11-8-23-17-15(24-11)16(21)26-20(22)27-17)12-4-2-10(3-5-12)18(31)25-13(19(32)33)6-7-14(29)30/h2-5,8,13H,6-7,9H2,1H3,(H,25,31)(H,29,30)(H,32,33)(H4,21,22,23,26,27)/t13-/m0/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Hebei Medical University
Curated by ChEMBL
| Assay Description
Inhibition of TS/AICARFTase/GARFTase in human KB cells assessed as reduction in cell proliferation in folate free medium after 72 hrs in presence of ... |
Eur J Med Chem 139: 531-541 (2017)
Article DOI: 10.1016/j.ejmech.2017.08.032
BindingDB Entry DOI: 10.7270/Q2WW7M6F |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50022232
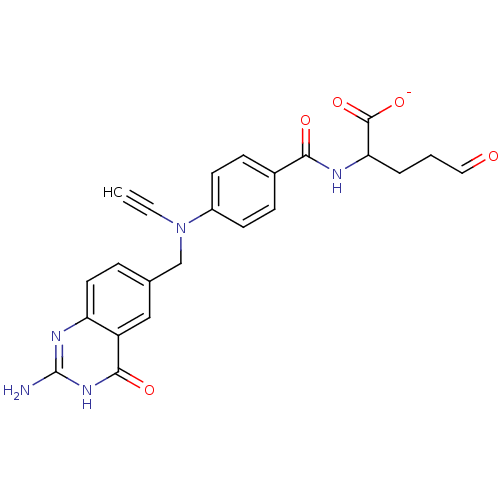
(2-{4-[(2-Amino-4-hydroxy-quinazolin-6-ylmethyl)-et...)Show SMILES Nc1nc2ccc(CN(C#C)c3ccc(cc3)C(=O)NC(CCC=O)C([O-])=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C23H21N5O5/c1-2-28(13-14-5-10-18-17(12-14)21(31)27-23(24)26-18)16-8-6-15(7-9-16)20(30)25-19(22(32)33)4-3-11-29/h1,5-12,19H,3-4,13H2,(H,25,30)(H,32,33)(H3,24,26,27,31)/p-1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidylate synthase of Streptococcus faecium |
J Med Chem 29: 1754-60 (1986)
BindingDB Entry DOI: 10.7270/Q2K074VD |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
University of South Alabama
Curated by ChEMBL
| Assay Description
Inhibition of pure human thymidyllate synthase from extract of Manca human lymphoma cells |
J Med Chem 32: 1284-9 (1989)
BindingDB Entry DOI: 10.7270/Q2P55MG5 |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Meerut Institute of Engineering and Technology
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase |
Eur J Med Chem 45: 1560-71 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.065
BindingDB Entry DOI: 10.7270/Q2D50P61 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50006689
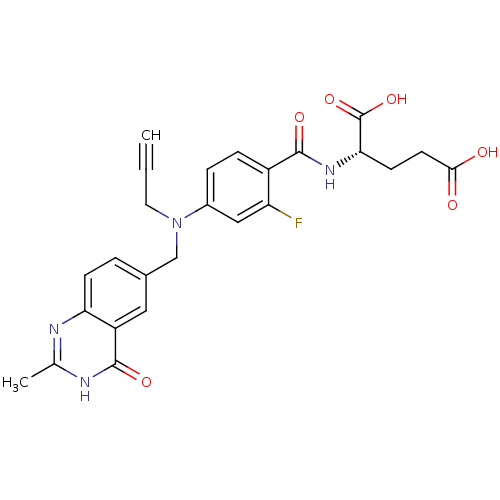
((S)-2-(2-fluoro-4-(((2-methyl-4-oxo-3,4-dihydroqui...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H23FN4O6/c1-3-10-30(13-15-4-7-20-18(11-15)24(34)28-14(2)27-20)16-5-6-17(19(26)12-16)23(33)29-21(25(35)36)8-9-22(31)32/h1,4-7,11-12,21H,8-10,13H2,2H3,(H,29,33)(H,31,32)(H,35,36)(H,27,28,34) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Meerut Institute of Engineering and Technology
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase |
Eur J Med Chem 45: 1560-71 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.065
BindingDB Entry DOI: 10.7270/Q2D50P61 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50078072
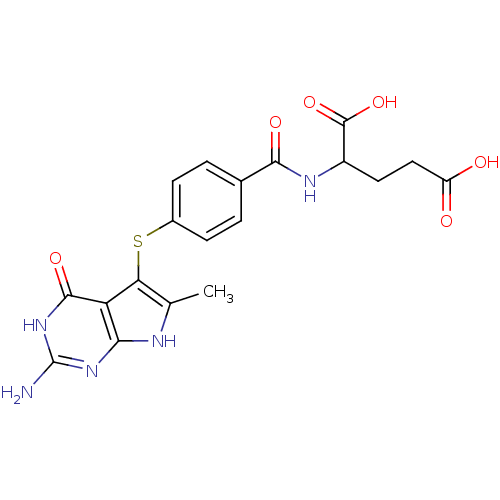
((S)-4-(4-(2-amino-6-methyl-4-oxo-4,7-dihydro-3H-py...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition at a given concentration against Lactobacillus casei Thymidylate synthase |
J Med Chem 42: 2272-9 (1999)
Article DOI: 10.1021/jm980586o
BindingDB Entry DOI: 10.7270/Q29Z9421 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50444517
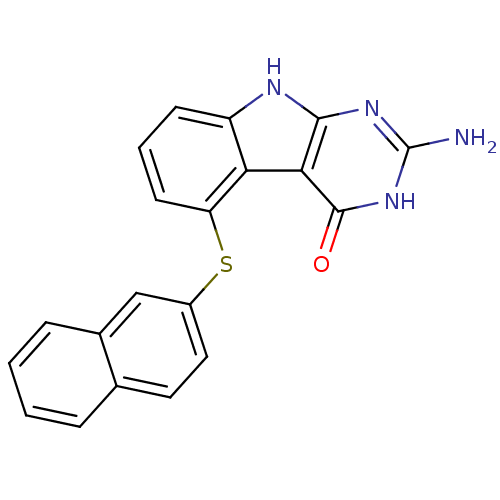
(CHEMBL3099582)Show SMILES Nc1nc2[nH]c3cccc(Sc4ccc5ccccc5c4)c3c2c(=O)[nH]1 Show InChI InChI=1S/C20H14N4OS/c21-20-23-18-17(19(25)24-20)16-14(22-18)6-3-7-15(16)26-13-9-8-11-4-1-2-5-12(11)10-13/h1-10H,(H4,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human TS using dUMP and methylene-THF as substrate by kaleidagraph analysis |
ACS Med Chem Lett 4: 1148-1151 (2013)
Article DOI: 10.1021/ml400208v
BindingDB Entry DOI: 10.7270/Q2FJ2J7P |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50444517

