 Found 465 hits of ic50 data for polymerid = 50001641
Found 465 hits of ic50 data for polymerid = 50001641 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heparanase
(Homo sapiens (Human)) | BDBM50614394
(CHEMBL5273238) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50614394

(CHEMBL5273238) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 0.0800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611959
(CHEMBL5276024) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50466629

(CHEMBL4280766)Show SMILES [H][C@]1(O[C@H]2[C@H](O)[C@@H](OS(O)(=O)=O)[C@]([H])(O[C@@H]3[C@@H](CO)O[C@H](O[C@@H](CO)C(O[C@H](O)CO)C(O)=O)[C@H](NC(C)=O)[C@H]3O)OC2C(O)=O)O[C@H](CO)[C@@H](O)[C@H](O)[C@H]1NC(C)=O |r| Show InChI InChI=1S/C28H46N2O26S/c1-7(35)29-13-16(39)15(38)9(3-31)49-27(13)54-21-18(41)22(56-57(46,47)48)28(55-23(21)25(44)45)53-19-10(4-32)50-26(14(17(19)40)30-8(2)36)51-11(5-33)20(24(42)43)52-12(37)6-34/h9-23,26-28,31-34,37-41H,3-6H2,1-2H3,(H,29,35)(H,30,36)(H,42,43)(H,44,45)(H,46,47,48)/t9-,10-,11+,12+,13-,14-,15-,16-,17-,18+,19-,20?,21+,22-,23?,26-,27-,28-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay |
J Med Chem 61: 10834-10859 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01497
BindingDB Entry DOI: 10.7270/Q29K4DWD |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50612819
(CHEMBL5291042) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611973

(CHEMBL5282692) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50614397
(CHEMBL5279386) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50093526
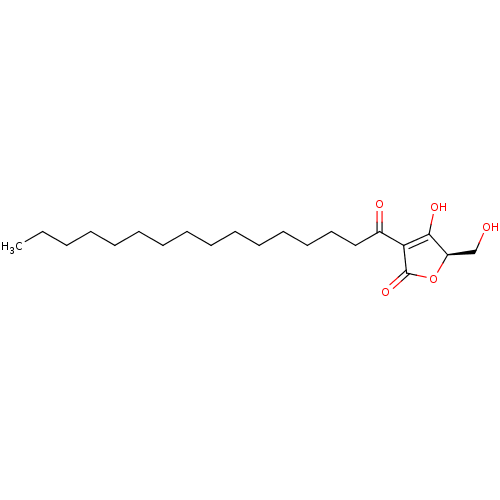
(CHEMBL426373 | RK-682)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(O)[C@@H](CO)OC1=O |r,c:17| Show InChI InChI=1S/C21H36O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-17(23)19-20(24)18(16-22)26-21(19)25/h18,22,24H,2-16H2,1H3/t18-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431651
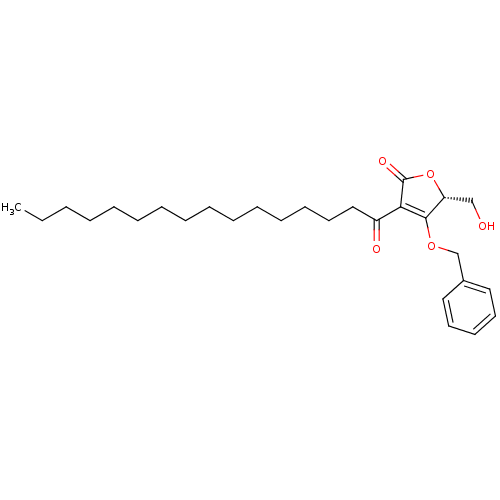
(CHEMBL2349236)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(OCc2ccccc2)[C@@H](CO)OC1=O |r,c:17| Show InChI InChI=1S/C28H42O5/c1-2-3-4-5-6-7-8-9-10-11-12-13-17-20-24(30)26-27(25(21-29)33-28(26)31)32-22-23-18-15-14-16-19-23/h14-16,18-19,25,29H,2-13,17,20-22H2,1H3/t25-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50614398

