 Found 2176 hits of ic50 for UniProtKB: P37231
Found 2176 hits of ic50 for UniProtKB: P37231 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM161153

(US9051265, 61)Show SMILES CC(C)c1cccc(c1)[C@H](C)NC(=O)c1ccc2n(Cc3cc(O[C@@H](C)C(O)=O)ccc3Cl)c(C)c(C)c2c1 |r| Show InChI InChI=1S/C32H35ClN2O4/c1-18(2)23-8-7-9-24(14-23)20(4)34-31(36)25-10-13-30-28(16-25)19(3)21(5)35(30)17-26-15-27(11-12-29(26)33)39-22(6)32(37)38/h7-16,18,20,22H,17H2,1-6H3,(H,34,36)(H,37,38)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.0600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of 5 nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan- PPAR Green, an... |
US Patent US9051265 (2015)
BindingDB Entry DOI: 10.7270/Q2FT8JSW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147275

(US8957093, 97)Show SMILES CC[C@@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C34H32N2O3/c1-4-31(26-10-6-5-7-11-26)35-33(37)27-18-19-32-30(20-27)22(2)23(3)36(32)21-24-14-16-25(17-15-24)28-12-8-9-13-29(28)34(38)39/h5-20,31H,4,21H2,1-3H3,(H,35,37)(H,38,39)/t31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147275