(CHEMBL3099582)Show SMILES Nc1nc2[nH]c3cccc(Sc4ccc5ccccc5c4)c3c2c(=O)[nH]1 Show InChI InChI=1S/C20H14N4OS/c21-20-23-18-17(19(25)24-20)16-14(22-18)6-3-7-15(16)26-13-9-8-11-4-1-2-5-12(11)10-13/h1-10H,(H4,21,22,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 29 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human TS using dUMP and methylene-THF as substrate by kaleidagraph analysis |
ACS Med Chem Lett 4: 1148-1151 (2013)
Article DOI: 10.1021/ml400208v
BindingDB Entry DOI: 10.7270/Q2FJ2J7P |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50040861
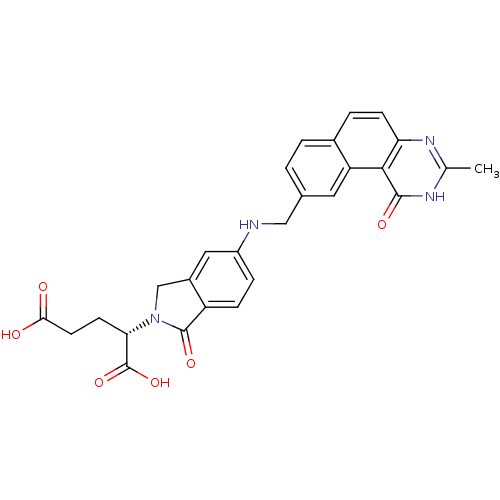
((S)-2-(5-(((1,2-DIHYDRO-3-METHYL-1-OXOBENZO(F)QUIN...)Show SMILES Cc1nc2ccc3ccc(CNc4ccc5C(=O)N(Cc5c4)[C@@H](CCC(O)=O)C(O)=O)cc3c2c(=O)[nH]1 Show InChI InChI=1S/C27H24N4O6/c1-14-29-21-7-4-16-3-2-15(10-20(16)24(21)25(34)30-14)12-28-18-5-6-19-17(11-18)13-31(26(19)35)22(27(36)37)8-9-23(32)33/h2-7,10-11,22,28H,8-9,12-13H2,1H3,(H,32,33)(H,36,37)(H,29,30,34)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human TS assessed as oxidation of tetrahydrofolate to dihydrofolate after 2 to 12 mins by spectrophotometry |
Bioorg Med Chem 19: 3585-94 (2011)
Article DOI: 10.1016/j.bmc.2011.03.067
BindingDB Entry DOI: 10.7270/Q22V2GGP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50350771