(CHEMBL5268939) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611974
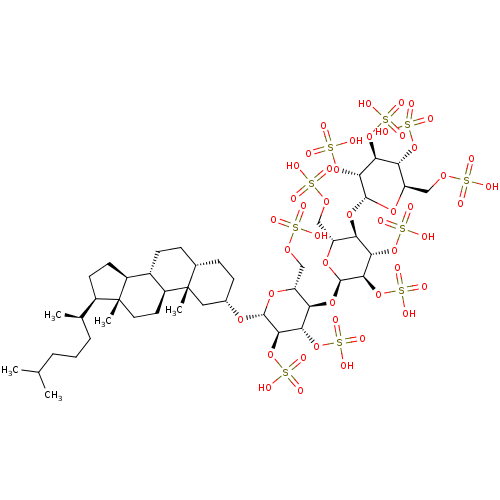
(CHEMBL5271999) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50612820
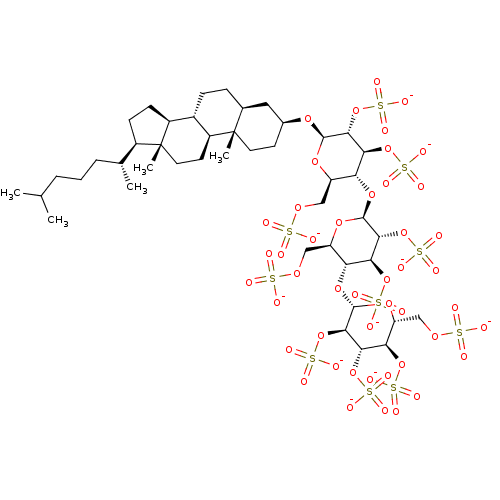
(CHEMBL5267246) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50175932
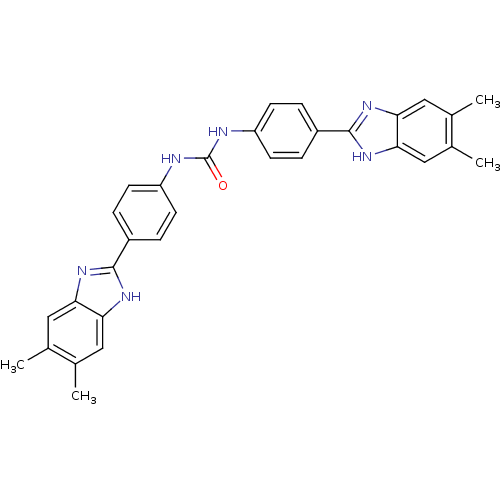
(1,3-bis-[4-(5,6-dimethyl-1H-benzoimidazol-2-yl)phe...)Show SMILES Cc1cc2nc([nH]c2cc1C)-c1ccc(NC(=O)Nc2ccc(cc2)-c2nc3cc(C)c(C)cc3[nH]2)cc1 Show InChI InChI=1S/C31H28N6O/c1-17-13-25-26(14-18(17)2)35-29(34-25)21-5-9-23(10-6-21)32-31(38)33-24-11-7-22(8-12-24)30-36-27-15-19(3)20(4)16-28(27)37-30/h5-16H,1-4H3,(H,34,35)(H,36,37)(H2,32,33,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibitory activity against heparanase from human platelets |
Bioorg Med Chem Lett 16: 409-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.069
BindingDB Entry DOI: 10.7270/Q2ST7PDB |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50175932

(1,3-bis-[4-(5,6-dimethyl-1H-benzoimidazol-2-yl)phe...)Show SMILES Cc1cc2nc([nH]c2cc1C)-c1ccc(NC(=O)Nc2ccc(cc2)-c2nc3cc(C)c(C)cc3[nH]2)cc1 Show InChI InChI=1S/C31H28N6O/c1-17-13-25-26(14-18(17)2)35-29(34-25)21-5-9-23(10-6-21)32-31(38)33-24-11-7-22(8-12-24)30-36-27-15-19(3)20(4)16-28(27)37-30/h5-16H,1-4H3,(H,34,35)(H,36,37)(H2,32,33,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 75 | n/a | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Inhibition of recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50466636
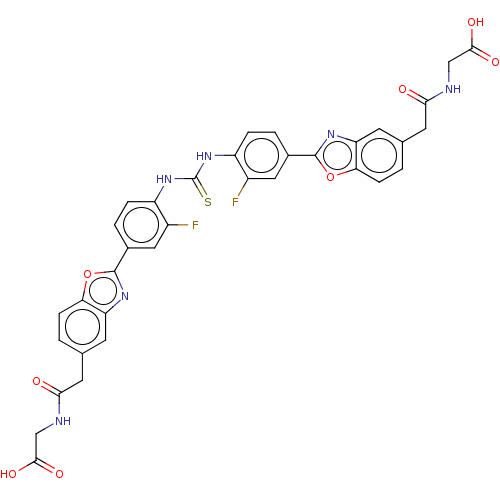
(CHEMBL4286441)Show SMILES OC(=O)CNC(=O)Cc1ccc2oc(nc2c1)-c1ccc(NC(=S)Nc2ccc(cc2F)-c2nc3cc(CC(=O)NCC(O)=O)ccc3o2)c(F)c1 Show InChI InChI=1S/C35H26F2N6O8S/c36-21-13-19(33-40-25-9-17(1-7-27(25)50-33)11-29(44)38-15-31(46)47)3-5-23(21)42-35(52)43-24-6-4-20(14-22(24)37)34-41-26-10-18(2-8-28(26)51-34)12-30(45)39-16-32(48)49/h1-10,13-14H,11-12,15-16H2,(H,38,44)(H,39,45)(H,46,47)(H,48,49)(H2,42,43,52) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay |
J Med Chem 61: 10834-10859 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01497
BindingDB Entry DOI: 10.7270/Q29K4DWD |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611970
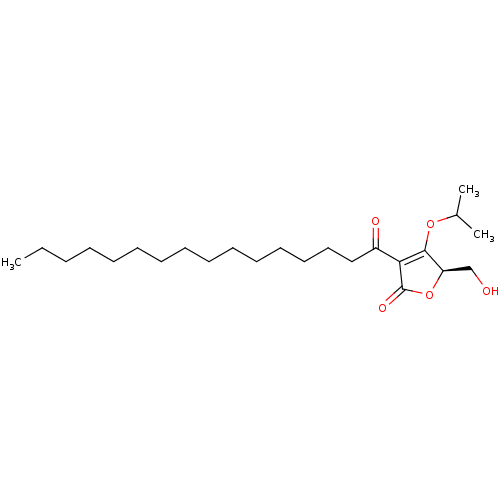
(CHEMBL5286960) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50104680
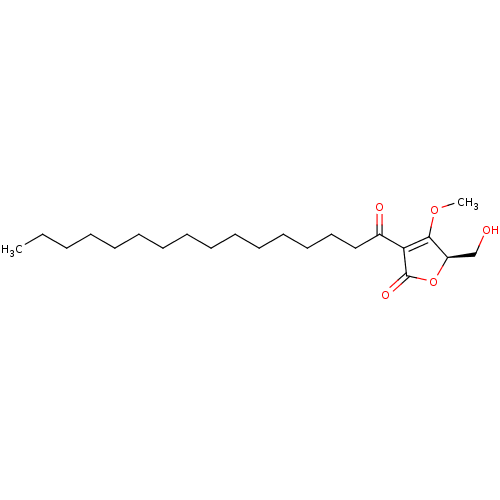
(3-Hexadecanoyl-5-hydroxymethyl-4-methoxy-5H-furan-...)Show SMILES CCCCCCCCCCCCCCCC(=O)C1=C(OC)[C@@H](CO)OC1=O |c:17| Show InChI InChI=1S/C22H38O5/c1-3-4-5-6-7-8-9-10-11-12-13-14-15-16-18(24)20-21(26-2)19(17-23)27-22(20)25/h19,23H,3-17H2,1-2H3/t19-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431662

