

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Vasopressin V1b receptor (RAT) | BDBM50205313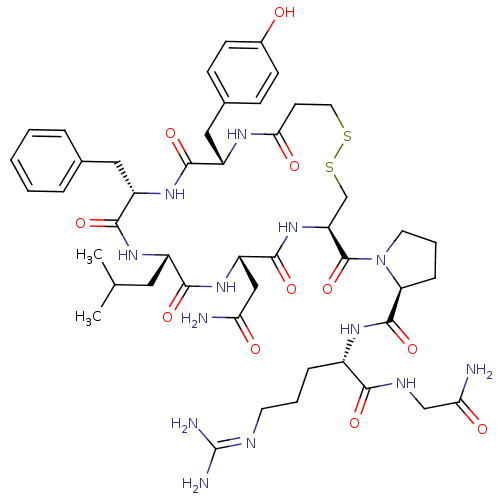 (CHEMBL265859 | d[Leu4]AVP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205305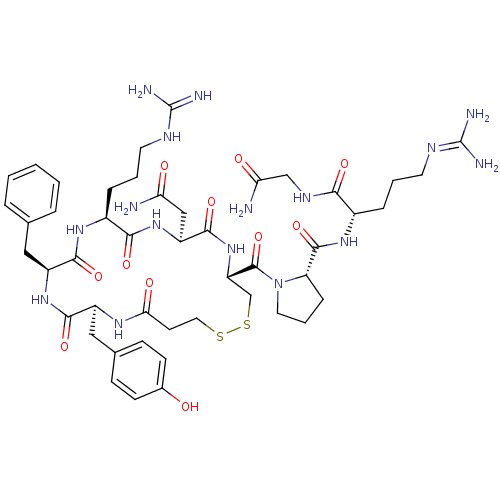 (CHEMBL375324 | d[Arg4]AVP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.130 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205309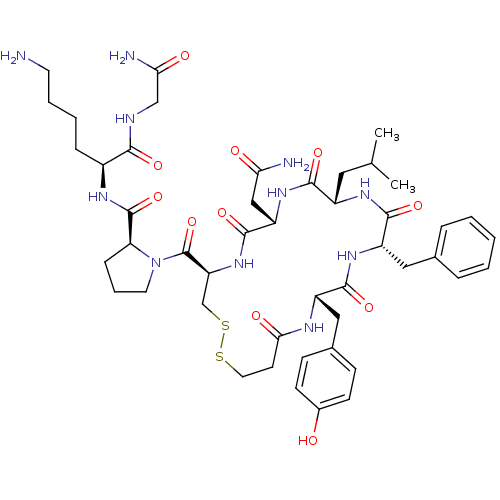 (CHEMBL412972 | d[Leu4,Lys8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205296 (CHEMBL385739 | d[Arg4,Dab8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205291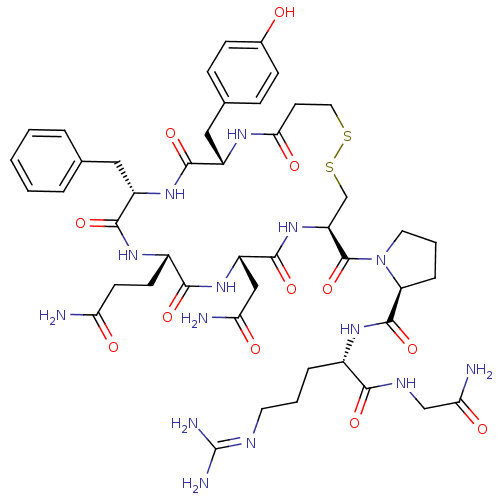 ((S)-1-((4R,7S,10S,13S,16S)-7-(2-amino-2-oxoethyl)-...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM35667 (AVP | CHEMBL373742 | US10131692, 44 (AVP) | [3H]Ar...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid UniChem Patents Similars | PubMed | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 282: 301-8 (1997) BindingDB Entry DOI: 10.7270/Q25T3J1N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205300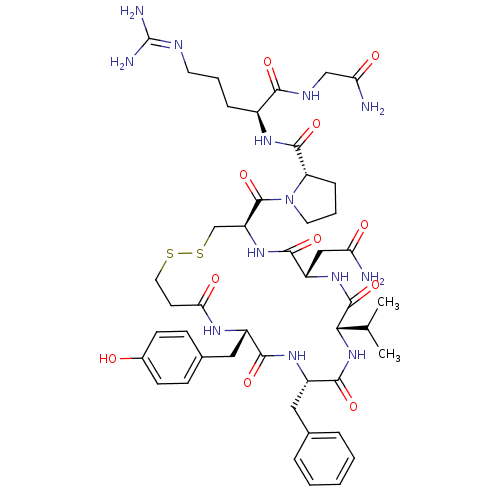 (CHEMBL221436 | d[Val4]AVP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205297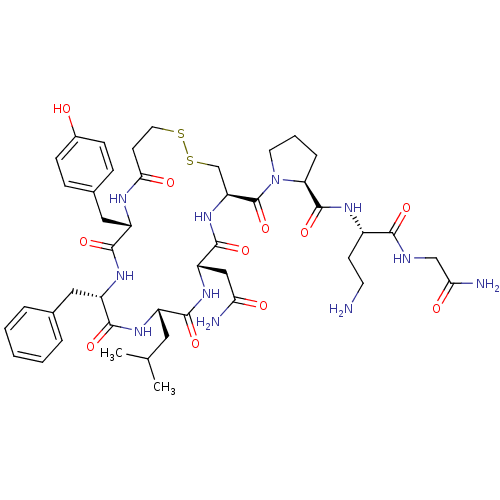 (CHEMBL412973 | d[Leu4,Dab8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205301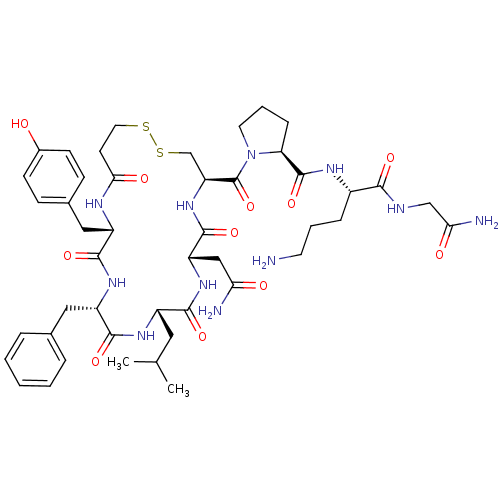 (CHEMBL375188 | d[Leu4,Orn8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205303 (CHEMBL373968 | d[Leu4,Dap8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.380 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205311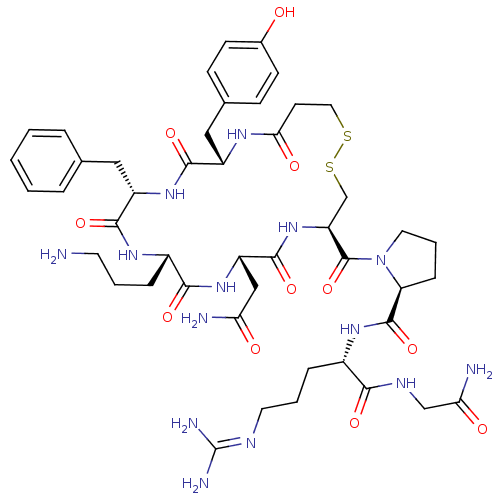 (CHEMBL375096 | d[Orn4]AVP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205306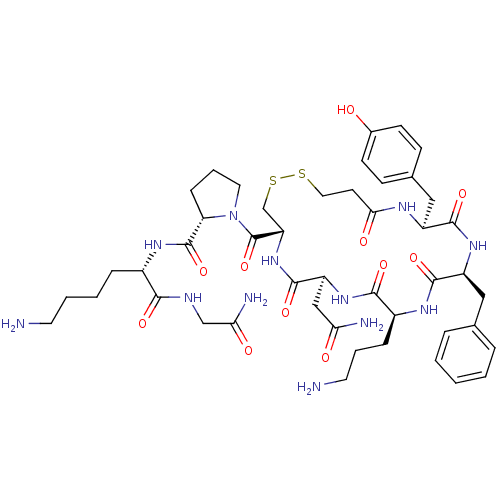 (CHEMBL375323 | d[Orn4,Lys8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.450 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205292 (CHEMBL385068 | d[Arg4,Orn8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205298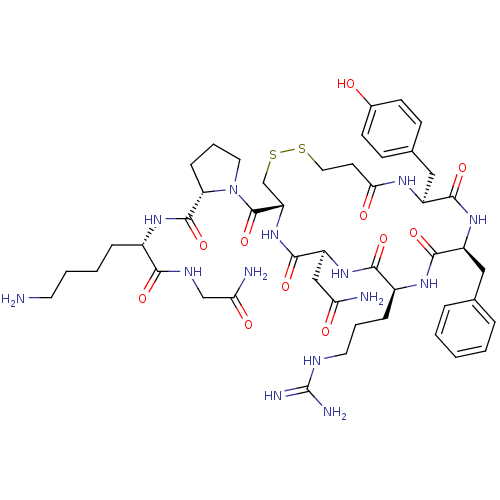 (CHEMBL375325 | d[Arg4,Lys8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205307 (CHEMBL412742 | d[Cha4,Dab8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205310 (CHEMBL219272 | d[Orn4,Orn8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.910 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
MSD Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in CHO cells by whole cell binding assay | Bioorg Med Chem Lett 21: 1871-5 (2011) Article DOI: 10.1016/j.bmcl.2010.12.081 BindingDB Entry DOI: 10.7270/Q2BR8SG4 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 