(US8957093, 97)Show SMILES CC[C@@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C34H32N2O3/c1-4-31(26-10-6-5-7-11-26)35-33(37)27-18-19-32-30(20-27)22(2)23(3)36(32)21-24-14-16-25(17-15-24)28-12-8-9-13-29(28)34(38)39/h5-20,31H,4,21H2,1-3H3,(H,35,37)(H,38,39)/t31-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591460
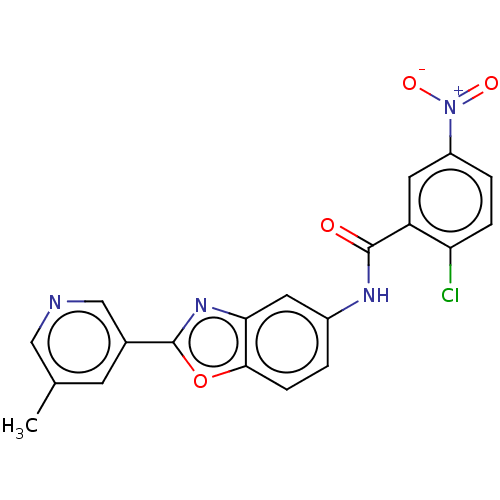
(CHEMBL5191837)Show SMILES Cc1cncc(c1)-c1nc2cc(NC(=O)c3cc(ccc3Cl)[N+]([O-])=O)ccc2o1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591456
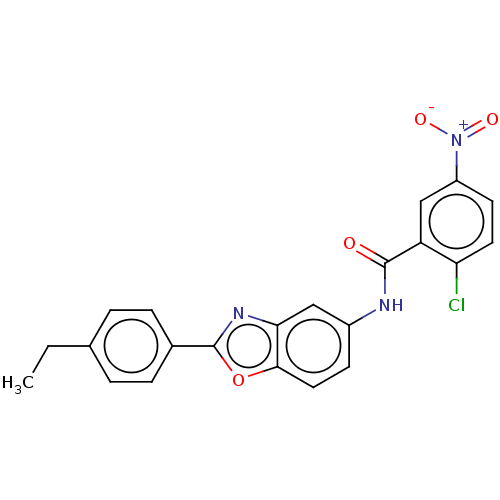
(CHEMBL5207130)Show SMILES CCc1ccc(cc1)-c1nc2cc(NC(=O)c3cc(ccc3Cl)[N+]([O-])=O)ccc2o1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.170 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM161156
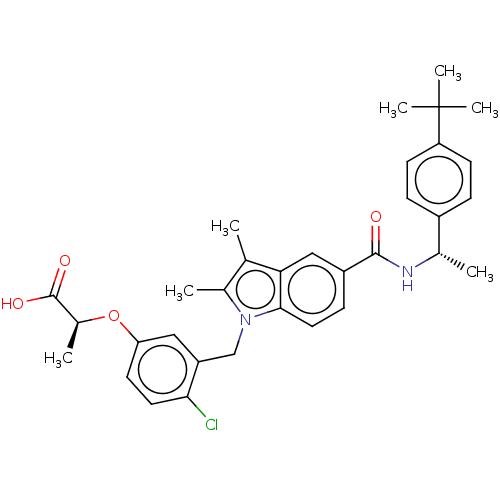
(US9051265, 64 | US9051265, 99)Show SMILES C[C@H](NC(=O)c1ccc2n(Cc3cc(O[C@@H](C)C(O)=O)ccc3Cl)c(C)c(C)c2c1)c1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C33H37ClN2O4/c1-19-21(3)36(18-25-16-27(13-14-29(25)34)40-22(4)32(38)39)30-15-10-24(17-28(19)30)31(37)35-20(2)23-8-11-26(12-9-23)33(5,6)7/h8-17,20,22H,18H2,1-7H3,(H,35,37)(H,38,39)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of 5 nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan- PPAR Green, an... |
US Patent US9051265 (2015)
BindingDB Entry DOI: 10.7270/Q2FT8JSW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM161150
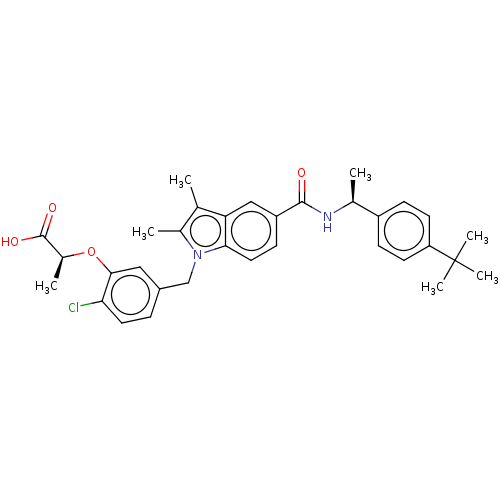
(US9051265, 58 | US9051265, 98)Show SMILES C[C@H](NC(=O)c1ccc2n(Cc3ccc(Cl)c(O[C@@H](C)C(O)=O)c3)c(C)c(C)c2c1)c1ccc(cc1)C(C)(C)C |r| Show InChI InChI=1S/C33H37ClN2O4/c1-19-21(3)36(18-23-8-14-28(34)30(16-23)40-22(4)32(38)39)29-15-11-25(17-27(19)29)31(37)35-20(2)24-9-12-26(13-10-24)33(5,6)7/h8-17,20,22H,18H2,1-7H3,(H,35,37)(H,38,39)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
PDB
UniChem
Similars
| PDB
US Patent
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of 5 nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan- PPAR Green, an... |
US Patent US9051265 (2015)
BindingDB Entry DOI: 10.7270/Q2FT8JSW |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591479
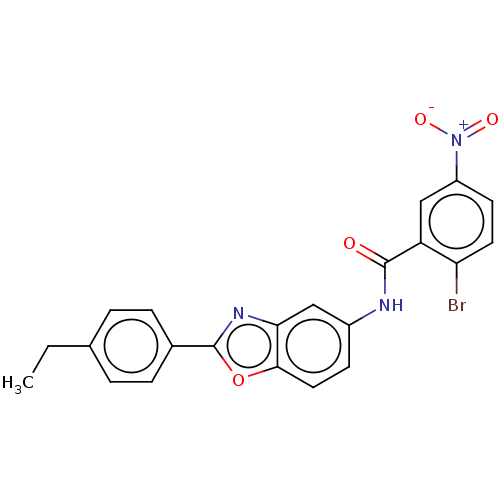
(CHEMBL5187164)Show SMILES CCc1ccc(cc1)-c1nc2cc(NC(=O)c3cc(ccc3Br)[N+]([O-])=O)ccc2o1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591459
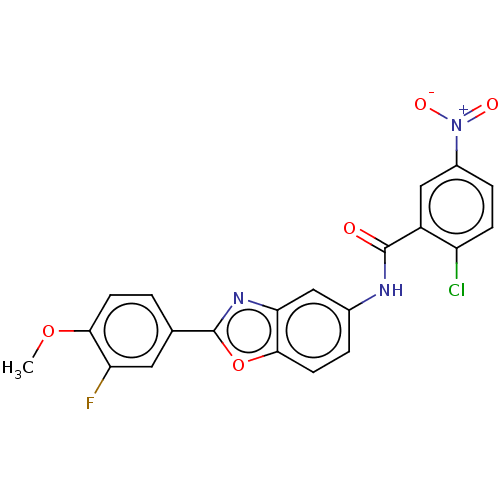
(CHEMBL5179281)Show SMILES COc1ccc(cc1F)-c1nc2cc(NC(=O)c3cc(ccc3Cl)[N+]([O-])=O)ccc2o1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591461
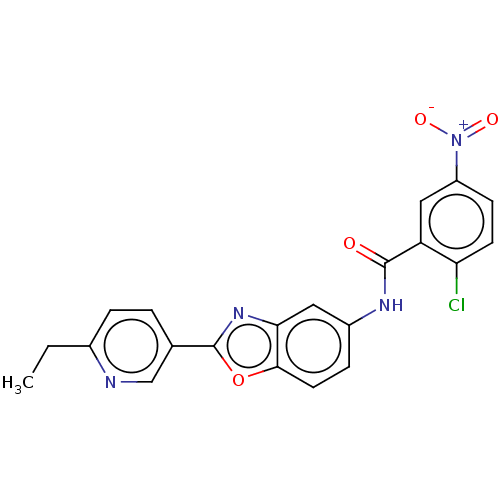
(CHEMBL5206512)Show SMILES CCc1ccc(cn1)-c1nc2cc(NC(=O)c3cc(ccc3Cl)[N+]([O-])=O)ccc2o1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591480
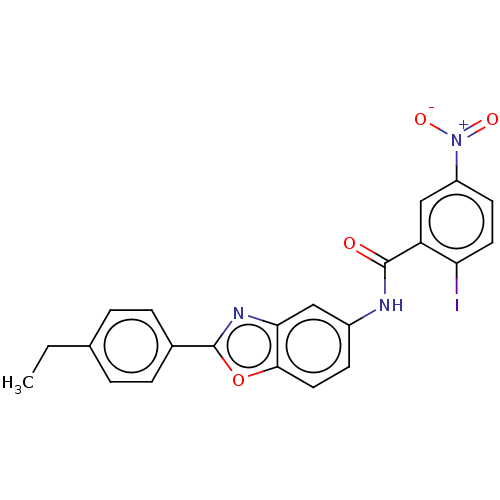
(CHEMBL5207464)Show SMILES CCc1ccc(cc1)-c1nc2cc(NC(=O)c3cc(ccc3I)[N+]([O-])=O)ccc2o1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM161155
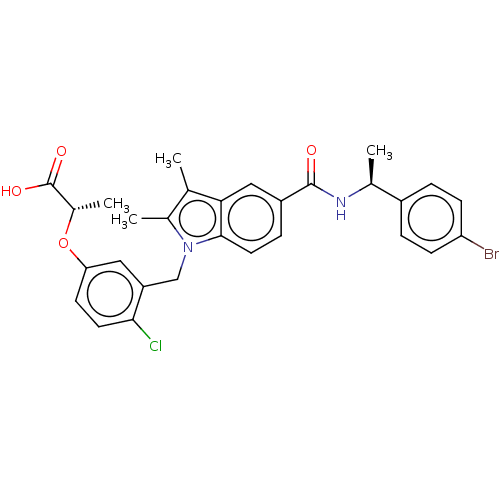
(US9051265, 63)Show SMILES C[C@H](Oc1ccc(Cl)c(Cn2c(C)c(C)c3cc(ccc23)C(=O)N[C@@H](C)c2ccc(Br)cc2)c1)C(O)=O |r| Show InChI InChI=1S/C29H28BrClN2O4/c1-16-18(3)33(15-22-13-24(10-11-26(22)31)37-19(4)29(35)36)27-12-7-21(14-25(16)27)28(34)32-17(2)20-5-8-23(30)9-6-20/h5-14,17,19H,15H2,1-4H3,(H,32,34)(H,35,36)/t17-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of 5 nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan- PPAR Green, an... |
US Patent US9051265 (2015)
BindingDB Entry DOI: 10.7270/Q2FT8JSW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591466
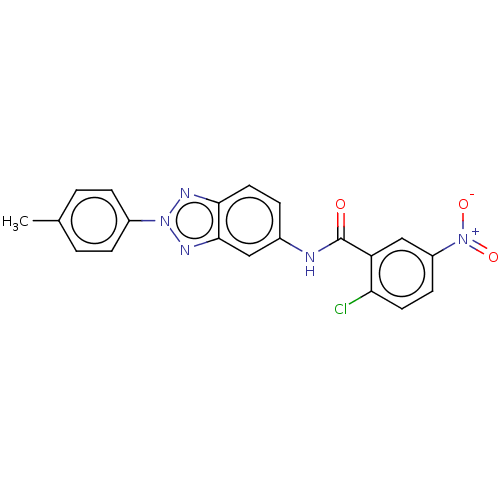
(CHEMBL5204936)Show SMILES Cc1ccc(cc1)-n1nc2ccc(NC(=O)c3cc(ccc3Cl)[N+]([O-])=O)cc2n1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50306508
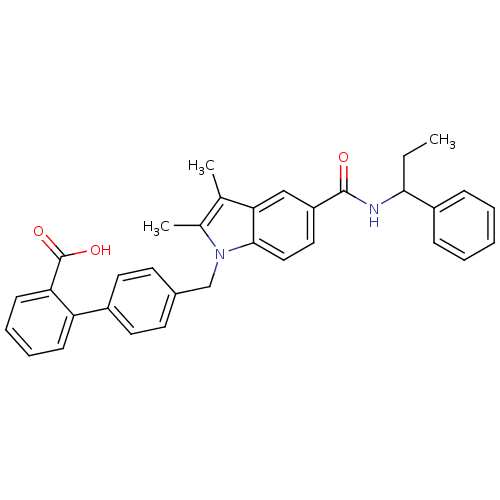
(4'-((2,3-dimethyl-5-(1-phenylpropylcarbamoyl)-1H-i...)Show SMILES CCC(NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccccc1 Show InChI InChI=1S/C34H32N2O3/c1-4-31(26-10-6-5-7-11-26)35-33(37)27-18-19-32-30(20-27)22(2)23(3)36(32)21-24-14-16-25(17-15-24)28-12-8-9-13-29(28)34(38)39/h5-20,31H,4,21H2,1-3H3,(H,35,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.370 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591456