(CHEMBL2349246)Show SMILES CCCCCCCCCCCCCCCCCC(=O)Nc1cc(=O)n([nH]1)-c1ccc(Oc2ccccc2)c(c1)S(O)(=O)=O Show InChI InChI=1S/C33H47N3O6S/c1-2-3-4-5-6-7-8-9-10-11-12-13-14-15-19-22-32(37)34-31-26-33(38)36(35-31)27-23-24-29(30(25-27)43(39,40)41)42-28-20-17-16-18-21-28/h16-18,20-21,23-26,35H,2-15,19,22H2,1H3,(H,34,37)(H,39,40,41) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Inhibition of recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50175931
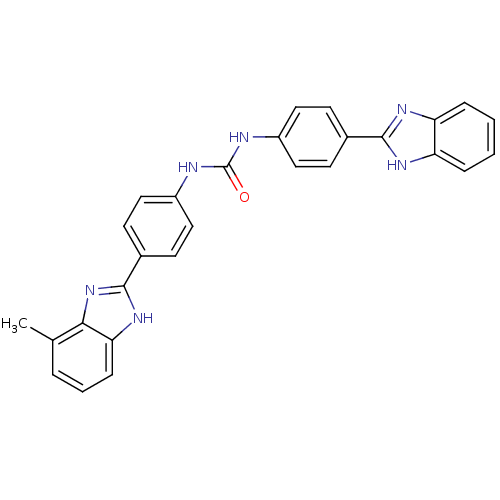
(1-(4-(1H-benzo[d]imidazol-2-yl)phenyl)-3-(4-(7-met...)Show SMILES Cc1cccc2[nH]c(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)-c2nc3ccccc3[nH]2)cc1 Show InChI InChI=1S/C28H22N6O/c1-17-5-4-8-24-25(17)34-27(33-24)19-11-15-21(16-12-19)30-28(35)29-20-13-9-18(10-14-20)26-31-22-6-2-3-7-23(22)32-26/h2-16H,1H3,(H,31,32)(H,33,34)(H2,29,30,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibitory activity against heparanase from human platelets |
Bioorg Med Chem Lett 16: 409-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.069
BindingDB Entry DOI: 10.7270/Q2ST7PDB |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50175938
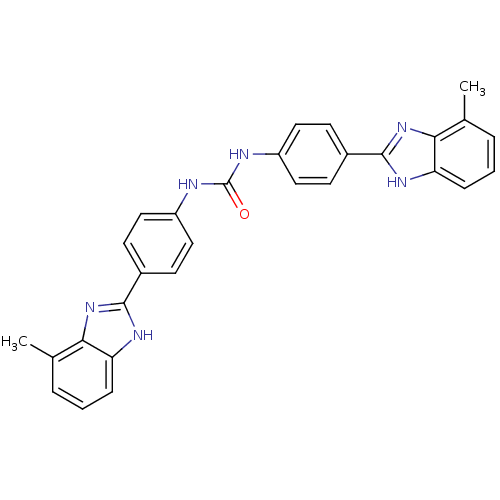
(1,3-bis(4-(7-methyl-1H-benzo[d]imidazol-2-yl)pheny...)Show SMILES Cc1cccc2[nH]c(nc12)-c1ccc(NC(=O)Nc2ccc(cc2)-c2nc3c(C)cccc3[nH]2)cc1 Show InChI InChI=1S/C29H24N6O/c1-17-5-3-7-23-25(17)34-27(32-23)19-9-13-21(14-10-19)30-29(36)31-22-15-11-20(12-16-22)28-33-24-8-4-6-18(2)26(24)35-28/h3-16H,1-2H3,(H,32,34)(H,33,35)(H2,30,31,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibitory activity against heparanase from human platelets |
Bioorg Med Chem Lett 16: 409-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.069
BindingDB Entry DOI: 10.7270/Q2ST7PDB |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50175936
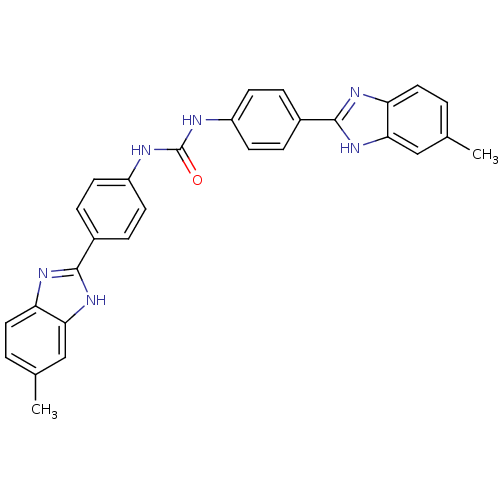
(1,3-bis(4-(6-methyl-1H-benzo[d]imidazol-2-yl)pheny...)Show SMILES Cc1ccc2nc([nH]c2c1)-c1ccc(NC(=O)Nc2ccc(cc2)-c2nc3ccc(C)cc3[nH]2)cc1 Show InChI InChI=1S/C29H24N6O/c1-17-3-13-23-25(15-17)34-27(32-23)19-5-9-21(10-6-19)30-29(36)31-22-11-7-20(8-12-22)28-33-24-14-4-18(2)16-26(24)35-28/h3-16H,1-2H3,(H,32,34)(H,33,35)(H2,30,31,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibitory activity against heparanase from human platelets |
Bioorg Med Chem Lett 16: 409-12 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.069
BindingDB Entry DOI: 10.7270/Q2ST7PDB |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50506597