300: 1122-30 (2002) Article DOI: 10.1124/jpet.300.3.1122 BindingDB Entry DOI: 10.7270/Q20V8BBW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205312 (CHEMBL265858 | d[Cha4]AVP) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205293 (CHEMBL221485 | d[Cha4,Lys8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205295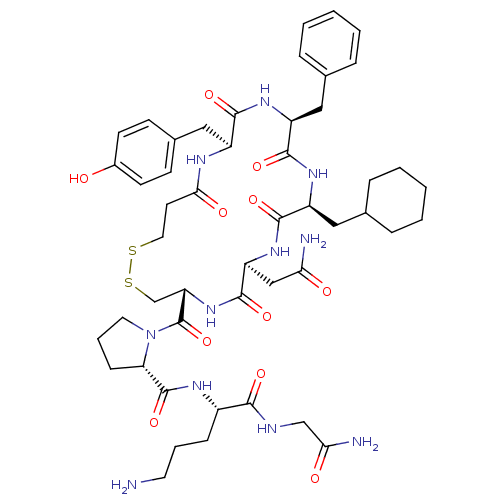 (CHEMBL263090 | d[Cha4,Orn8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299343 ((2S,4R)-1-((R)-5-chloro-1-(2,4-dimethoxyphenylsulf...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Sanofi-Synthelabo Recherche Curated by PDSP Ki Database | J Pharmacol Exp Ther 300: 1122-30 (2002) Article DOI: 10.1124/jpet.300.3.1122 BindingDB Entry DOI: 10.7270/Q20V8BBW | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205299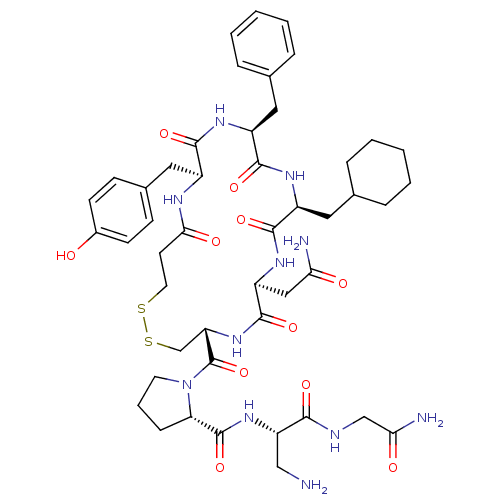 (CHEMBL375187 | d[Cha4,Dap8]VP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299342 (CHEMBL1204126 | CHEMBL1204403 | CHEMBL572709 | N-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299342 (CHEMBL1204126 | CHEMBL1204403 | CHEMBL572709 | N-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 21 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299349 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM85094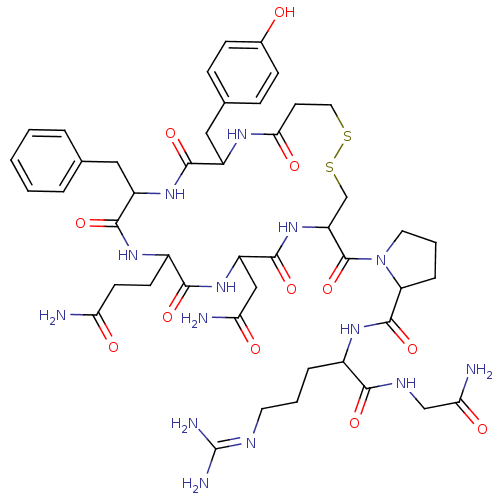 (CAS_62357-86-2 | NSC_64759 | dDAVP) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 25.1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 282: 301-8 (1997) BindingDB Entry DOI: 10.7270/Q25T3J1N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299358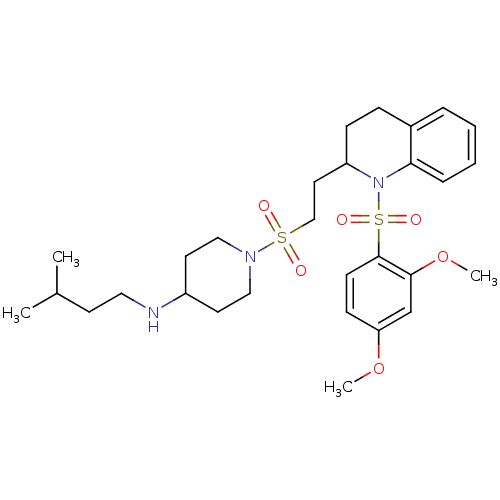 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM85096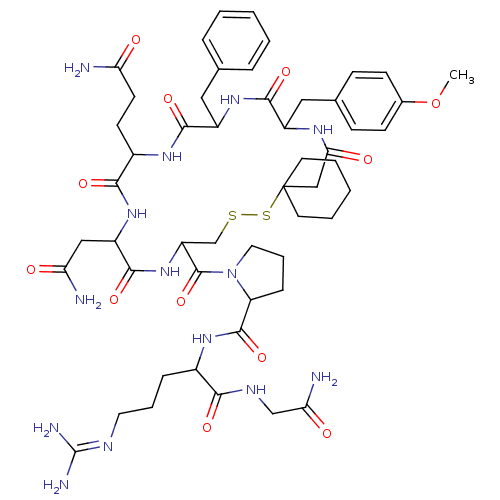 (CAS_105077 | NSC_105077 | d(CH2)5Tyr(Me)AVP) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Similars | PubMed | 37.6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 282: 301-8 (1997) BindingDB Entry DOI: 10.7270/Q25T3J1N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50013775 ((oxytocin-OT) cyclo[Cys-Tyr-Ile-Gln-Asn-Cys]-Pro-L...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | 39.4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Yamanouchi Pharmaceutical Co., Ltd. Curated by PDSP Ki Database | J Pharmacol Exp Ther 282: 301-8 (1997) BindingDB Entry DOI: 10.7270/Q25T3J1N | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50326716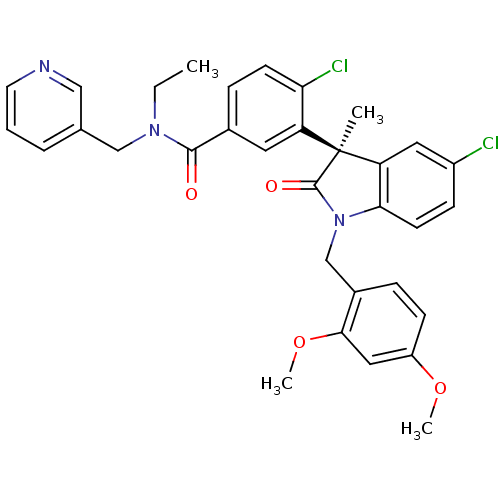 ((R)-4-chloro-3-(5-chloro-1-(2,4-dimethoxybenzyl)-3...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