(CHEMBL5207130)Show SMILES CCc1ccc(cc1)-c1nc2cc(NC(=O)c3cc(ccc3Cl)[N+]([O-])=O)ccc2o1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147214
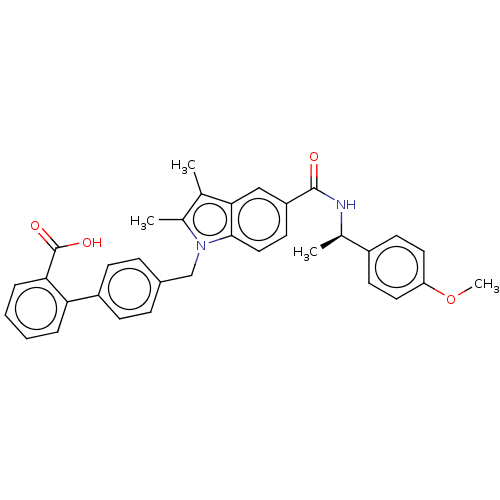
(US8957093, 23)Show SMILES COc1ccc(cc1)[C@@H](C)NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1 |r| Show InChI InChI=1S/C34H32N2O4/c1-21-23(3)36(20-24-9-11-26(12-10-24)29-7-5-6-8-30(29)34(38)39)32-18-15-27(19-31(21)32)33(37)35-22(2)25-13-16-28(40-4)17-14-25/h5-19,22H,20H2,1-4H3,(H,35,37)(H,38,39)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147216

(US8957093, 25)Show SMILES Cc1c(C)c2cc(ccc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NC(C)(C)c1ccccc1 Show InChI InChI=1S/C34H32N2O3/c1-22-23(2)36(21-24-14-16-25(17-15-24)28-12-8-9-13-29(28)33(38)39)31-19-18-26(20-30(22)31)32(37)35-34(3,4)27-10-6-5-7-11-27/h5-20H,21H2,1-4H3,(H,35,37)(H,38,39) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147221
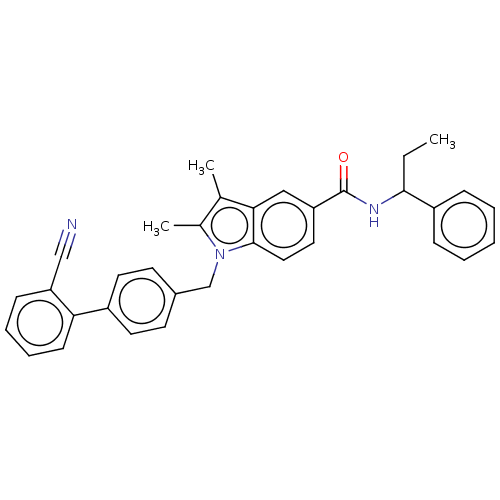
(US8957093, 34)Show SMILES CCC(NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C#N)c(C)c(C)c2c1)c1ccccc1 Show InChI InChI=1S/C34H31N3O/c1-4-32(27-10-6-5-7-11-27)36-34(38)28-18-19-33-31(20-28)23(2)24(3)37(33)22-25-14-16-26(17-15-25)30-13-9-8-12-29(30)21-35/h5-20,32H,4,22H2,1-3H3,(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147214

(US8957093, 23)Show SMILES COc1ccc(cc1)[C@@H](C)NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1 |r| Show InChI InChI=1S/C34H32N2O4/c1-21-23(3)36(20-24-9-11-26(12-10-24)29-7-5-6-8-30(29)34(38)39)32-18-15-27(19-31(21)32)33(37)35-22(2)25-13-16-28(40-4)17-14-25/h5-19,22H,20H2,1-4H3,(H,35,37)(H,38,39)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.540 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50591465
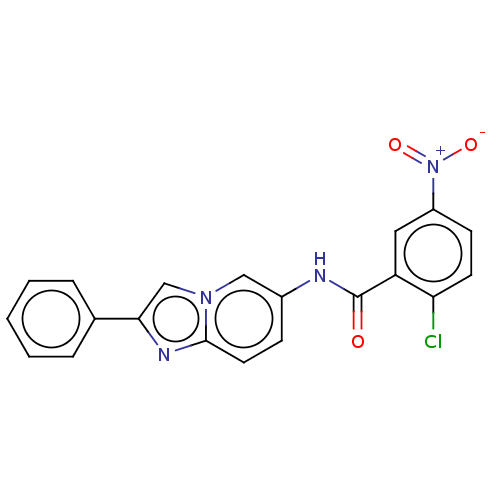
(CHEMBL5201278)Show SMILES [O-][N+](=O)c1ccc(Cl)c(c1)C(=O)Nc1ccc2nc(cn2c1)-c1ccccc1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.2c01379
BindingDB Entry DOI: 10.7270/Q2W099XX |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM161154