(CHEMBL4576477)Show SMILES COc1ccc(cc1Br)C(=O)Nc1ccc(CNc2ccc(cc2F)-c2nc3ccccc3[nH]2)cc1 Show InChI InChI=1S/C28H22BrFN4O2/c1-36-26-13-9-19(14-21(26)29)28(35)32-20-10-6-17(7-11-20)16-31-23-12-8-18(15-22(23)30)27-33-24-4-2-3-5-25(24)34-27/h2-15,31H,16H2,1H3,(H,32,35)(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 160 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HPSE GS3 (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 based colorimetry |
J Med Chem 61: 6918-6936 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00908
BindingDB Entry DOI: 10.7270/Q2MS3X1W |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50466651
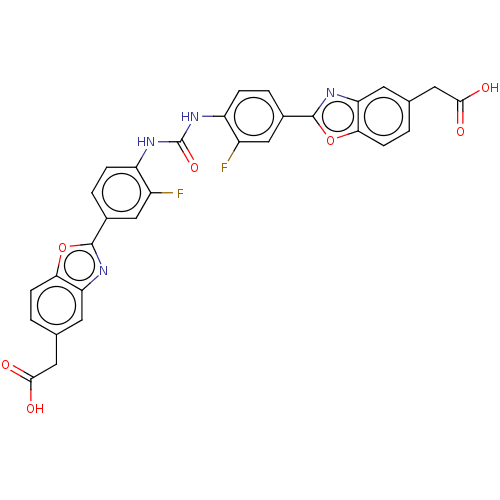
(CHEMBL4283251)Show SMILES OC(=O)Cc1ccc2oc(nc2c1)-c1ccc(NC(=O)Nc2ccc(cc2F)-c2nc3cc(CC(O)=O)ccc3o2)c(F)c1 Show InChI InChI=1S/C31H20F2N4O7/c32-19-13-17(29-34-23-9-15(11-27(38)39)1-7-25(23)43-29)3-5-21(19)36-31(42)37-22-6-4-18(14-20(22)33)30-35-24-10-16(12-28(40)41)2-8-26(24)44-30/h1-10,13-14H,11-12H2,(H,38,39)(H,40,41)(H2,36,37,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
"Sapienza" Universit£ di Roma
Curated by ChEMBL
| Assay Description
Inhibition of recombinant HPSE (unknown origin) using fondaparinux as substrate incubated for 3 hrs in absence of light by WST1 assay |
J Med Chem 61: 10834-10859 (2018)
Article DOI: 10.1021/acs.jmedchem.8b01497
BindingDB Entry DOI: 10.7270/Q29K4DWD |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165639
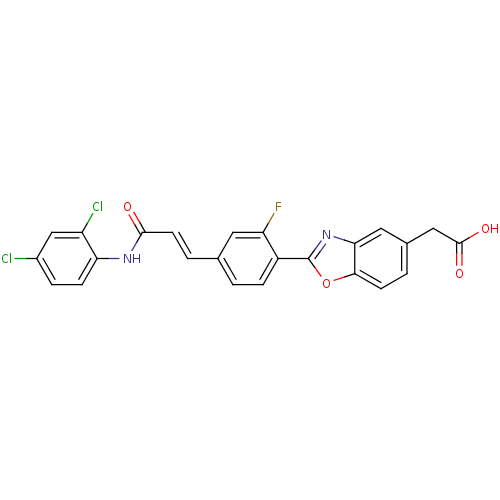
((2-{4-[2-(2,4-Dichloro-phenylcarbamoyl)-vinyl]-2-f...)Show SMILES OC(=O)Cc1ccc2oc(nc2c1)-c1ccc(\C=C\C(=O)Nc2ccc(Cl)cc2Cl)cc1F Show InChI InChI=1S/C24H15Cl2FN2O4/c25-15-4-6-19(17(26)12-15)28-22(30)8-3-13-1-5-16(18(27)9-13)24-29-20-10-14(11-23(31)32)2-7-21(20)33-24/h1-10,12H,11H2,(H,28,30)(H,31,32)/b8-3+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165669

