 Found 75 hits of ic50 for UniProtKB: P23921
Found 75 hits of ic50 for UniProtKB: P23921 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000150
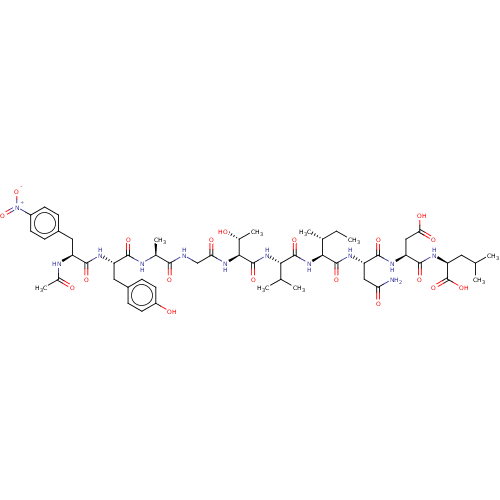
(CHEMBL440253 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1ccc(cc1)[N+]([O-])=O)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C54H78N12O19/c1-10-27(6)44(52(80)61-37(22-40(55)70)49(77)60-38(23-42(72)73)50(78)62-39(54(82)83)19-25(2)3)65-51(79)43(26(4)5)64-53(81)45(29(8)67)63-41(71)24-56-46(74)28(7)57-47(75)36(21-32-13-17-34(69)18-14-32)59-48(76)35(58-30(9)68)20-31-11-15-33(16-12-31)66(84)85/h11-18,25-29,35-39,43-45,67,69H,10,19-24H2,1-9H3,(H2,55,70)(H,56,74)(H,57,75)(H,58,68)(H,59,76)(H,60,77)(H,61,80)(H,62,78)(H,63,71)(H,64,81)(H,65,79)(H,72,73)(H,82,83)/t27-,28+,29-,35+,36+,37+,38+,39+,43+,44+,45+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000141
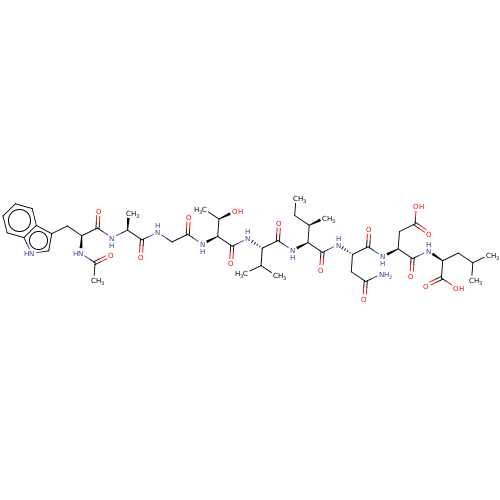
(2-{2-[2-(2-{2-[2-(2-{2-[2-Acetylamino-3-(1H-indol-...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1c[nH]c2ccccc12)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C47H71N11O15/c1-10-23(6)38(45(70)54-31(17-34(48)61)42(67)53-32(18-36(63)64)43(68)55-33(47(72)73)15-21(2)3)58-44(69)37(22(4)5)57-46(71)39(25(8)59)56-35(62)20-50-40(65)24(7)51-41(66)30(52-26(9)60)16-27-19-49-29-14-12-11-13-28(27)29/h11-14,19,21-25,30-33,37-39,49,59H,10,15-18,20H2,1-9H3,(H2,48,61)(H,50,65)(H,51,66)(H,52,60)(H,53,67)(H,54,70)(H,55,68)(H,56,62)(H,57,71)(H,58,69)(H,63,64)(H,72,73)/t23-,24+,25-,30+,31+,32+,33+,37+,38+,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000153

(CHEMBL386066 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H70N10O17/c1-9-22(6)36(43(69)50-28(16-32(46)60)40(66)49-29(17-34(62)63)41(67)51-30(45(71)72)14-20(2)3)55-42(68)35(21(4)5)54-44(70)37(23(7)57)53-33(61)18-47-38(64)31(19-56)52-39(65)27(48-24(8)58)15-25-10-12-26(59)13-11-25/h10-13,20-23,27-31,35-37,56-57,59H,9,14-19H2,1-8H3,(H2,46,60)(H,47,64)(H,48,58)(H,49,66)(H,50,69)(H,51,67)(H,52,65)(H,53,61)(H,54,70)(H,55,68)(H,62,63)(H,71,72)/t22-,23-,27+,28+,29+,30+,31+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000152

(CHEMBL277280 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)[C@@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C46H72N10O16/c1-10-22(5)36(43(68)52-30(18-33(47)60)41(66)51-31(19-35(62)63)42(67)53-32(46(71)72)16-21(3)4)55-44(69)37(23(6)11-2)56-45(70)38(25(8)57)54-34(61)20-48-39(64)24(7)49-40(65)29(50-26(9)58)17-27-12-14-28(59)15-13-27/h12-15,21-25,29-32,36-38,57,59H,10-11,16-20H2,1-9H3,(H2,47,60)(H,48,64)(H,49,65)(H,50,58)(H,51,66)(H,52,68)(H,53,67)(H,54,61)(H,55,69)(H,56,70)(H,62,63)(H,71,72)/t22-,23+,24+,25-,29+,30+,31+,32+,36+,37+,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000146

(CHEMBL414299 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)c(I)c1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H69IN10O16/c1-10-21(6)36(43(69)52-28(16-32(47)60)40(66)51-29(17-34(62)63)41(67)53-30(45(71)72)13-19(2)3)56-42(68)35(20(4)5)55-44(70)37(23(8)57)54-33(61)18-48-38(64)22(7)49-39(65)27(50-24(9)58)15-25-11-12-31(59)26(46)14-25/h11-12,14,19-23,27-30,35-37,57,59H,10,13,15-18H2,1-9H3,(H2,47,60)(H,48,64)(H,49,65)(H,50,58)(H,51,66)(H,52,69)(H,53,67)(H,54,61)(H,55,70)(H,56,68)(H,62,63)(H,71,72)/t21-,22+,23-,27+,28+,29+,30+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000165
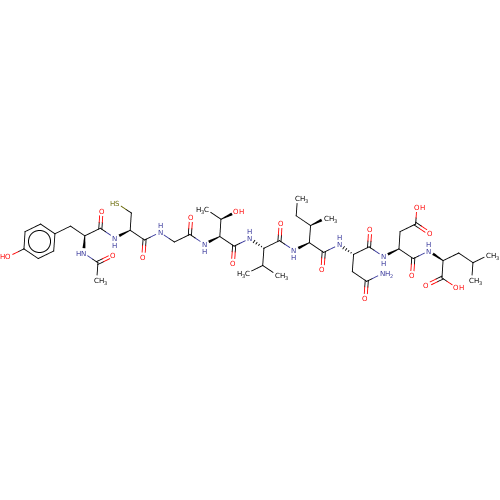
(CHEMBL437817 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CS)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H70N10O16S/c1-9-22(6)36(43(68)50-28(16-32(46)59)40(65)49-29(17-34(61)62)41(66)51-30(45(70)71)14-20(2)3)55-42(67)35(21(4)5)54-44(69)37(23(7)56)53-33(60)18-47-38(63)31(19-72)52-39(64)27(48-24(8)57)15-25-10-12-26(58)13-11-25/h10-13,20-23,27-31,35-37,56,58,72H,9,14-19H2,1-8H3,(H2,46,59)(H,47,63)(H,48,57)(H,49,65)(H,50,68)(H,51,66)(H,52,64)(H,53,60)(H,54,69)(H,55,67)(H,61,62)(H,70,71)/t22-,23-,27+,28+,29+,30+,31+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000154