DrugMolDesign Curated by ChEMBL | Assay Description Displacement of [3H]-vasopressin from rat vasopressin V1b receptor expressed in CHO cells | J Med Chem 53: 6525-38 (2010) Article DOI: 10.1021/jm901812z BindingDB Entry DOI: 10.7270/Q20R9PMZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299341 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 56 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299359 (CHEMBL575797 | N-(cyclopropylmethyl)-1-(2-(1-(2,4-...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 62 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299351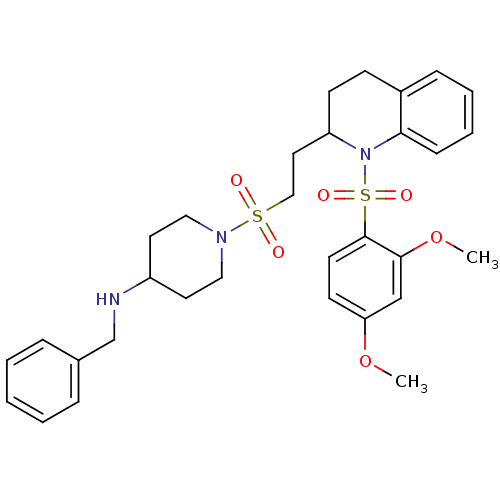 (CHEMBL583083 | N-benzyl-1-(2-(1-(2,4-dimethoxyphen...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 83 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299356 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 91 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50205294 (CHEMBL414074 | d[D-3-Pal2]AVP) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 112 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
INSERM Curated by ChEMBL | Assay Description Displacement of [3H]AVP from rat vasopressin V1b receptor expressed in At-T20 cells | J Med Chem 50: 835-47 (2007) Article DOI: 10.1021/jm060928n BindingDB Entry DOI: 10.7270/Q2G161NH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299350 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 145 