((2-{4-[2-(3-Bromo-phenylcarbamoyl)-vinyl]-2-fluoro...)Show SMILES OC(=O)Cc1ccc2oc(nc2c1)-c1ccc(\C=C\C(=O)Nc2cccc(Br)c2)cc1F Show InChI InChI=1S/C24H16BrFN2O4/c25-16-2-1-3-17(13-16)27-22(29)9-6-14-4-7-18(19(26)10-14)24-28-20-11-15(12-23(30)31)5-8-21(20)32-24/h1-11,13H,12H2,(H,27,29)(H,30,31)/b9-6+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human Heparanase |
Bioorg Med Chem Lett 15: 2295-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.014
BindingDB Entry DOI: 10.7270/Q2BP029G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50614402

(CHEMBL5285151) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165653

((2-{4-[2-(3,4-Dichloro-phenylcarbamoyl)-vinyl]-2-f...)Show SMILES OC(=O)Cc1ccc2oc(nc2c1)-c1ccc(\C=C\C(=O)Nc2ccc(Cl)c(Cl)c2)cc1F Show InChI InChI=1S/C24H15Cl2FN2O4/c25-17-6-4-15(12-18(17)26)28-22(30)8-3-13-1-5-16(19(27)9-13)24-29-20-10-14(11-23(31)32)2-7-21(20)33-24/h1-10,12H,11H2,(H,28,30)(H,31,32)/b8-3+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human Heparanase |
Bioorg Med Chem Lett 15: 2295-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.014
BindingDB Entry DOI: 10.7270/Q2BP029G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165660
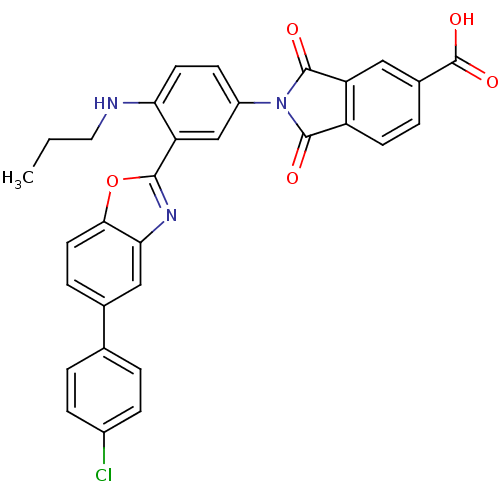
(2-(3-(5-(4-chlorophenyl)benzo[d]oxazol-2-yl)-4-(pr...)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(Cl)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22ClN3O5/c1-2-13-33-25-11-9-21(35-29(36)22-10-5-19(31(38)39)14-23(22)30(35)37)16-24(25)28-34-26-15-18(6-12-27(26)40-28)17-3-7-20(32)8-4-17/h3-12,14-16,33H,2,13H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human Heparanase |
Bioorg Med Chem Lett 15: 2295-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.014
BindingDB Entry DOI: 10.7270/Q2BP029G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165639

((2-{4-[2-(2,4-Dichloro-phenylcarbamoyl)-vinyl]-2-f...)Show SMILES OC(=O)Cc1ccc2oc(nc2c1)-c1ccc(\C=C\C(=O)Nc2ccc(Cl)cc2Cl)cc1F Show InChI InChI=1S/C24H15Cl2FN2O4/c25-15-4-6-19(17(26)12-15)28-22(30)8-3-13-1-5-16(18(27)9-13)24-29-20-10-14(11-23(31)32)2-7-21(20)33-24/h1-10,12H,11H2,(H,28,30)(H,31,32)/b8-3+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vitro inhibitory concentration against human Heparanase |
Bioorg Med Chem Lett 15: 2295-9 (2005)
Article DOI: 10.1016/j.bmcl.2005.03.014
BindingDB Entry DOI: 10.7270/Q2BP029G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147513

(2-[3-[5-(4-Chloro-phenyl)-benzooxazol-2-yl]-4-(2-m...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(Cl)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22ClN3O6/c1-40-13-12-33-25-10-8-21(35-29(36)22-9-4-19(31(38)39)14-23(22)30(35)37)16-24(25)28-34-26-15-18(5-11-27(26)41-28)17-2-6-20(32)7-3-17/h2-11,14-16,33H,12-13H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147546
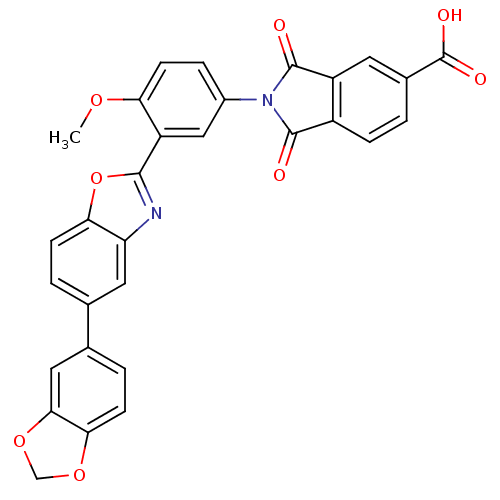
(2-(3-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H18N2O8/c1-37-23-9-5-18(32-28(33)19-6-2-17(30(35)36)10-20(19)29(32)34)13-21(23)27-31-22-11-15(3-7-24(22)40-27)16-4-8-25-26(12-16)39-14-38-25/h2-13H,14H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165660

(2-(3-(5-(4-chlorophenyl)benzo[d]oxazol-2-yl)-4-(pr...)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(Cl)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22ClN3O5/c1-2-13-33-25-11-9-21(35-29(36)22-10-5-19(31(38)39)14-23(22)30(35)37)16-24(25)28-34-26-15-18(6-12-27(26)40-28)17-3-7-20(32)8-4-17/h3-12,14-16,33H,2,13H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Inhibition of recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50431663
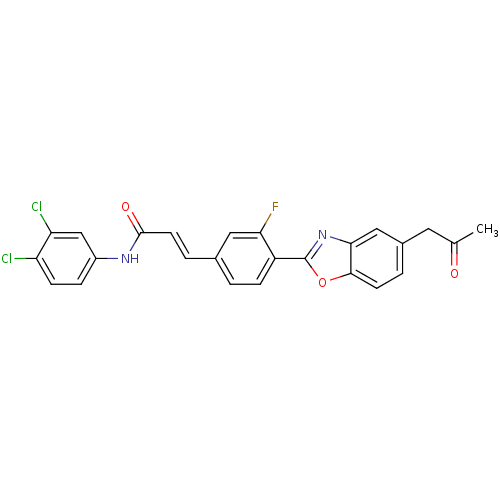
(CHEMBL2349247)Show SMILES CC(=O)Cc1ccc2oc(nc2c1)-c1ccc(\C=C\C(=O)Nc2ccc(Cl)c(Cl)c2)cc1F Show InChI InChI=1S/C25H17Cl2FN2O3/c1-14(31)10-16-3-8-23-22(12-16)30-25(33-23)18-6-2-15(11-21(18)28)4-9-24(32)29-17-5-7-19(26)20(27)13-17/h2-9,11-13H,10H2,1H3,(H,29,32)/b9-4+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Inhibition of recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147546

(2-(3-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H18N2O8/c1-37-23-9-5-18(32-28(33)19-6-2-17(30(35)36)10-20(19)29(32)34)13-21(23)27-31-22-11-15(3-7-24(22)40-27)16-4-8-25-26(12-16)39-14-38-25/h2-13H,14H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sharif University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of heparanase |
Eur J Med Chem 43: 548-56 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.014
BindingDB Entry DOI: 10.7270/Q2PC33KM |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165653

((2-{4-[2-(3,4-Dichloro-phenylcarbamoyl)-vinyl]-2-f...)Show SMILES OC(=O)Cc1ccc2oc(nc2c1)-c1ccc(\C=C\C(=O)Nc2ccc(Cl)c(Cl)c2)cc1F Show InChI InChI=1S/C24H15Cl2FN2O4/c25-17-6-4-15(12-18(17)26)28-22(30)8-3-13-1-5-16(19(27)9-13)24-29-20-10-14(11-23(31)32)2-7-21(20)33-24/h1-10,12H,11H2,(H,28,30)(H,31,32)/b8-3+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sharif University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of heparanase |
Eur J Med Chem 43: 548-56 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.014
BindingDB Entry DOI: 10.7270/Q2PC33KM |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165660

(2-(3-(5-(4-chlorophenyl)benzo[d]oxazol-2-yl)-4-(pr...)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(Cl)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22ClN3O5/c1-2-13-33-25-11-9-21(35-29(36)22-10-5-19(31(38)39)14-23(22)30(35)37)16-24(25)28-34-26-15-18(6-12-27(26)40-28)17-3-7-20(32)8-4-17/h3-12,14-16,33H,2,13H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sharif University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of heparanase |
Eur J Med Chem 43: 548-56 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.014
BindingDB Entry DOI: 10.7270/Q2PC33KM |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165669

((2-{4-[2-(3-Bromo-phenylcarbamoyl)-vinyl]-2-fluoro...)Show SMILES OC(=O)Cc1ccc2oc(nc2c1)-c1ccc(\C=C\C(=O)Nc2cccc(Br)c2)cc1F Show InChI InChI=1S/C24H16BrFN2O4/c25-16-2-1-3-17(13-16)27-22(29)9-6-14-4-7-18(19(26)10-14)24-28-20-11-15(12-23(30)31)5-8-21(20)32-24/h1-11,13H,12H2,(H,27,29)(H,30,31)/b9-6+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sharif University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of heparanase |
Eur J Med Chem 43: 548-56 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.014
BindingDB Entry DOI: 10.7270/Q2PC33KM |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50165639