(US9051265, 62)Show SMILES C[C@H](Oc1ccc(Cl)c(Cn2c(C)c(C)c3cc(ccc23)C(=O)N[C@@H](C)c2cccc(c2)C2CC2)c1)C(O)=O |r| Show InChI InChI=1S/C32H33ClN2O4/c1-18-20(3)35(17-26-15-27(11-12-29(26)33)39-21(4)32(37)38)30-13-10-25(16-28(18)30)31(36)34-19(2)23-6-5-7-24(14-23)22-8-9-22/h5-7,10-16,19,21-22H,8-9,17H2,1-4H3,(H,34,36)(H,37,38)/t19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of 5 nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan- PPAR Green, an... |
US Patent US9051265 (2015)
BindingDB Entry DOI: 10.7270/Q2FT8JSW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM161148

(US9051265, 56)Show SMILES C[C@H](Oc1cc(Cn2c(C)c(C)c3cc(ccc23)C(=O)N[C@@H](C)c2cccc(c2)C2CC2)ccc1Cl)C(O)=O |r| Show InChI InChI=1S/C32H33ClN2O4/c1-18-20(3)35(17-22-8-12-28(33)30(14-22)39-21(4)32(37)38)29-13-11-26(16-27(18)29)31(36)34-19(2)24-6-5-7-25(15-24)23-9-10-23/h5-8,11-16,19,21,23H,9-10,17H2,1-4H3,(H,34,36)(H,37,38)/t19-,21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of 5 nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan- PPAR Green, an... |
US Patent US9051265 (2015)
BindingDB Entry DOI: 10.7270/Q2FT8JSW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM161147

(US9051265, 55)Show SMILES CC(C)c1cccc(c1)[C@H](C)NC(=O)c1ccc2n(Cc3ccc(Cl)c(O[C@@H](C)C(O)=O)c3)c(C)c(C)c2c1 |r| Show InChI InChI=1S/C32H35ClN2O4/c1-18(2)24-8-7-9-25(15-24)20(4)34-31(36)26-11-13-29-27(16-26)19(3)21(5)35(29)17-23-10-12-28(33)30(14-23)39-22(6)32(37)38/h7-16,18,20,22H,17H2,1-6H3,(H,34,36)(H,37,38)/t20-,22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of 5 nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan- PPAR Green, an... |
US Patent US9051265 (2015)
BindingDB Entry DOI: 10.7270/Q2FT8JSW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147198
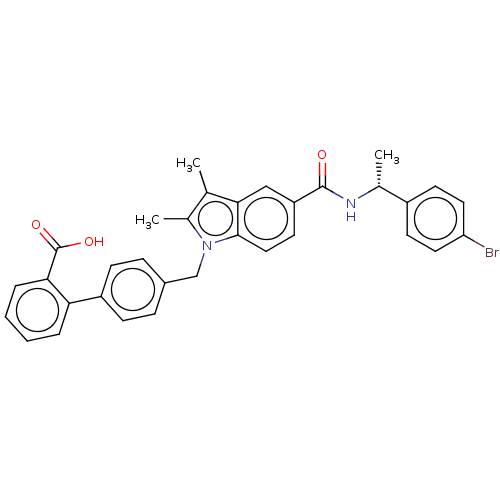
(US8957093, 6)Show SMILES C[C@@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C33H29BrN2O3/c1-20-22(3)36(19-23-8-10-25(11-9-23)28-6-4-5-7-29(28)33(38)39)31-17-14-26(18-30(20)31)32(37)35-21(2)24-12-15-27(34)16-13-24/h4-18,21H,19H2,1-3H3,(H,35,37)(H,38,39)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM161149
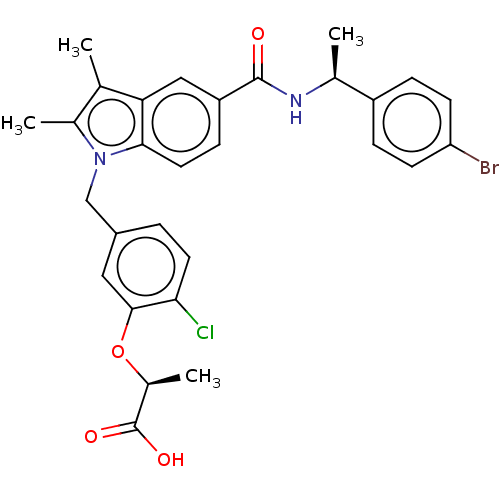
(US9051265, 57)Show SMILES C[C@H](Oc1cc(Cn2c(C)c(C)c3cc(ccc23)C(=O)N[C@@H](C)c2ccc(Br)cc2)ccc1Cl)C(O)=O |r| Show InChI InChI=1S/C29H28BrClN2O4/c1-16-18(3)33(15-20-5-11-25(31)27(13-20)37-19(4)29(35)36)26-12-8-22(14-24(16)26)28(34)32-17(2)21-6-9-23(30)10-7-21/h5-14,17,19H,15H2,1-4H3,(H,32,34)(H,35,36)/t17-,19-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of 5 nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan- PPAR Green, an... |
US Patent US9051265 (2015)
BindingDB Entry DOI: 10.7270/Q2FT8JSW |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147198

(US8957093, 6)Show SMILES C[C@@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccc(Br)cc1 |r| Show InChI InChI=1S/C33H29BrN2O3/c1-20-22(3)36(19-23-8-10-25(11-9-23)28-6-4-5-7-29(28)33(38)39)31-17-14-26(18-30(20)31)32(37)35-21(2)24-12-15-27(34)16-13-24/h4-18,21H,19H2,1-3H3,(H,35,37)(H,38,39)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.730 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147291

(US8957093, 113)Show SMILES C[C@@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C33H29FN2O3/c1-20-22(3)36(19-23-8-10-25(11-9-23)28-6-4-5-7-29(28)33(38)39)31-17-14-26(18-30(20)31)32(37)35-21(2)24-12-15-27(34)16-13-24/h4-18,21H,19H2,1-3H3,(H,35,37)(H,38,39)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.790 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147291