(CHEMBL1738740)Show SMILES Nc1nc2sc3cccc(CNc4ccc5C(=O)N(Cc5c4)[C@@H](CCC(O)=O)C(O)=O)c3c2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H21N5O6S/c25-24-27-20(32)19-18-11(2-1-3-16(18)36-21(19)28-24)9-26-13-4-5-14-12(8-13)10-29(22(14)33)15(23(34)35)6-7-17(30)31/h1-5,8,15,26H,6-7,9-10H2,(H,30,31)(H,34,35)(H3,25,27,28,32)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human TS assessed as oxidation of tetrahydrofolate to dihydrofolate after 2 to 12 mins by spectrophotometry |
Bioorg Med Chem 19: 3585-94 (2011)
Article DOI: 10.1016/j.bmc.2011.03.067
BindingDB Entry DOI: 10.7270/Q22V2GGP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition at a given concentration against Lactobacillus casei TS |
J Med Chem 42: 2272-9 (1999)
Article DOI: 10.1021/jm980586o
BindingDB Entry DOI: 10.7270/Q29Z9421 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50008294

(2-(4-(((2-amino-4-oxo-1,4-dihydroquinazolin-6-yl)m...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)NC(CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidylate Synthase from human recombinant sources |
J Med Chem 38: 4495-502 (1995)
BindingDB Entry DOI: 10.7270/Q2GX49MK |
More data for this
Ligand-Target Pair | 
3D Structure (docked) |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Compound was evaluated for the inhibition at a given concentration against recombinant human TS |
J Med Chem 42: 2272-9 (1999)
Article DOI: 10.1021/jm980586o
BindingDB Entry DOI: 10.7270/Q29Z9421 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50014496

((S)-2-(2-fluoro-4-(((2-methoxy-4-oxo-3,4-dihydroqu...)Show SMILES COc1nc2ccc(CN(CC#C)c3ccc(C(=O)N[C@@H](CCC(O)=O)C(O)=O)c(F)c3)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H23FN4O7/c1-3-10-30(13-14-4-7-19-17(11-14)23(34)29-25(28-19)37-2)15-5-6-16(18(26)12-15)22(33)27-20(24(35)36)8-9-21(31)32/h1,4-7,11-12,20H,8-10,13H2,2H3,(H,27,33)(H,31,32)(H,35,36)(H,28,29,34)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Meerut Institute of Engineering and Technology
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase |
Eur J Med Chem 45: 1560-71 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.065
BindingDB Entry DOI: 10.7270/Q2D50P61 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18771