(2-{2-[2-(2-{2-[2-(2-{2-[2-Acetylamino-3-(4-hydroxy...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H70N10O16/c1-10-22(6)36(43(68)51-29(17-32(46)59)40(65)50-30(18-34(61)62)41(66)52-31(45(70)71)15-20(2)3)55-42(67)35(21(4)5)54-44(69)37(24(8)56)53-33(60)19-47-38(63)23(7)48-39(64)28(49-25(9)57)16-26-11-13-27(58)14-12-26/h11-14,20-24,28-31,35-37,56,58H,10,15-19H2,1-9H3,(H2,46,59)(H,47,63)(H,48,64)(H,49,57)(H,50,65)(H,51,68)(H,52,66)(H,53,60)(H,54,69)(H,55,67)(H,61,62)(H,70,71)/t22-,23+,24-,28+,29+,30+,31+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000149
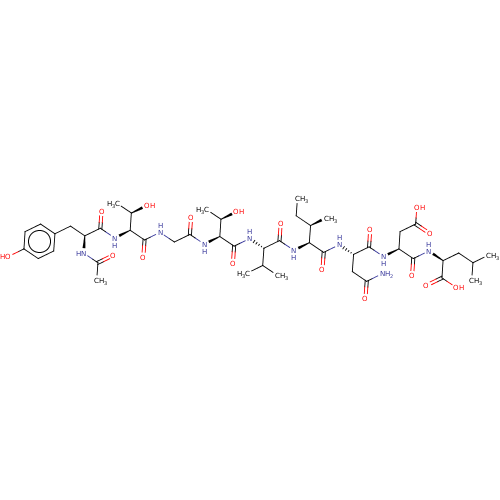
(CHEMBL261954 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C46H72N10O17/c1-10-22(6)36(44(70)51-29(17-32(47)61)39(65)50-30(18-34(63)64)40(66)52-31(46(72)73)15-20(2)3)55-43(69)35(21(4)5)54-45(71)38(24(8)58)53-33(62)19-48-42(68)37(23(7)57)56-41(67)28(49-25(9)59)16-26-11-13-27(60)14-12-26/h11-14,20-24,28-31,35-38,57-58,60H,10,15-19H2,1-9H3,(H2,47,61)(H,48,68)(H,49,59)(H,50,65)(H,51,70)(H,52,66)(H,53,62)(H,54,71)(H,55,69)(H,56,67)(H,63,64)(H,72,73)/t22-,23-,24-,28+,29+,30+,31+,35+,36+,37+,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000166

(2-{2-[2-(2-{2-[2-(2-{2-[2-Acetylamino-3-(4-hydroxy...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C46H72N10O15/c1-11-24(8)38(45(69)52-30(18-33(47)59)41(65)51-31(19-35(61)62)42(66)53-32(46(70)71)16-21(2)3)56-44(68)37(23(6)7)55-43(67)36(22(4)5)54-34(60)20-48-39(63)25(9)49-40(64)29(50-26(10)57)17-27-12-14-28(58)15-13-27/h12-15,21-25,29-32,36-38,58H,11,16-20H2,1-10H3,(H2,47,59)(H,48,63)(H,49,64)(H,50,57)(H,51,65)(H,52,69)(H,53,66)(H,54,60)(H,55,67)(H,56,68)(H,61,62)(H,70,71)/t24-,25+,29+,30+,31+,32+,36+,37+,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000163