(US8957093, 113)Show SMILES C[C@@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccc(F)cc1 |r| Show InChI InChI=1S/C33H29FN2O3/c1-20-22(3)36(19-23-8-10-25(11-9-23)28-6-4-5-7-29(28)33(38)39)31-17-14-26(18-30(20)31)32(37)35-21(2)24-12-15-27(34)16-13-24/h4-18,21H,19H2,1-3H3,(H,35,37)(H,38,39)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147292

(US8957093, 114)Show SMILES CCC[C@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccccc1 |r| Show InChI InChI=1S/C35H34N2O3/c1-4-10-32(27-11-6-5-7-12-27)36-34(38)28-19-20-33-31(21-28)23(2)24(3)37(33)22-25-15-17-26(18-16-25)29-13-8-9-14-30(29)35(39)40/h5-9,11-21,32H,4,10,22H2,1-3H3,(H,36,38)(H,39,40)/t32-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147210

(US8957093, 19)Show SMILES COc1ccc(cc1)[C@H](C)NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1 |r| Show InChI InChI=1S/C34H32N2O4/c1-21-23(3)36(20-24-9-11-26(12-10-24)29-7-5-6-8-30(29)34(38)39)32-18-15-27(19-31(21)32)33(37)35-22(2)25-13-16-28(40-4)17-14-25/h5-19,22H,20H2,1-4H3,(H,35,37)(H,38,39)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147210

(US8957093, 19)Show SMILES COc1ccc(cc1)[C@H](C)NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1 |r| Show InChI InChI=1S/C34H32N2O4/c1-21-23(3)36(20-24-9-11-26(12-10-24)29-7-5-6-8-30(29)34(38)39)32-18-15-27(19-31(21)32)33(37)35-22(2)25-13-16-28(40-4)17-14-25/h5-19,22H,20H2,1-4H3,(H,35,37)(H,38,39)/t22-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.900 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147263

(US8957093, 83)Show SMILES Cc1c(C)c2cc(ccc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1cccs1 Show InChI InChI=1S/C30H26N2O3S/c1-19-20(2)32(18-21-9-11-22(12-10-21)25-7-3-4-8-26(25)30(34)35)28-14-13-23(16-27(19)28)29(33)31-17-24-6-5-15-36-24/h3-16H,17-18H2,1-2H3,(H,31,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147263

(US8957093, 83)Show SMILES Cc1c(C)c2cc(ccc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1cccs1 Show InChI InChI=1S/C30H26N2O3S/c1-19-20(2)32(18-21-9-11-22(12-10-21)25-7-3-4-8-26(25)30(34)35)28-14-13-23(16-27(19)28)29(33)31-17-24-6-5-15-36-24/h3-16H,17-18H2,1-2H3,(H,31,33)(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147370
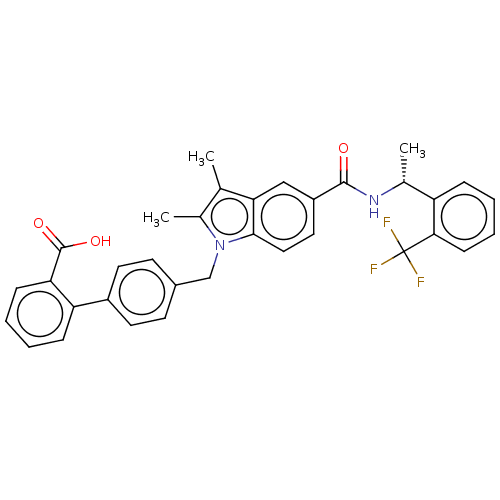
(US8957093, 207)Show SMILES C[C@@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccccc1C(F)(F)F |r| Show InChI InChI=1S/C34H29F3N2O3/c1-20-22(3)39(19-23-12-14-24(15-13-23)27-9-4-5-10-28(27)33(41)42)31-17-16-25(18-29(20)31)32(40)38-21(2)26-8-6-7-11-30(26)34(35,36)37/h4-18,21H,19H2,1-3H3,(H,38,40)(H,41,42)/t21-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50306515

(4'-((5-(benzylcarbamoyl)-2,3-dimethyl-1H-indol-1-y...)Show SMILES Cc1c(C)c2cc(ccc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C32H28N2O3/c1-21-22(2)34(20-24-12-14-25(15-13-24)27-10-6-7-11-28(27)32(36)37)30-17-16-26(18-29(21)30)31(35)33-19-23-8-4-3-5-9-23/h3-18H,19-20H2,1-2H3,(H,33,35)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
US Patent
| Assay Description
The assay was performed according to manufacturer protocol. A mixture of nM GST-PPARG-LBD, 5 nM Tb-GST-antibody, 5 nM Fluormone Pan-PPAR Green, and s... |
US Patent US8957093 (2015)
BindingDB Entry DOI: 10.7270/Q2X63KNR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM147416
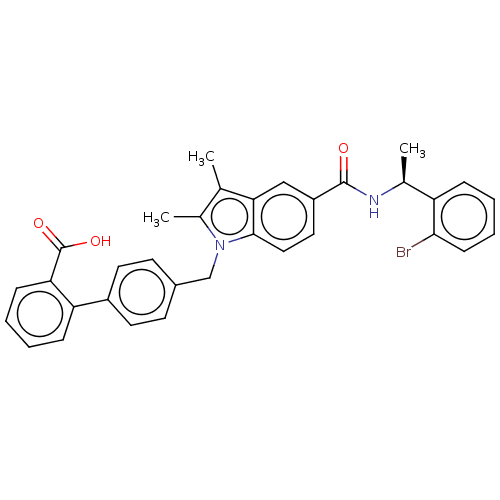
(US8957093, 254)Show SMILES C[C@H](NC(=O)c1ccc2n(Cc3ccc(cc3)-c3ccccc3C(O)=O)c(C)c(C)c2c1)c1ccccc1Br |r| Show InChI InChI=1S/C33H29BrN2O3/c1-20-22(3)36(19-23-12-14-24(15-13-23)27-9-4-5-10-28(27)33(38)39)31-17-16-25(18-29(20)31)32(37)35-21(2)26-8-6-7-11-30(26)34/h4-18,21H,19H2,1-3H3,(H,35,37)(H,38,39)/t21-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50306515