((2S)-2-[(4-{[(2-amino-4-oxo-1,4-dihydroquinazolin-...)Show SMILES Nc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 |r| Show InChI InChI=1S/C24H23N5O6/c1-2-11-29(13-14-3-8-18-17(12-14)22(33)28-24(25)27-18)16-6-4-15(5-7-16)21(32)26-19(23(34)35)9-10-20(30)31/h1,3-8,12,19H,9-11,13H2,(H,26,32)(H,30,31)(H,34,35)(H3,25,27,28,33)/t19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 37 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Ability to inhibit thymidylate synthase derived from human leukemia K562 cells |
J Med Chem 30: 675-8 (1987)
BindingDB Entry DOI: 10.7270/Q2G44QW6 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM31776
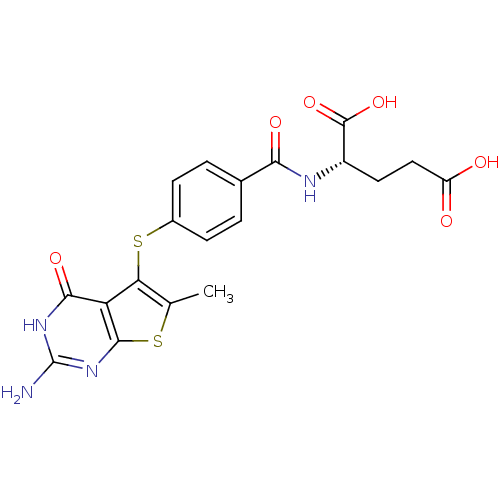
(thieno[2,3-d]pyrimidine deriv., 1)Show SMILES Cc1sc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H18N4O6S2/c1-8-14(13-16(27)22-19(20)23-17(13)30-8)31-10-4-2-9(3-5-10)15(26)21-11(18(28)29)6-7-12(24)25/h2-5,11H,6-7H2,1H3,(H,21,26)(H,24,25)(H,28,29)(H3,20,22,23,27)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | 7.4 | 30 |
Duquesne University
| Assay Description
TS was assayed spectrophotometrically in the reaction buffer solution containing (6R, 6S)-5, 10-CH2H4folate. The reaction was initiated by the additi... |
J Med Chem 52: 4892-902 (2009)
Article DOI: 10.1021/jm900490a
BindingDB Entry DOI: 10.7270/Q2CR5RPK |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50012036
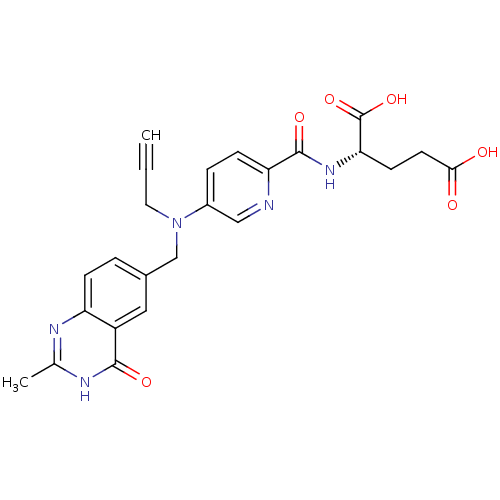
((S)-2-(5-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(nc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C24H23N5O6/c1-3-10-29(13-15-4-6-18-17(11-15)22(32)27-14(2)26-18)16-5-7-19(25-12-16)23(33)28-20(24(34)35)8-9-21(30)31/h1,4-7,11-12,20H,8-10,13H2,2H3,(H,28,33)(H,30,31)(H,34,35)(H,26,27,32)/t20-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Meerut Institute of Engineering and Technology
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase |
Eur J Med Chem 45: 1560-71 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.065
BindingDB Entry DOI: 10.7270/Q2D50P61 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50252968

(CHEMBL522363 | N-{4-[(2-Amino-6-methyl-4-oxo-3,4-d...)Show SMILES Cc1sc2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N(CCC(O)=O)C(O)=O Show InChI InChI=1S/C18H16N4O6S2/c1-8-13(12-14(25)20-17(19)21-15(12)29-8)30-10-4-2-9(3-5-10)16(26)22(18(27)28)7-6-11(23)24/h2-5H,6-7H2,1H3,(H,23,24)(H,27,28)(H3,19,20,21,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
J Med Chem 51: 5789-97 (2008)
Article DOI: 10.1021/jm8006933
BindingDB Entry DOI: 10.7270/Q2FF3T7B |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50006687