(CHEMBL386716 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CS)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C44H68N10O15S/c1-9-22(6)36(43(67)51-28(16-32(45)57)39(63)50-29(17-34(59)60)40(64)52-30(44(68)69)14-20(2)3)54-42(66)35(21(4)5)53-41(65)31(19-70)49-33(58)18-46-37(61)23(7)47-38(62)27(48-24(8)55)15-25-10-12-26(56)13-11-25/h10-13,20-23,27-31,35-36,56,70H,9,14-19H2,1-8H3,(H2,45,57)(H,46,61)(H,47,62)(H,48,55)(H,49,58)(H,50,63)(H,51,67)(H,52,64)(H,53,65)(H,54,66)(H,59,60)(H,68,69)/t22-,23+,27+,28+,29+,30+,31+,35+,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000164
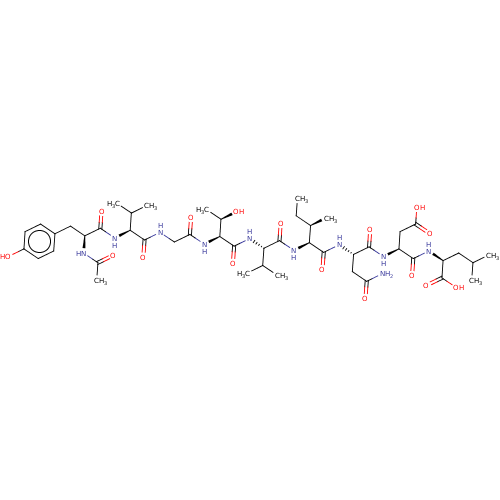
(CHEMBL405583 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C47H74N10O16/c1-11-24(8)38(45(70)52-30(18-33(48)61)40(65)51-31(19-35(63)64)41(66)53-32(47(72)73)16-21(2)3)57-44(69)37(23(6)7)56-46(71)39(25(9)58)54-34(62)20-49-43(68)36(22(4)5)55-42(67)29(50-26(10)59)17-27-12-14-28(60)15-13-27/h12-15,21-25,29-32,36-39,58,60H,11,16-20H2,1-10H3,(H2,48,61)(H,49,68)(H,50,59)(H,51,65)(H,52,70)(H,53,66)(H,54,62)(H,55,67)(H,56,71)(H,57,69)(H,63,64)(H,72,73)/t24-,25-,29+,30+,31+,32+,36+,37+,38+,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000157
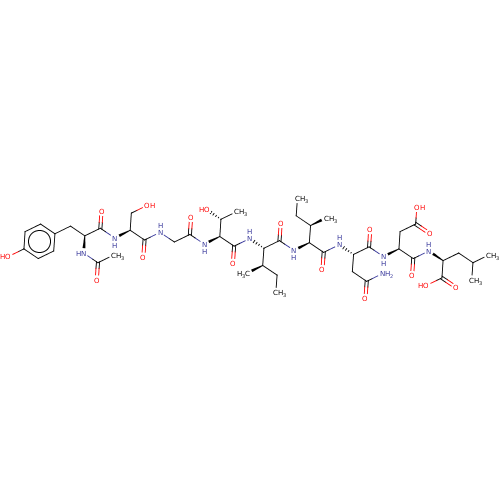
(CHEMBL261962 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](CO)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)[C@H](C)CC)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C46H72N10O17/c1-9-22(5)36(43(69)51-29(17-33(47)61)41(67)50-30(18-35(63)64)42(68)52-31(46(72)73)15-21(3)4)55-44(70)37(23(6)10-2)56-45(71)38(24(7)58)54-34(62)19-48-39(65)32(20-57)53-40(66)28(49-25(8)59)16-26-11-13-27(60)14-12-26/h11-14,21-24,28-32,36-38,57-58,60H,9-10,15-20H2,1-8H3,(H2,47,61)(H,48,65)(H,49,59)(H,50,67)(H,51,69)(H,52,68)(H,53,66)(H,54,62)(H,55,70)(H,56,71)(H,63,64)(H,72,73)/t22-,23-,24-,28+,29+,30+,31+,32+,36+,37+,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000137
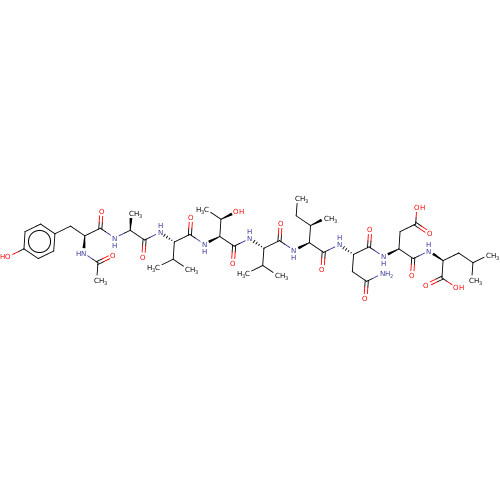
(CHEMBL263845 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C48H76N10O16/c1-12-24(8)38(46(71)53-31(19-34(49)62)42(67)52-32(20-35(63)64)43(68)54-33(48(73)74)17-21(2)3)57-44(69)37(23(6)7)56-47(72)39(26(10)59)58-45(70)36(22(4)5)55-40(65)25(9)50-41(66)30(51-27(11)60)18-28-13-15-29(61)16-14-28/h13-16,21-26,30-33,36-39,59,61H,12,17-20H2,1-11H3,(H2,49,62)(H,50,66)(H,51,60)(H,52,67)(H,53,71)(H,54,68)(H,55,65)(H,56,72)(H,57,69)(H,58,70)(H,63,64)(H,73,74)/t24-,25+,26-,30+,31+,32+,33+,36+,37+,38+,39+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000145

(CHEMBL386344 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@H](CO)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C44H68N10O16/c1-9-22(6)36(43(68)51-28(16-32(45)58)39(64)50-29(17-34(60)61)40(65)52-30(44(69)70)14-20(2)3)54-42(67)35(21(4)5)53-41(66)31(19-55)49-33(59)18-46-37(62)23(7)47-38(63)27(48-24(8)56)15-25-10-12-26(57)13-11-25/h10-13,20-23,27-31,35-36,55,57H,9,14-19H2,1-8H3,(H2,45,58)(H,46,62)(H,47,63)(H,48,56)(H,49,59)(H,50,64)(H,51,68)(H,52,65)(H,53,66)(H,54,67)(H,60,61)(H,69,70)/t22-,23+,27+,28+,29+,30+,31+,35+,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247442
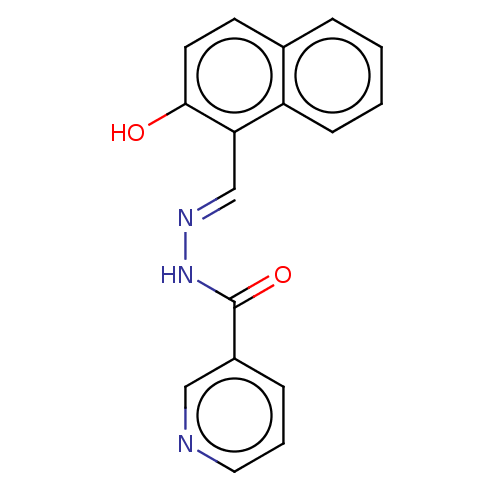
(CHEMBL3193213)Show InChI InChI=1S/C17H13N3O2/c21-16-8-7-12-4-1-2-6-14(12)15(16)11-19-20-17(22)13-5-3-9-18-10-13/h1-11,21H,(H,20,22)/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000148
(2-{2-[2-(2-{2-[2-(2-{2-[2-Acetylamino-3-(4-fluoro-...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(F)cc1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H69FN10O15/c1-10-22(6)36(43(68)52-29(17-32(47)59)40(65)51-30(18-34(61)62)41(66)53-31(45(70)71)15-20(2)3)56-42(67)35(21(4)5)55-44(69)37(24(8)57)54-33(60)19-48-38(63)23(7)49-39(64)28(50-25(9)58)16-26-11-13-27(46)14-12-26/h11-14,20-24,28-31,35-37,57H,10,15-19H2,1-9H3,(H2,47,59)(H,48,63)(H,49,64)(H,50,58)(H,51,65)(H,52,68)(H,53,66)(H,54,60)(H,55,69)(H,56,67)(H,61,62)(H,70,71)/t22-,23+,24-,28+,29+,30+,31+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000134