(4'-((5-(benzylcarbamoyl)-2,3-dimethyl-1H-indol-1-y...)Show SMILES Cc1c(C)c2cc(ccc2n1Cc1ccc(cc1)-c1ccccc1C(O)=O)C(=O)NCc1ccccc1 Show InChI InChI=1S/C32H28N2O3/c1-21-22(2)34(20-24-12-14-25(15-13-24)27-10-6-7-11-28(27)32(36)37)30-17-16-26(18-29(21)30)31(35)33-19-23-8-4-3-5-9-23/h3-18H,19-20H2,1-2H3,(H,33,35)(H,36,37) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Binding affinity to GST-tagged PPAR-gamma-LBD (unknown origin) after 2 hrs by Lantha screen assay using fluormone Pan-PPAR green probe |
ACS Med Chem Lett 6: 998-1003 (2015)
Article DOI: 10.1021/acsmedchemlett.5b00218
BindingDB Entry DOI: 10.7270/Q29026SZ |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50268271
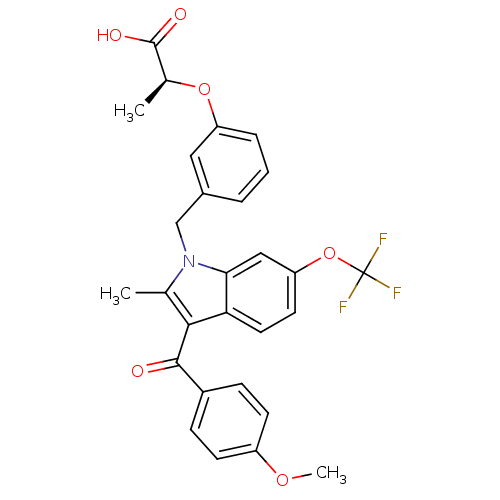
((S)-2-(3-((3-(4-methoxybenzoyl)-2-methyl-6-(triflu...)Show SMILES COc1ccc(cc1)C(=O)c1c(C)n(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 |r| Show InChI InChI=1S/C28H24F3NO6/c1-16-25(26(33)19-7-9-20(36-3)10-8-19)23-12-11-22(38-28(29,30)31)14-24(23)32(16)15-18-5-4-6-21(13-18)37-17(2)27(34)35/h4-14,17H,15H2,1-3H3,(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50267990
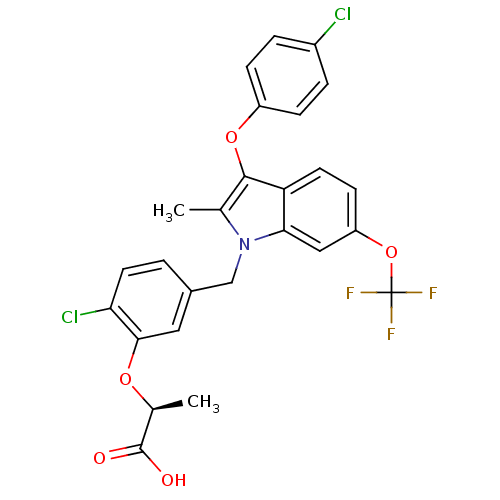
((S)-2-(2-chloro-5-((3-(4-chlorophenoxy)-2-methyl-6...)Show SMILES C[C@H](Oc1cc(Cn2c(C)c(Oc3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)ccc1Cl)C(O)=O |r| Show InChI InChI=1S/C26H20Cl2F3NO5/c1-14-24(36-18-6-4-17(27)5-7-18)20-9-8-19(37-26(29,30)31)12-22(20)32(14)13-16-3-10-21(28)23(11-16)35-15(2)25(33)34/h3-12,15H,13H2,1-2H3,(H,33,34)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50277469
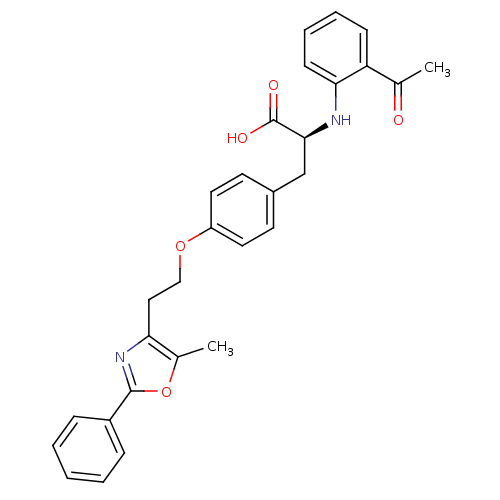
(2-(S)-2-(2-benzoyl-phenylamino)-3-{4-[2-(5-methyl-...)Show SMILES CC(=O)c1ccccc1N[C@@H](Cc1ccc(OCCc2nc(oc2C)-c2ccccc2)cc1)C(O)=O |r| Show InChI InChI=1S/C29H28N2O5/c1-19(32)24-10-6-7-11-26(24)30-27(29(33)34)18-21-12-14-23(15-13-21)35-17-16-25-20(2)36-28(31-25)22-8-4-3-5-9-22/h3-15,27,30H,16-18H2,1-2H3,(H,33,34)/t27-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd
Curated by ChEMBL
| Assay Description
Displacement of radio labeled 2(S)-(2-benzoyl-phenylamino)-3-{4-[1,1-ditritio-2-(5-methyl-2-phenyl-oxazol-4-yl)-ethoxy]-phenyl}-propionic acid from G... |
Bioorg Med Chem Lett 19: 2468-73 (2009)
Article DOI: 10.1016/j.bmcl.2009.03.036
BindingDB Entry DOI: 10.7270/Q2QC03C3 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244556
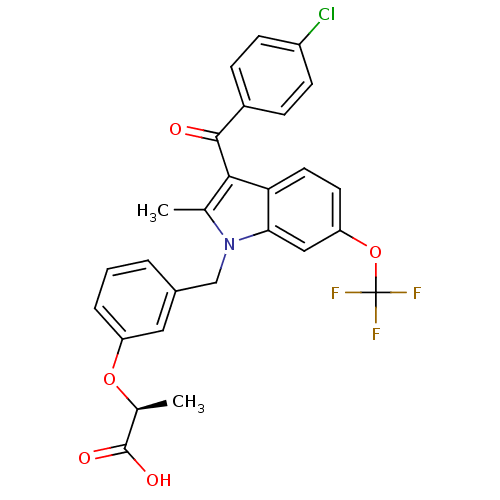
((2S)-2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES C[C@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C27H21ClF3NO5/c1-15-24(25(33)18-6-8-19(28)9-7-18)22-11-10-21(37-27(29,30)31)13-23(22)32(15)14-17-4-3-5-20(12-17)36-16(2)26(34)35/h3-13,16H,14H2,1-2H3,(H,34,35)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50293854
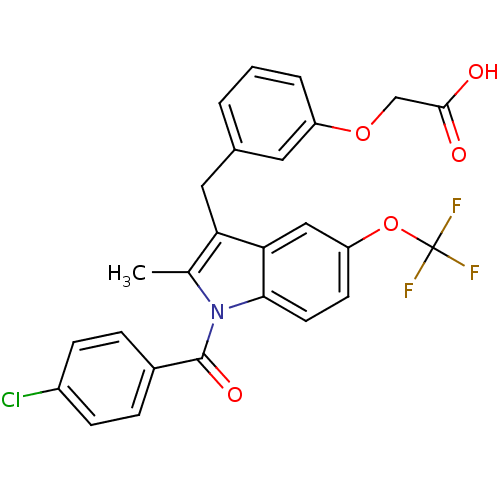
(2-(3-((1-(4-chlorobenzoyl)-2-methyl-5-(trifluorome...)Show SMILES Cc1c(Cc2cccc(OCC(O)=O)c2)c2cc(OC(F)(F)F)ccc2n1C(=O)c1ccc(Cl)cc1 Show InChI InChI=1S/C26H19ClF3NO5/c1-15-21(12-16-3-2-4-19(11-16)35-14-24(32)33)22-13-20(36-26(28,29)30)9-10-23(22)31(15)25(34)17-5-7-18(27)8-6-17/h2-11,13H,12,14H2,1H3,(H,32,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human PPARgamma receptor by scintillation proximity assay |
J Med Chem 52: 4443-53 (2009)
Article DOI: 10.1021/jm900367w
BindingDB Entry DOI: 10.7270/Q2028RK2 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50244556

((2S)-2-[3-[[3-(4-Chlorobenzoyl)-2-methyl-6-(triflu...)Show SMILES C[C@H](Oc1cccc(Cn2c(C)c(C(=O)c3ccc(Cl)cc3)c3ccc(OC(F)(F)F)cc23)c1)C(O)=O |r| Show InChI InChI=1S/C27H21ClF3NO5/c1-15-24(25(33)18-6-8-19(28)9-7-18)22-11-10-21(37-27(29,30)31)13-23(22)32(15)14-17-4-3-5-20(12-17)36-16(2)26(34)35/h3-13,16H,14H2,1-2H3,(H,34,35)/t16-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H]2AD-5075 from GST-tagged human PPARgamma receptor expressed in Escherichia coli BL21 |
Bioorg Med Chem Lett 18: 4798-801 (2008)
Article DOI: 10.1016/j.bmcl.2008.07.103
BindingDB Entry DOI: 10.7270/Q2BC3ZC5 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157917

((2S)-2-(3-{[1-(4-METHOXYBENZOYL)-2-METHYL-5-(TRIFL...)Show SMILES COc1ccc(cc1)C(=O)n1c(C)c(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 |r| Show InChI InChI=1S/C28H24F3NO6/c1-16-23(14-18-5-4-6-21(13-18)37-17(2)27(34)35)24-15-22(38-28(29,30)31)11-12-25(24)32(16)26(33)19-7-9-20(36-3)10-8-19/h4-13,15,17H,14H2,1-3H3,(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Displacement of [3H2]nTZD3 from human recombinant GST-fused PPARgamma expressed in Escherichia coli by scintillation proximity assay |
J Med Chem 52: 3846-54 (2009)
Article DOI: 10.1021/jm900097m
BindingDB Entry DOI: 10.7270/Q2GF0TDR |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166300
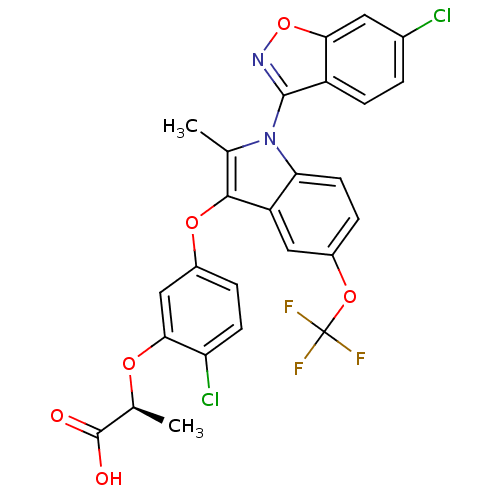
((S)-2-{2-Chloro-5-[1-(6-chloro-benzo[d]isoxazol-3-...)Show SMILES C[C@H](Oc1cc(Oc2c(C)n(-c3noc4cc(Cl)ccc34)c3ccc(OC(F)(F)F)cc23)ccc1Cl)C(O)=O |wU:1.0,(11.52,1.27,;11.04,-.19,;9.55,-.52,;8.51,.64,;7.01,.32,;5.98,1.43,;4.48,1.11,;4,-.36,;4.91,-1.61,;6.44,-1.61,;4,-2.84,;4.41,-4.33,;3.45,-5.51,;4.3,-6.81,;5.77,-6.42,;7.06,-7.25,;8.43,-6.56,;9.72,-7.37,;8.5,-5,;7.22,-4.17,;5.86,-4.89,;2.54,-2.38,;1.22,-3.14,;-.13,-2.38,;-.13,-.84,;-1.48,-.05,;-2.8,-.84,;-4.15,-1.63,;-3.59,.5,;-2.01,-2.17,;1.22,-.05,;2.54,-.82,;6.46,2.92,;7.95,3.24,;9,2.11,;10.5,2.41,;12.09,-1.33,;11.6,-2.79,;13.6,-1.01,)| Show InChI InChI=1S/C26H17Cl2F3N2O6/c1-12-23(37-15-4-7-19(28)22(11-15)36-13(2)25(34)35)18-10-16(38-26(29,30)31)5-8-20(18)33(12)24-17-6-3-14(27)9-21(17)39-32-24/h3-11,13H,1-2H3,(H,34,35)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50166292
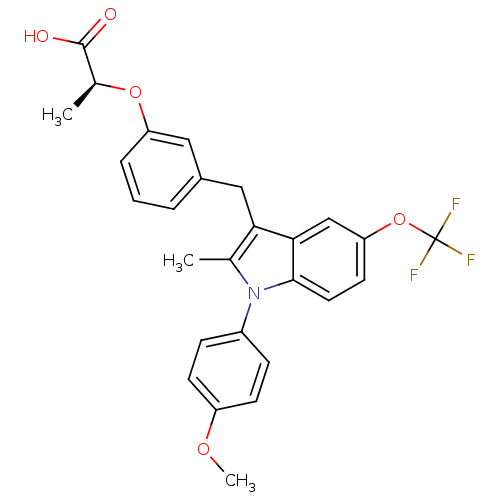
((S)-2-{3-[1-(4-Methoxy-phenyl)-2-methyl-5-trifluor...)Show SMILES COc1ccc(cc1)-n1c(C)c(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 Show InChI InChI=1S/C27H24F3NO5/c1-16-23(14-18-5-4-6-21(13-18)35-17(2)26(32)33)24-15-22(36-27(28,29)30)11-12-25(24)31(16)19-7-9-20(34-3)10-8-19/h4-13,15,17H,14H2,1-3H3,(H,32,33)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human peroxisome proliferator activated receptor gamma binding |
Bioorg Med Chem Lett 15: 2437-40 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.092
BindingDB Entry DOI: 10.7270/Q2CF9PM8 |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50193734
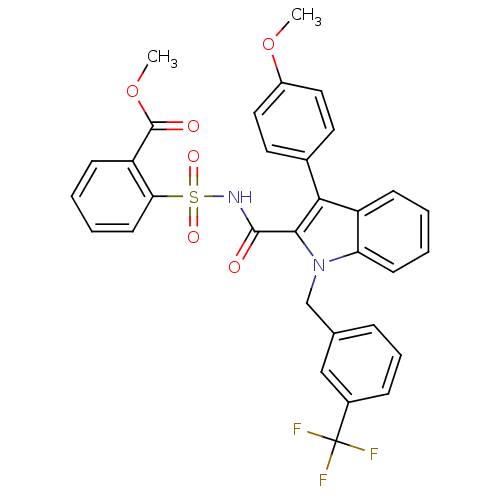
(2-{[3-(4-methoxy-phenyl)-1-(3-trifluoromethyl-benz...)Show SMILES COC(=O)c1ccccc1S(=O)(=O)NC(=O)c1c(-c2ccc(OC)cc2)c2ccccc2n1Cc1cccc(c1)C(F)(F)F Show InChI InChI=1S/C32H25F3N2O6S/c1-42-23-16-14-21(15-17-23)28-24-10-3-5-12-26(24)37(19-20-8-7-9-22(18-20)32(33,34)35)29(28)30(38)36-44(40,41)27-13-6-4-11-25(27)31(39)43-2/h3-18H,19H2,1-2H3,(H,36,38) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Procter and Gamble Pharmaceuticals
Curated by ChEMBL
| Assay Description
Displacement of Fluormone from PPAR gamma |
Bioorg Med Chem Lett 16: 5659-63 (2006)
Article DOI: 10.1016/j.bmcl.2006.08.003
BindingDB Entry DOI: 10.7270/Q2K35T8B |
More data for this
Ligand-Target Pair | |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157926