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299352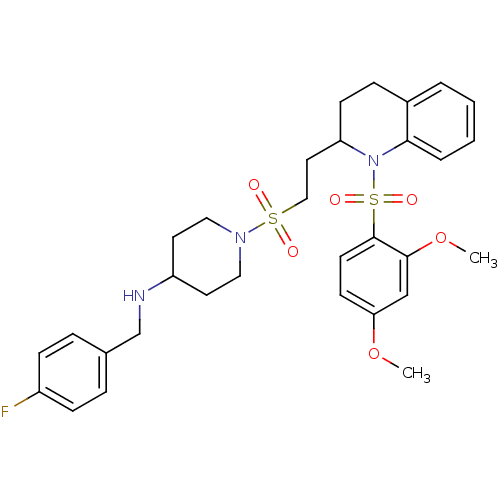 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 158 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299357 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 227 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299354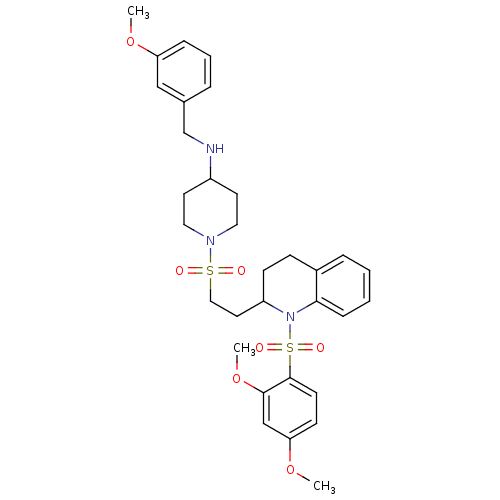 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 270 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299340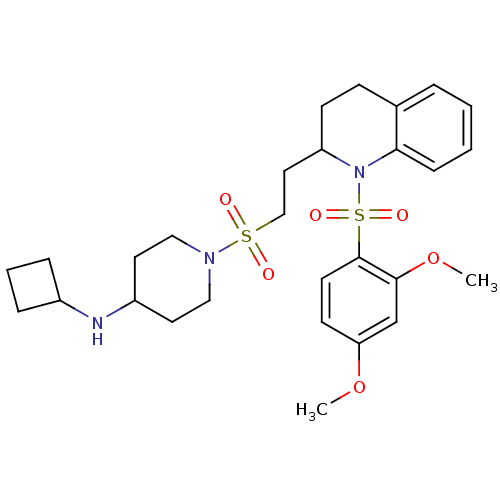 (CHEMBL576341 | N-cyclobutyl-1-(2-(1-(2,4-dimethoxy...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 557 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299353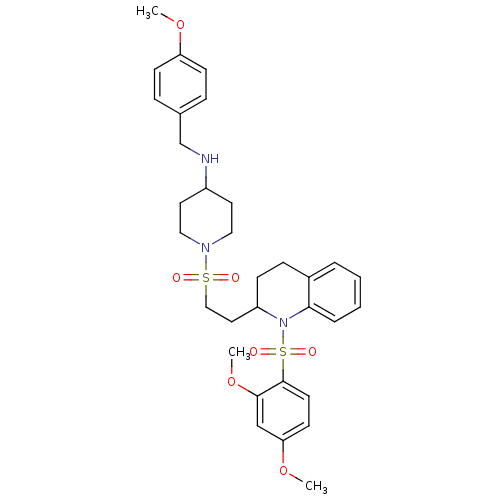 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 637 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299345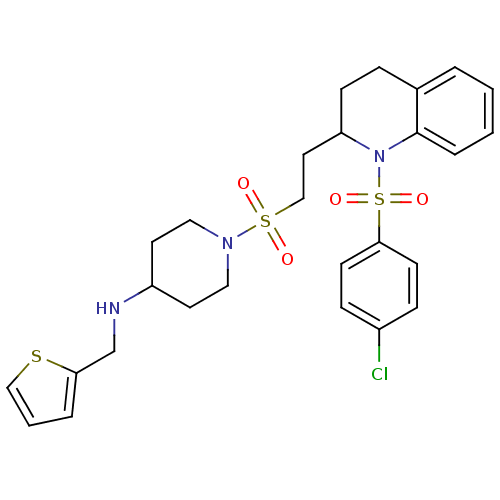 (1-(2-(1-(4-chlorophenylsulfonyl)-1,2,3,4-tetrahydr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 813 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299342 (CHEMBL1204126 | CHEMBL1204403 | CHEMBL572709 | N-(...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 874 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299355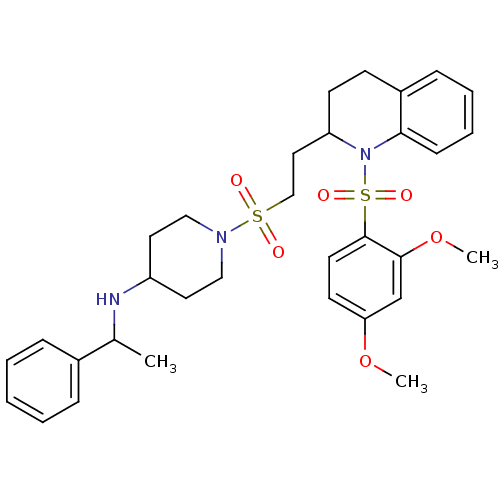 (1-(2-(1-(2,4-dimethoxyphenylsulfonyl)-1,2,3,4-tetr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 