(2-{2-[2-(2-{2-[2-(2-{2-[2-Acetylamino-3-(4-chloro-...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(Cl)cc1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H69ClN10O15/c1-10-22(6)36(43(68)52-29(17-32(47)59)40(65)51-30(18-34(61)62)41(66)53-31(45(70)71)15-20(2)3)56-42(67)35(21(4)5)55-44(69)37(24(8)57)54-33(60)19-48-38(63)23(7)49-39(64)28(50-25(9)58)16-26-11-13-27(46)14-12-26/h11-14,20-24,28-31,35-37,57H,10,15-19H2,1-9H3,(H2,47,59)(H,48,63)(H,49,64)(H,50,58)(H,51,65)(H,52,68)(H,53,66)(H,54,60)(H,55,69)(H,56,67)(H,61,62)(H,70,71)/t22-,23+,24-,28+,29+,30+,31+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247435
(CHEMBL4073197)Show InChI InChI=1S/C18H14ClN3O2/c19-15-9-12(5-7-16(15)20)18(24)22-21-10-14-13-4-2-1-3-11(13)6-8-17(14)23/h1-10,23H,20H2,(H,22,24)/b21-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247434
(CHEMBL1958235)Show SMILES Cc1ccc(cc1)S(=O)(=O)N\N=C\c1c(O)ccc2ccccc12 Show InChI InChI=1S/C18H16N2O3S/c1-13-6-9-15(10-7-13)24(22,23)20-19-12-17-16-5-3-2-4-14(16)8-11-18(17)21/h2-12,20-21H,1H3/b19-12+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247436
(CHEMBL4100050)Show InChI InChI=1S/C18H14N2O3/c21-15-8-7-13-9-12(5-6-14(13)10-15)11-19-20-18(23)16-3-1-2-4-17(16)22/h1-11,21-22H,(H,20,23)/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000159
(CHEMBL331824 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1cc(I)c(O)c(I)c1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H68I2N10O16/c1-10-20(6)35(43(70)53-28(15-31(48)60)40(67)52-29(16-33(62)63)41(68)54-30(45(72)73)11-18(2)3)57-42(69)34(19(4)5)56-44(71)36(22(8)58)55-32(61)17-49-38(65)21(7)50-39(66)27(51-23(9)59)14-24-12-25(46)37(64)26(47)13-24/h12-13,18-22,27-30,34-36,58,64H,10-11,14-17H2,1-9H3,(H2,48,60)(H,49,65)(H,50,66)(H,51,59)(H,52,67)(H,53,70)(H,54,68)(H,55,61)(H,56,71)(H,57,69)(H,62,63)(H,72,73)/t20-,21+,22-,27+,28+,29+,30+,34+,35+,36+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000151
(2-{2-[2-(2-{2-[2-(2-{2-[2-Acetylamino-3-(4-hydroxy...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H69N9O17/c1-10-22(6)36(43(68)50-30(18-34(61)62)40(65)49-29(17-33(59)60)41(66)51-31(45(70)71)15-20(2)3)54-42(67)35(21(4)5)53-44(69)37(24(8)55)52-32(58)19-46-38(63)23(7)47-39(64)28(48-25(9)56)16-26-11-13-27(57)14-12-26/h11-14,20-24,28-31,35-37,55,57H,10,15-19H2,1-9H3,(H,46,63)(H,47,64)(H,48,56)(H,49,65)(H,50,68)(H,51,66)(H,52,58)(H,53,69)(H,54,67)(H,59,60)(H,61,62)(H,70,71)/t22-,23+,24-,28+,29+,30+,31+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247408
(CHEMBL4067105)Show InChI InChI=1S/C18H14N2O3/c21-16-8-4-3-7-14(16)18(23)20-19-11-15-13-6-2-1-5-12(13)9-10-17(15)22/h1-11,21-22H,(H,20,23)/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247441
(CHEMBL4067759)Show InChI InChI=1S/C17H15N3O2/c1-11-5-4-7-13-12(9-18-16(11)13)10-19-20-17(22)14-6-2-3-8-15(14)21/h2-10,18,21H,1H3,(H,20,22)/b19-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000161
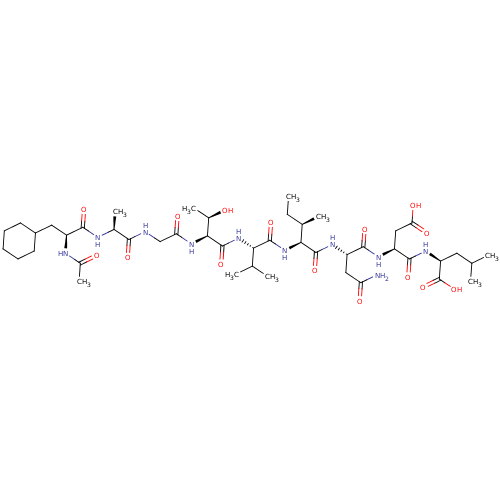
(2-[2-(2-{2-[2-(2-{2-[2-(2-Acetylamino-3-cyclohexyl...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CC1CCCCC1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C45H76N10O15/c1-10-23(6)36(43(67)51-29(18-32(46)58)40(64)50-30(19-34(60)61)41(65)52-31(45(69)70)16-21(2)3)55-42(66)35(22(4)5)54-44(68)37(25(8)56)53-33(59)20-47-38(62)24(7)48-39(63)28(49-26(9)57)17-27-14-12-11-13-15-27/h21-25,27-31,35-37,56H,10-20H2,1-9H3,(H2,46,58)(H,47,62)(H,48,63)(H,49,57)(H,50,64)(H,51,67)(H,52,65)(H,53,59)(H,54,68)(H,55,66)(H,60,61)(H,69,70)/t23-,24+,25-,28+,29+,30+,31+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.13E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000138
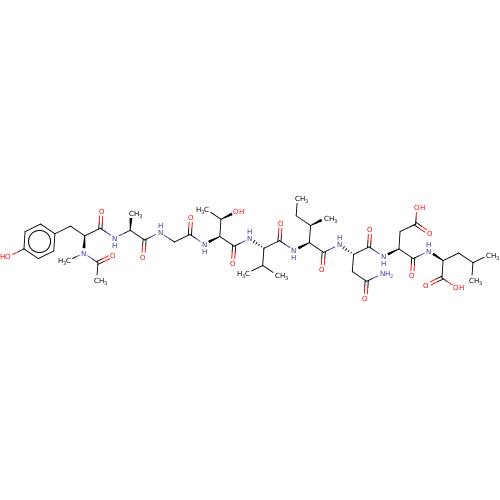
(CHEMBL384014 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)N(C)C(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C46H72N10O16/c1-11-23(6)37(44(69)51-29(18-33(47)60)40(65)50-30(19-35(62)63)41(66)52-31(46(71)72)16-21(2)3)55-43(68)36(22(4)5)54-45(70)38(25(8)57)53-34(61)20-48-39(64)24(7)49-42(67)32(56(10)26(9)58)17-27-12-14-28(59)15-13-27/h12-15,21-25,29-32,36-38,57,59H,11,16-20H2,1-10H3,(H2,47,60)(H,48,64)(H,49,67)(H,50,65)(H,51,69)(H,52,66)(H,53,61)(H,54,70)(H,55,68)(H,62,63)(H,71,72)/t23-,24+,25-,29+,30+,31+,32+,36+,37+,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.21E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
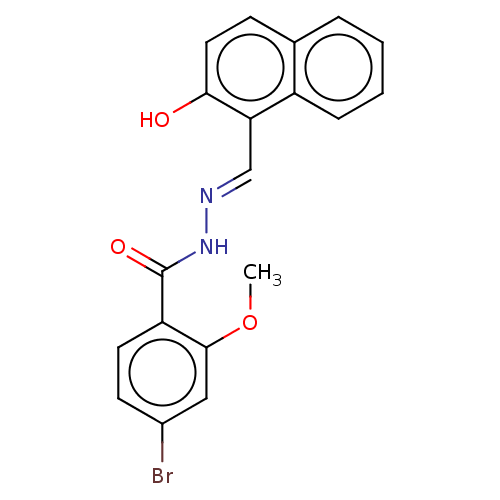
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247433
(CHEMBL4059739)Show InChI InChI=1S/C19H15BrN2O3/c1-25-18-10-13(20)7-8-15(18)19(24)22-21-11-16-14-5-3-2-4-12(14)6-9-17(16)23/h2-11,23H,1H3,(H,22,24)/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000162
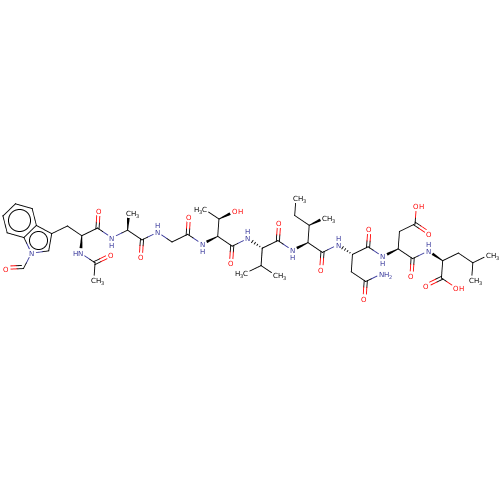
(CHEMBL263439 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](Cc1cn(C=O)c2ccccc12)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C48H71N11O16/c1-10-24(6)39(46(72)54-31(17-35(49)63)43(69)53-32(18-37(65)66)44(70)55-33(48(74)75)15-22(2)3)58-45(71)38(23(4)5)57-47(73)40(26(8)61)56-36(64)19-50-41(67)25(7)51-42(68)30(52-27(9)62)16-28-20-59(21-60)34-14-12-11-13-29(28)34/h11-14,20-26,30-33,38-40,61H,10,15-19H2,1-9H3,(H2,49,63)(H,50,67)(H,51,68)(H,52,62)(H,53,69)(H,54,72)(H,55,70)(H,56,64)(H,57,73)(H,58,71)(H,65,66)(H,74,75)/t24-,25+,26-,30+,31+,32+,33+,38+,39+,40+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000155

(CHEMBL265013 | Ribonucleotide reductase inhibiting...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)[C@H](C)NC(=O)[C@H](Cc1ccc(O)cc1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C46H72N10O16/c1-11-22(6)36(44(69)52-30(18-33(47)60)41(66)51-31(19-34(61)62)42(67)53-32(46(71)72)16-20(2)3)55-43(68)35(21(4)5)54-45(70)37(25(9)57)56-39(64)24(8)48-38(63)23(7)49-40(65)29(50-26(10)58)17-27-12-14-28(59)15-13-27/h12-15,20-25,29-32,35-37,57,59H,11,16-19H2,1-10H3,(H2,47,60)(H,48,63)(H,49,65)(H,50,58)(H,51,66)(H,52,69)(H,53,67)(H,54,70)(H,55,68)(H,56,64)(H,61,62)(H,71,72)/t22-,23+,24+,25-,29+,30+,31+,32+,35+,36+,37+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentaration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
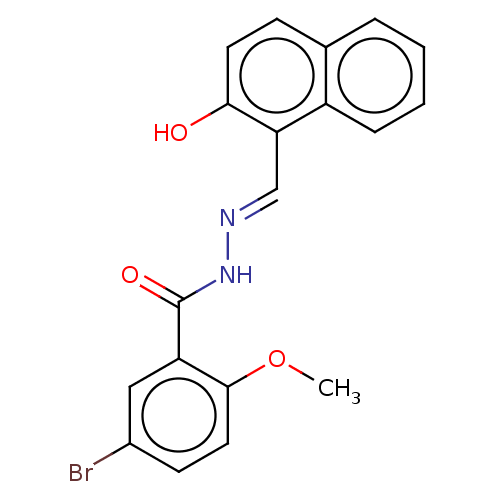
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247432
(CHEMBL4077024)Show InChI InChI=1S/C19H15BrN2O3/c1-25-18-9-7-13(20)10-15(18)19(24)22-21-11-16-14-5-3-2-4-12(14)6-8-17(16)23/h2-11,23H,1H3,(H,22,24)/b21-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.66E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247427
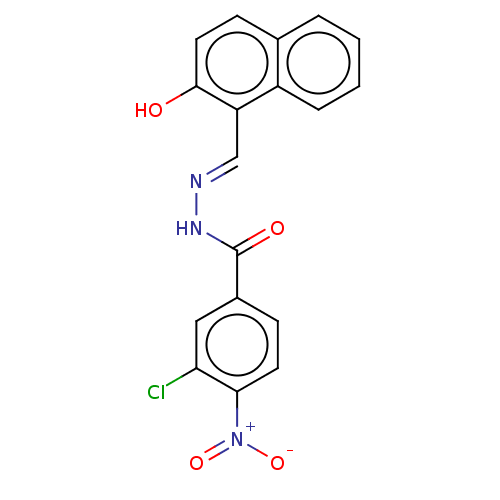
(CHEMBL4064659)Show SMILES Oc1ccc2ccccc2c1\C=N\NC(=O)c1ccc(c(Cl)c1)[N+]([O-])=O Show InChI InChI=1S/C18H12ClN3O4/c19-15-9-12(5-7-16(15)22(25)26)18(24)21-20-10-14-13-4-2-1-3-11(13)6-8-17(14)23/h1-10,23H,(H,21,24)/b20-10+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.67E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
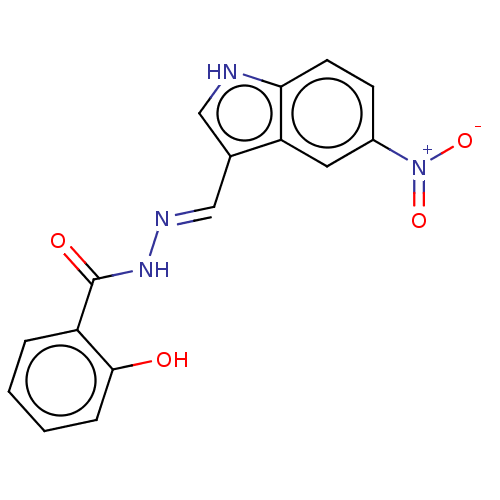
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247440
(CHEMBL4091609)Show SMILES Oc1ccccc1C(=O)N\N=C\c1c[nH]c2ccc(cc12)[N+]([O-])=O Show InChI InChI=1S/C16H12N4O4/c21-15-4-2-1-3-12(15)16(22)19-18-9-10-8-17-14-6-5-11(20(23)24)7-13(10)14/h1-9,17,21H,(H,19,22)/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
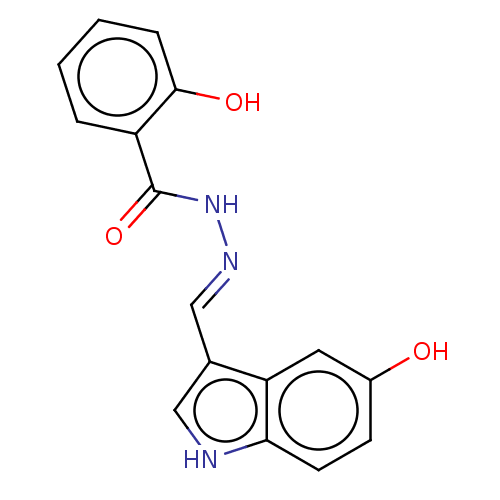
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247426
(CHEMBL4097201)Show InChI InChI=1S/C16H13N3O3/c20-11-5-6-14-13(7-11)10(8-17-14)9-18-19-16(22)12-3-1-2-4-15(12)21/h1-9,17,20-21H,(H,19,22)/b18-9+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247424
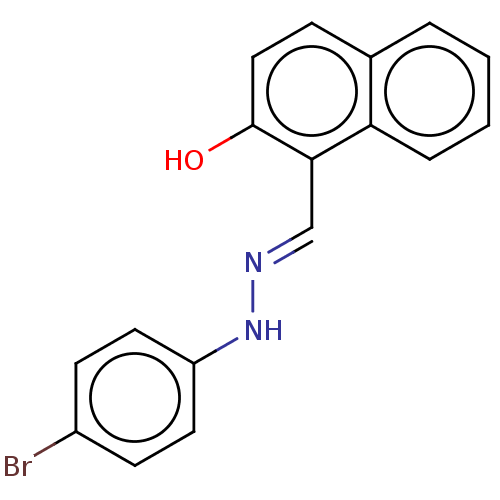
(CHEMBL4101599)Show InChI InChI=1S/C17H13BrN2O/c18-13-6-8-14(9-7-13)20-19-11-16-15-4-2-1-3-12(15)5-10-17(16)21/h1-11,20-21H/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500167
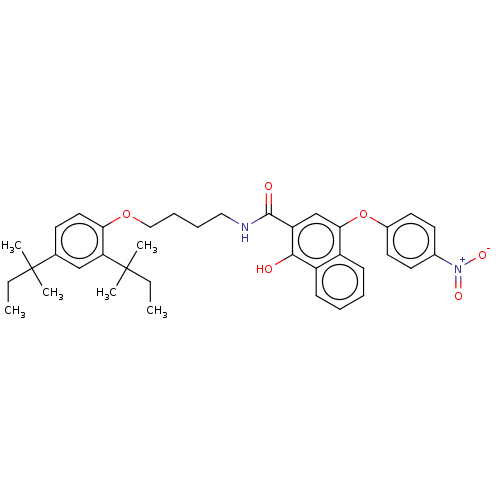
(CHEMBL3747291)Show SMILES CCC(C)(C)c1ccc(OCCCCNC(=O)c2cc(Oc3ccc(cc3)[N+]([O-])=O)c3ccccc3c2O)c(c1)C(C)(C)CC Show InChI InChI=1S/C37H44N2O6/c1-7-36(3,4)25-15-20-32(31(23-25)37(5,6)8-2)44-22-12-11-21-38-35(41)30-24-33(28-13-9-10-14-29(28)34(30)40)45-27-18-16-26(17-19-27)39(42)43/h9-10,13-20,23-24,40H,7-8,11-12,21-22H2,1-6H3,(H,38,41) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.18E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500166
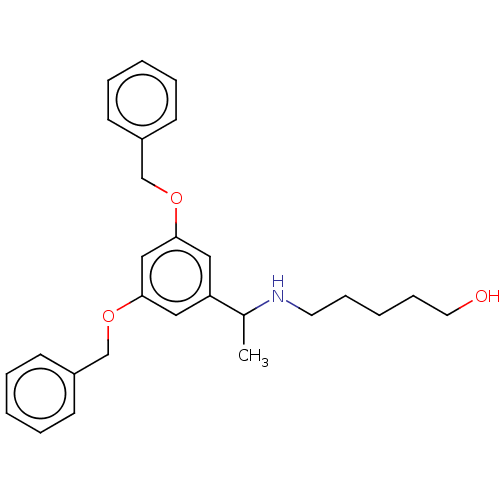
(CHEMBL3747319)Show SMILES CC(NCCCCCO)c1cc(OCc2ccccc2)cc(OCc2ccccc2)c1 Show InChI InChI=1S/C27H33NO3/c1-22(28-15-9-4-10-16-29)25-17-26(30-20-23-11-5-2-6-12-23)19-27(18-25)31-21-24-13-7-3-8-14-24/h2-3,5-8,11-14,17-19,22,28-29H,4,9-10,15-16,20-21H2,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
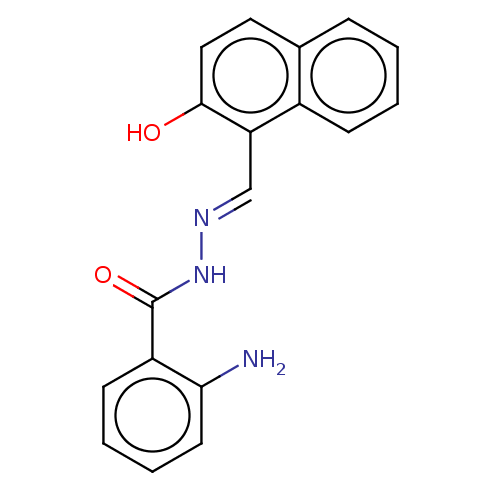
(Homo sapiens (Human)) | BDBM50247425
(CHEMBL4070699)Show InChI InChI=1S/C18H15N3O2/c19-16-8-4-3-7-14(16)18(23)21-20-11-15-13-6-2-1-5-12(13)9-10-17(15)22/h1-11,22H,19H2,(H,21,23)/b20-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500171

(CHEMBL600902 | GNF-Pf-721)Show SMILES OC(COc1ccc(OCC(O)CN2CCN(CC2)C2c3ccccc3-c3ccccc23)cc1)CN1CCN(CC1)C1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C46H50N4O4/c51-33(29-47-21-25-49(26-22-47)45-41-13-5-1-9-37(41)38-10-2-6-14-42(38)45)31-53-35-17-19-36(20-18-35)54-32-34(52)30-48-23-27-50(28-24-48)46-43-15-7-3-11-39(43)40-12-4-8-16-44(40)46/h1-20,33-34,45-46,51-52H,21-32H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500170
(CHEMBL3747217)Show SMILES CCOC(=O)C[C@@H](CC(=O)NCCN[C@H](C)c1ccc(O)c(c1)C(=O)OC)OC(C)=O |r| Show InChI InChI=1S/C21H30N2O8/c1-5-30-20(27)12-16(31-14(3)24)11-19(26)23-9-8-22-13(2)15-6-7-18(25)17(10-15)21(28)29-4/h6-7,10,13,16,22,25H,5,8-9,11-12H2,1-4H3,(H,23,26)/t13-,16-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000139
(2-[2-(2-{2-[2-(2-{2-[2-(2-Acetylamino-4-phenyl-but...)Show SMILES CC[C@@H](C)[C@H](NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)CNC(=O)[C@H](C)NC(=O)[C@H](CCc1ccccc1)NC(C)=O)[C@@H](C)O)C(C)C)C(=O)N[C@@H](CC(N)=O)C(=O)N[C@@H](CC(O)=O)C(=O)N[C@@H](CC(C)C)C(O)=O Show InChI InChI=1S/C46H72N10O15/c1-10-24(6)37(44(68)52-30(19-33(47)59)41(65)51-31(20-35(61)62)42(66)53-32(46(70)71)18-22(2)3)56-43(67)36(23(4)5)55-45(69)38(26(8)57)54-34(60)21-48-39(63)25(7)49-40(64)29(50-27(9)58)17-16-28-14-12-11-13-15-28/h11-15,22-26,29-32,36-38,57H,10,16-21H2,1-9H3,(H2,47,59)(H,48,63)(H,49,64)(H,50,58)(H,51,65)(H,52,68)(H,53,66)(H,54,60)(H,55,69)(H,56,67)(H,61,62)(H,70,71)/t24-,25+,26-,29+,30+,31+,32+,36+,37+,38+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration against HSV-1 ribonucleotide reductase R1 protein binding |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247408