((2-{4-[2-(2,4-Dichloro-phenylcarbamoyl)-vinyl]-2-f...)Show SMILES OC(=O)Cc1ccc2oc(nc2c1)-c1ccc(\C=C\C(=O)Nc2ccc(Cl)cc2Cl)cc1F Show InChI InChI=1S/C24H15Cl2FN2O4/c25-15-4-6-19(17(26)12-15)28-22(30)8-3-13-1-5-16(18(27)9-13)24-29-20-10-14(11-23(31)32)2-7-21(20)33-24/h1-10,12H,11H2,(H,28,30)(H,31,32)/b8-3+ | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Sharif University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of heparanase |
Eur J Med Chem 43: 548-56 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.014
BindingDB Entry DOI: 10.7270/Q2PC33KM |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50412102
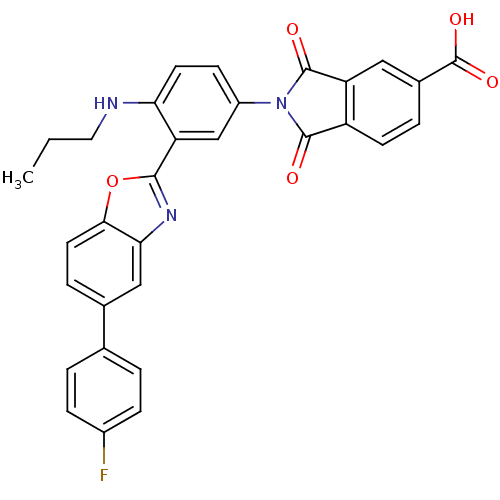
(CHEMBL495255)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(F)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22FN3O5/c1-2-13-33-25-11-9-21(35-29(36)22-10-5-19(31(38)39)14-23(22)30(35)37)16-24(25)28-34-26-15-18(6-12-27(26)40-28)17-3-7-20(32)8-4-17/h3-12,14-16,33H,2,13H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147546

(2-(3-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H18N2O8/c1-37-23-9-5-18(32-28(33)19-6-2-17(30(35)36)10-20(19)29(32)34)13-21(23)27-31-22-11-15(3-7-24(22)40-27)16-4-8-25-26(12-16)39-14-38-25/h2-13H,14H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147546

(2-(3-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H18N2O8/c1-37-23-9-5-18(32-28(33)19-6-2-17(30(35)36)10-20(19)29(32)34)13-21(23)27-31-22-11-15(3-7-24(22)40-27)16-4-8-25-26(12-16)39-14-38-25/h2-13H,14H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611966
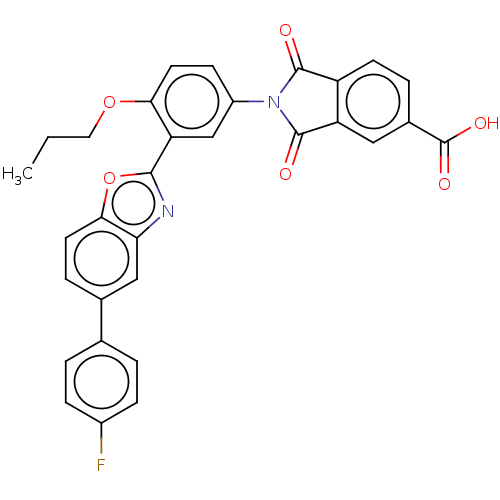
(CHEMBL5277782) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611968

(CHEMBL5266599) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50175811
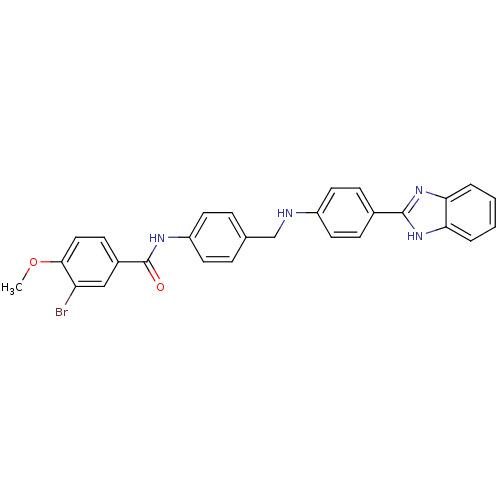
(CHEMBL200215 | N-(4-{[4-(1H-benzoimidazol-2-yl)phe...)Show SMILES COc1ccc(cc1Br)C(=O)Nc1ccc(CNc2ccc(cc2)-c2nc3ccccc3[nH]2)cc1 Show InChI InChI=1S/C28H23BrN4O2/c1-35-26-15-10-20(16-23(26)29)28(34)31-22-11-6-18(7-12-22)17-30-21-13-8-19(9-14-21)27-32-24-4-2-3-5-25(24)33-27/h2-16,30H,17H2,1H3,(H,31,34)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
ImClone Systems Inc
Curated by ChEMBL
| Assay Description
Inhibitory activity against heparanase from human platelets |
Bioorg Med Chem Lett 16: 404-8 (2005)
Article DOI: 10.1016/j.bmcl.2005.09.070
BindingDB Entry DOI: 10.7270/Q2FJ2G9R |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50175811