((S)-2-(4-(((2-methyl-4-oxo-3,4-dihydroquinazolin-6...)Show SMILES Cc1nc2ccc(CN(CC#C)c3ccc(cc3)C(=O)N[C@@H](CCC(O)=O)C(O)=O)cc2c(=O)[nH]1 Show InChI InChI=1S/C25H24N4O6/c1-3-12-29(14-16-4-9-20-19(13-16)24(33)27-15(2)26-20)18-7-5-17(6-8-18)23(32)28-21(25(34)35)10-11-22(30)31/h1,4-9,13,21H,10-12,14H2,2H3,(H,28,32)(H,30,31)(H,34,35)(H,26,27,33)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 40 | n/a | n/a | n/a | n/a | n/a | n/a |
Meerut Institute of Engineering and Technology
Curated by ChEMBL
| Assay Description
Inhibition of thymidylate synthase |
Eur J Med Chem 45: 1560-71 (2010)
Article DOI: 10.1016/j.ejmech.2009.12.065
BindingDB Entry DOI: 10.7270/Q2D50P61 |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18797
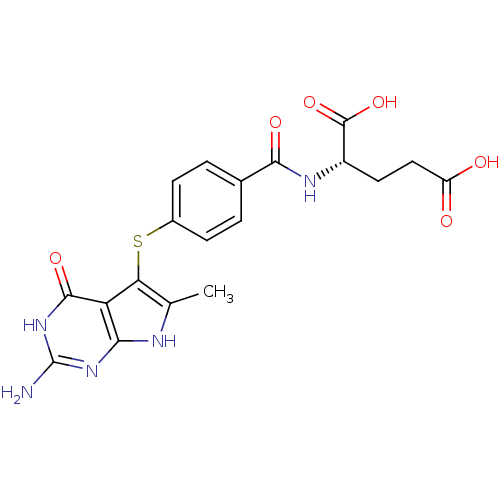
((2S)-2-{[4-({2-amino-6-methyl-4-oxo-3H,4H,7H-pyrro...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human TS assessed as oxidation of tetrahydrofolate to dihydrofolate after 2 to 12 mins by spectrophotometry |
Bioorg Med Chem 19: 3585-94 (2011)
Article DOI: 10.1016/j.bmc.2011.03.067
BindingDB Entry DOI: 10.7270/Q22V2GGP |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM18797

((2S)-2-{[4-({2-amino-6-methyl-4-oxo-3H,4H,7H-pyrro...)Show SMILES Cc1[nH]c2nc(N)[nH]c(=O)c2c1Sc1ccc(cc1)C(=O)N[C@@H](CCC(O)=O)C(O)=O |r| Show InChI InChI=1S/C19H19N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H4,20,21,23,24,28)/t11-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibition of human thymidylate synthase |
J Med Chem 51: 5789-97 (2008)
Article DOI: 10.1021/jm8006933
BindingDB Entry DOI: 10.7270/Q2FF3T7B |
More data for this
Ligand-Target Pair | |
Thymidylate synthase
(Homo sapiens (Human)) | BDBM50029547

(2-[4-(2-Amino-6-methyl-4-oxo-4,4a,7,7a-tetrahydro-...)Show SMILES CC1=NC2N=C(N)NC(=O)C2C1Sc1ccc(cc1)C(=O)NC(CCC(O)=O)C(O)=O |t:1,4| Show InChI InChI=1S/C19H21N5O6S/c1-8-14(13-15(21-8)23-19(20)24-17(13)28)31-10-4-2-9(3-5-10)16(27)22-11(18(29)30)6-7-12(25)26/h2-5,11,13-15H,6-7H2,1H3,(H,22,27)(H,25,26)(H,29,30)(H3,20,23,24,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 42 | n/a | n/a | n/a | n/a | n/a | n/a |
Duquesne University
Curated by ChEMBL
| Assay Description
Inhibitory activity against thymidylate Synthase from human recombinant sources |
J Med Chem 38: 4495-502 (1995)
BindingDB Entry DOI: 10.7270/Q2GX49MK |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data