(CHEMBL4067105)Show InChI InChI=1S/C18H14N2O3/c21-16-8-4-3-7-14(16)18(23)20-19-11-15-13-6-2-1-5-12(13)9-10-17(15)22/h1-11,21-22H,(H,20,23)/b19-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate by boronate affinity chromatography |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500168

(CHEMBL3746072)Show SMILES CC(C)(NC(=O)CN1C(=O)c2ccccc2C1=O)C(=O)NCCCC[C@H](NC(=O)CC(O)(C(F)(F)F)C(F)(F)F)C(O)=O |r| Show InChI InChI=1S/C25H28F6N4O8/c1-22(2,34-17(37)12-35-18(38)13-7-3-4-8-14(13)19(35)39)21(42)32-10-6-5-9-15(20(40)41)33-16(36)11-23(43,24(26,27)28)25(29,30)31/h3-4,7-8,15,43H,5-6,9-12H2,1-2H3,(H,32,42)(H,33,36)(H,34,37)(H,40,41)/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.22E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500174
(CHEMBL3745866)Show SMILES CCCCCCCCCCCCCCCC(=O)N[C@@H](CCCCN)C(=O)N[C@@H](CCCCN)C(=O)OC |r| Show InChI InChI=1S/C29H58N4O4/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-22-27(34)32-25(20-16-18-23-30)28(35)33-26(29(36)37-2)21-17-19-24-31/h25-26H,3-24,30-31H2,1-2H3,(H,32,34)(H,33,35)/t25-,26-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247439
(CHEMBL4075660)Show InChI InChI=1S/C18H14N2O/c21-18-11-10-15-8-4-5-9-16(15)17(18)13-20-19-12-14-6-2-1-3-7-14/h1-13,21H/b19-12+,20-13+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.94E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50247423