(2-{3-[1-(4-Methoxy-benzoyl)-2-methyl-5-trifluorome...)Show SMILES COc1ccc(cc1)C(=O)n1c(C)c(Cc2cccc(OC(C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 Show InChI InChI=1S/C28H24F3NO6/c1-16-23(14-18-5-4-6-21(13-18)37-17(2)27(34)35)24-15-22(38-28(29,30)31)11-12-25(24)32(16)26(33)19-7-9-20(36-3)10-8-19/h4-13,15,17H,14H2,1-3H3,(H,34,35) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor gamma |
Bioorg Med Chem Lett 15: 357-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.068
BindingDB Entry DOI: 10.7270/Q27W6BNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157917

((2S)-2-(3-{[1-(4-METHOXYBENZOYL)-2-METHYL-5-(TRIFL...)Show SMILES COc1ccc(cc1)C(=O)n1c(C)c(Cc2cccc(O[C@@H](C)C(O)=O)c2)c2cc(OC(F)(F)F)ccc12 |r| Show InChI InChI=1S/C28H24F3NO6/c1-16-23(14-18-5-4-6-21(13-18)37-17(2)27(34)35)24-15-22(38-28(29,30)31)11-12-25(24)32(16)26(33)19-7-9-20(36-3)10-8-19/h4-13,15,17H,14H2,1-3H3,(H,34,35)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| MMDB
PDB
Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor gamma |
Bioorg Med Chem Lett 15: 357-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.068
BindingDB Entry DOI: 10.7270/Q27W6BNP |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Peroxisome proliferator-activated receptor gamma
(Homo sapiens (Human)) | BDBM50157931
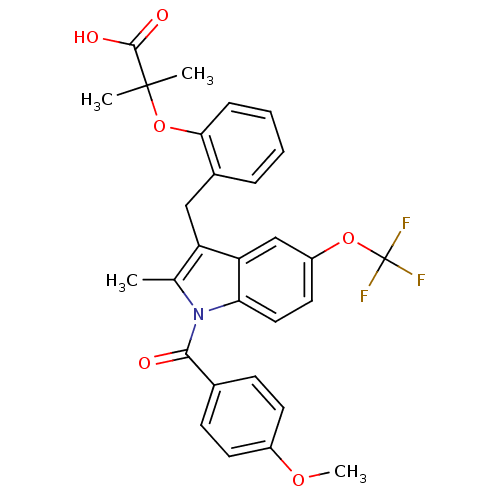
(2-{2-[1-(4-Methoxy-benzoyl)-2-methyl-5-trifluorome...)Show SMILES COc1ccc(cc1)C(=O)n1c(C)c(Cc2ccccc2OC(C)(C)C(O)=O)c2cc(OC(F)(F)F)ccc12 Show InChI InChI=1S/C29H26F3NO6/c1-17-22(15-19-7-5-6-8-25(19)39-28(2,3)27(35)36)23-16-21(38-29(30,31)32)13-14-24(23)33(17)26(34)18-9-11-20(37-4)12-10-18/h5-14,16H,15H2,1-4H3,(H,35,36) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
In vitro inhibition of human Peroxisome proliferator activated receptor gamma |
Bioorg Med Chem Lett 15: 357-62 (2004)
Article DOI: 10.1016/j.bmcl.2004.10.068
BindingDB Entry DOI: 10.7270/Q27W6BNP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data