(CHEMBL200215 | N-(4-{[4-(1H-benzoimidazol-2-yl)phe...)Show SMILES COc1ccc(cc1Br)C(=O)Nc1ccc(CNc2ccc(cc2)-c2nc3ccccc3[nH]2)cc1 Show InChI InChI=1S/C28H23BrN4O2/c1-35-26-15-10-20(16-23(26)29)28(34)31-22-11-6-18(7-12-22)17-30-21-13-8-19(9-14-21)27-32-24-4-2-3-5-25(24)33-27/h2-16,30H,17H2,1H3,(H,31,34)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Structural Biochemistry Laboratory, Centro de Investigaci�n Pr�ncipe Felipe, 46012 Valencia, Spain. rgozalbes@cipf.es
Curated by ChEMBL
| Assay Description
Inhibition of recombinant heparanase catalytic stie (unknown origin) expressed in Escherichia coli BL21 (DE3) |
Bioorg Med Chem 21: 1944-51 (2013)
Article DOI: 10.1016/j.bmc.2013.01.033
BindingDB Entry DOI: 10.7270/Q2HQ419G |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147534

(1,3-Dioxo-2-[3-(5-phenyl-benzooxazol-2-yl)-4-propy...)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H23N3O5/c1-2-14-32-25-12-10-21(34-29(35)22-11-8-20(31(37)38)15-23(22)30(34)36)17-24(25)28-33-26-16-19(9-13-27(26)39-28)18-6-4-3-5-7-18/h3-13,15-17,32H,2,14H2,1H3,(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147534

(1,3-Dioxo-2-[3-(5-phenyl-benzooxazol-2-yl)-4-propy...)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H23N3O5/c1-2-14-32-25-12-10-21(34-29(35)22-11-8-20(31(37)38)15-23(22)30(34)36)17-24(25)28-33-26-16-19(9-13-27(26)39-28)18-6-4-3-5-7-18/h3-13,15-17,32H,2,14H2,1H3,(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50375289

(CHEMBL258980)Show SMILES CO[C@H]1O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](O[C@H]2O[C@H](COS([O-])(=O)=O)[C@@H](OS([O-])(=O)=O)[C@H](OS([O-])(=O)=O)[C@@H]2OS([O-])(=O)=O)[C@@H]1OS([O-])(=O)=O Show InChI InChI=1S/C13H24O32S7/c1-35-12-10(44-51(29,30)31)8(6(41-48(20,21)22)4(38-12)2-36-46(14,15)16)40-13-11(45-52(32,33)34)9(43-50(26,27)28)7(42-49(23,24)25)5(39-13)3-37-47(17,18)19/h4-13H,2-3H2,1H3,(H,14,15,16)(H,17,18,19)(H,20,21,22)(H,23,24,25)(H,26,27,28)(H,29,30,31)(H,32,33,34)/p-7/t4-,5-,6-,7-,8+,9+,10+,11+,12+,13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Progen Pharmaceuticals Limited
Curated by ChEMBL
| Assay Description
Inhibition of human platelet heparanase |
Bioorg Med Chem 16: 699-709 (2008)
Article DOI: 10.1016/j.bmc.2007.10.044
BindingDB Entry DOI: 10.7270/Q2SQ918D |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50611965
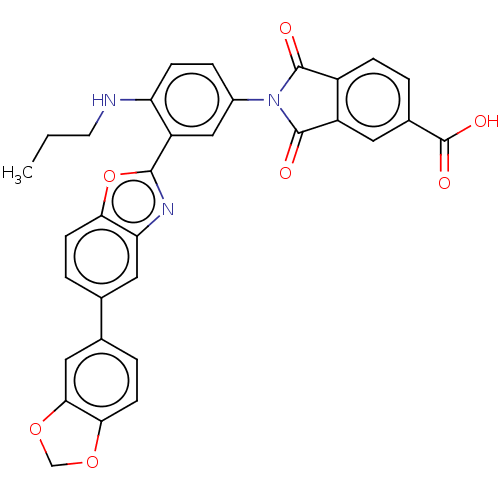
(CHEMBL5271688) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147534

(1,3-Dioxo-2-[3-(5-phenyl-benzooxazol-2-yl)-4-propy...)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H23N3O5/c1-2-14-32-25-12-10-21(34-29(35)22-11-8-20(31(37)38)15-23(22)30(34)36)17-24(25)28-33-26-16-19(9-13-27(26)39-28)18-6-4-3-5-7-18/h3-13,15-17,32H,2,14H2,1H3,(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 251 | n/a | n/a | n/a | n/a | n/a | n/a |
Sharif University of Technology
Curated by ChEMBL
| Assay Description
Inhibition of heparanase |
Eur J Med Chem 43: 548-56 (2008)
Article DOI: 10.1016/j.ejmech.2007.04.014
BindingDB Entry DOI: 10.7270/Q2PC33KM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data