(CHEMBL4064137)Show SMILES Oc1ccc2ccccc2c1\C=N\NS(=O)(=O)c1ccc(cc1)[N+]([O-])=O Show InChI InChI=1S/C17H13N3O5S/c21-17-10-5-12-3-1-2-4-15(12)16(17)11-18-19-26(24,25)14-8-6-13(7-9-14)20(22)23/h1-11,19,21H/b18-11+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human RRM1 expressed in Escherichia coli BL21-codon plus(DE3)-RIL using [14C]-ADP as substrate after 3 mins by liquid scintillation cou... |
J Med Chem 61: 666-680 (2018)
Article DOI: 10.1021/acs.jmedchem.7b00530
BindingDB Entry DOI: 10.7270/Q2W098CB |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500165
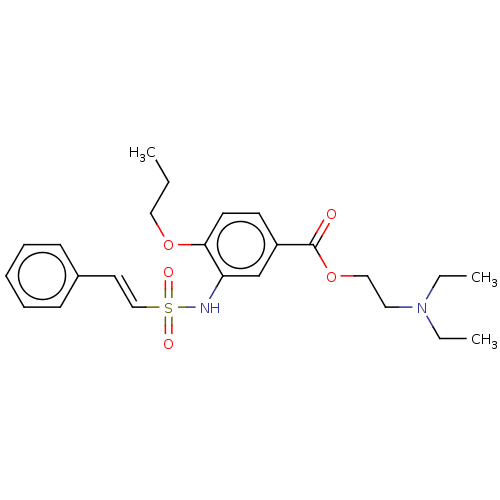
(CHEMBL3746526)Show SMILES CCCOc1ccc(cc1NS(=O)(=O)\C=C\c1ccccc1)C(=O)OCCN(CC)CC Show InChI InChI=1S/C24H32N2O5S/c1-4-16-30-23-13-12-21(24(27)31-17-15-26(5-2)6-3)19-22(23)25-32(28,29)18-14-20-10-8-7-9-11-20/h7-14,18-19,25H,4-6,15-17H2,1-3H3/b18-14+ | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500172
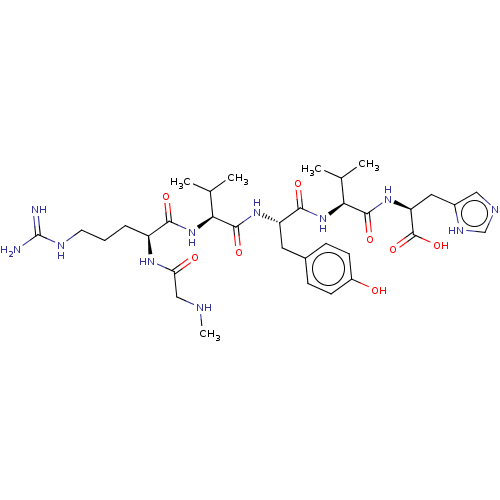
(CHEMBL3746454)Show SMILES CNCC(=O)N[C@@H](CCCNC(N)=N)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1ccc(O)cc1)C(=O)N[C@@H](C(C)C)C(=O)N[C@@H](Cc1cnc[nH]1)C(O)=O |r| Show InChI InChI=1S/C34H53N11O8/c1-18(2)27(44-29(48)23(41-26(47)16-37-5)7-6-12-39-34(35)36)31(50)42-24(13-20-8-10-22(46)11-9-20)30(49)45-28(19(3)4)32(51)43-25(33(52)53)14-21-15-38-17-40-21/h8-11,15,17-19,23-25,27-28,37,46H,6-7,12-14,16H2,1-5H3,(H,38,40)(H,41,47)(H,42,50)(H,43,51)(H,44,48)(H,45,49)(H,52,53)(H4,35,36,39)/t23-,24-,25-,27-,28-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500173

(CHEMBL3746940)Show InChI InChI=1S/C18H39N3O/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-21-18(22)17(20)14-15-19/h17H,2-16,19-20H2,1H3,(H,21,22)/t17-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.72E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50000140
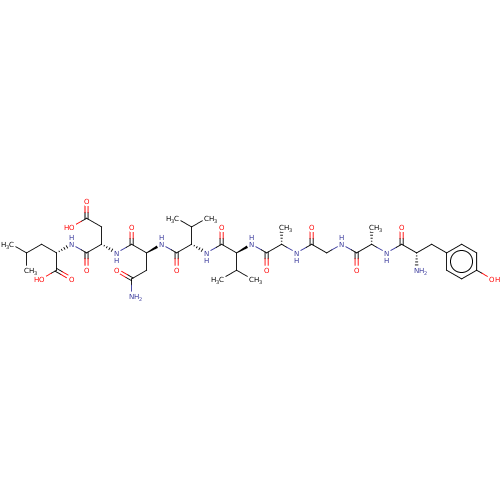
(2-{2-[2-(2-{2-[2-(2-{2-[2-Amino-3-(4-hydroxy-pheny...)Show SMILES CC(C)C[C@H](NC(=O)[C@H](CC(O)=O)NC(=O)[C@H](CC(N)=O)NC(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@H](C)NC(=O)CNC(=O)[C@H](C)NC(=O)[C@@H](N)Cc1ccc(O)cc1)C(C)C)C(C)C)C(O)=O Show InChI InChI=1S/C41H64N10O14/c1-18(2)13-28(41(64)65)49-38(61)27(16-31(55)56)47-37(60)26(15-29(43)53)48-39(62)32(19(3)4)51-40(63)33(20(5)6)50-35(58)22(8)45-30(54)17-44-34(57)21(7)46-36(59)25(42)14-23-9-11-24(52)12-10-23/h9-12,18-22,25-28,32-33,52H,13-17,42H2,1-8H3,(H2,43,53)(H,44,57)(H,45,54)(H,46,59)(H,47,60)(H,48,62)(H,49,61)(H,50,58)(H,51,63)(H,55,56)(H,64,65)/t21-,22-,25-,26-,27-,28-,32-,33-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 6.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Notre-Dame Hospital Research
Curated by ChEMBL
| Assay Description
Inhibitory concentration of the compound against HSV-1 ribonucleotide reductase R1 protein binding; Range 30-60 |
J Med Chem 35: 346-50 (1992)
BindingDB Entry DOI: 10.7270/Q2348JB2 |
More data for this
Ligand-Target Pair | |
Ribonucleoside-diphosphate reductase large subunit
(Homo sapiens (Human)) | BDBM50500169

(CHEMBL3746715)Show SMILES CCOC(=O)C(NC(=O)CN[C@H]1CCCc2sccc12)C(=O)OCC |r| Show InChI InChI=1S/C17H24N2O5S/c1-3-23-16(21)15(17(22)24-4-2)19-14(20)10-18-12-6-5-7-13-11(12)8-9-25-13/h8-9,12,15,18H,3-7,10H2,1-2H3,(H,19,20)/t12-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.17E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Case Western Reserve University
Curated by ChEMBL
| Assay Description
Inhibition of human ribonucleotide reductase M1 subunit expressed in Escherichia coli BL21 (DE3) using 14C-ADP as substrate by liquid scintillation c... |
J Med Chem 58: 9498-509 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00929
BindingDB Entry DOI: 10.7270/Q2MW2M5M |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data