999 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299344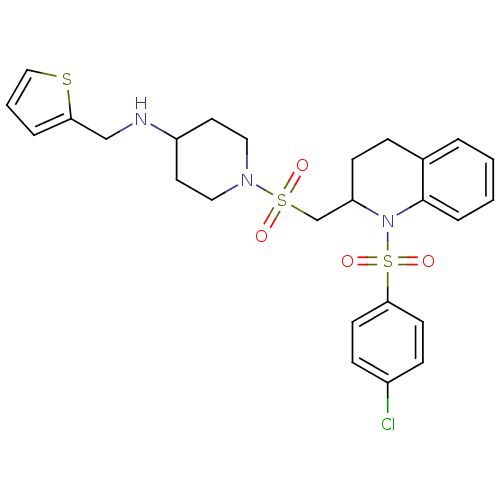 (1-((1-(4-chlorophenylsulfonyl)-1,2,3,4-tetrahydroq...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.69E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299339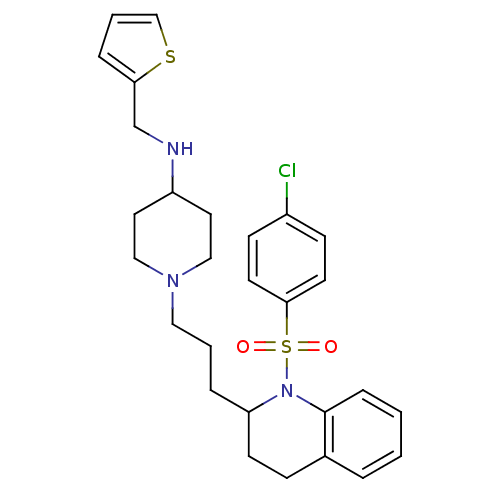 (1-(3-(1-(4-chlorophenylsulfonyl)-1,2,3,4-tetrahydr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.78E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299337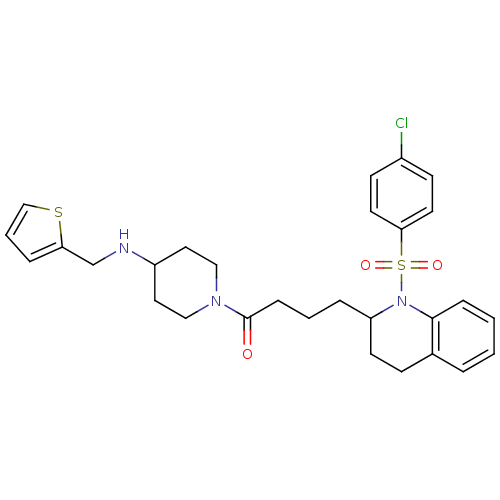 (4-(1-(4-chlorophenylsulfonyl)-1,2,3,4-tetrahydroqu...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.24E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vasopressin V1b receptor (RAT) | BDBM50299346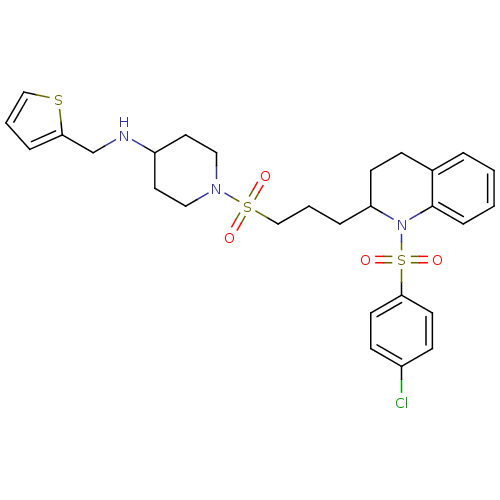 (1-(3-(1-(4-chlorophenylsulfonyl)-1,2,3,4-tetrahydr...) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Schering-Plough Research Institute Curated by ChEMBL | Assay Description Displacement of [3H]Arg8-vasopressin from rat vasopressin V1b receptor expressed in CHO-K1 cells by Packard Topcount scintillation counter | Bioorg Med Chem Lett 19: 6018-22 (2009) Article DOI: 10.1016/j.bmcl.2009.09.050 BindingDB Entry DOI: 10.7270/Q2J9679W | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |