

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kcal/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM392076 (US10301272, Example 7/9) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Phenex Pharmaceuticals AG Curated by ChEMBL | Assay Description Displacement of [3H]-SCH 23390 from human recombinant dopamine D1 receptor after 60 mins by scintillation counting analysis | Bioorg Med Chem Lett 28: 1446-1455 (2018) Article DOI: 10.1016/j.bmcl.2018.03.093 BindingDB Entry DOI: 10.7270/Q27W6FPC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86180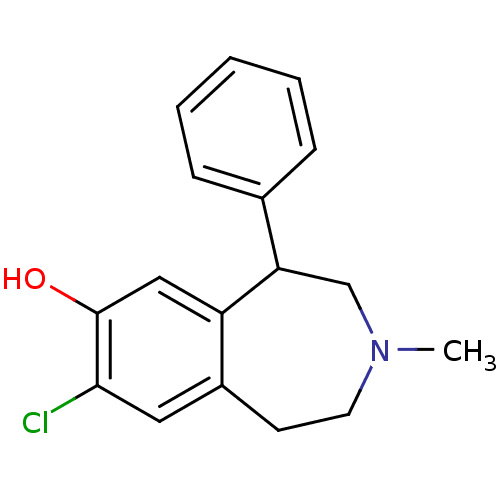 (CAS_87075-17-0 | NSC_5018 | SCH 23390 | SCH23390 |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human recombinant Dopamine D1 receptor expressed in CHO cells | Bioorg Med Chem 24: 1793-810 (2016) Article DOI: 10.1016/j.bmc.2016.03.006 BindingDB Entry DOI: 10.7270/Q2J67JS7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86180 (CAS_87075-17-0 | NSC_5018 | SCH 23390 | SCH23390 |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Jagiellonian University Medical College Curated by ChEMBL | Assay Description Displacement of [3H]SCH 23390 from human recombinant dopamine D1 receptor expressed in CHO cells measured after 60 mins by scintillation counting met... | Bioorg Med Chem 25: 471-482 (2017) Article DOI: 10.1016/j.bmc.2016.11.014 BindingDB Entry DOI: 10.7270/Q2CF9S3S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.820 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human dopamine D1 receptor | Bioorg Med Chem 18: 7675-99 (2010) Article DOI: 10.1016/j.bmc.2010.07.034 BindingDB Entry DOI: 10.7270/Q2DF6S69 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50004823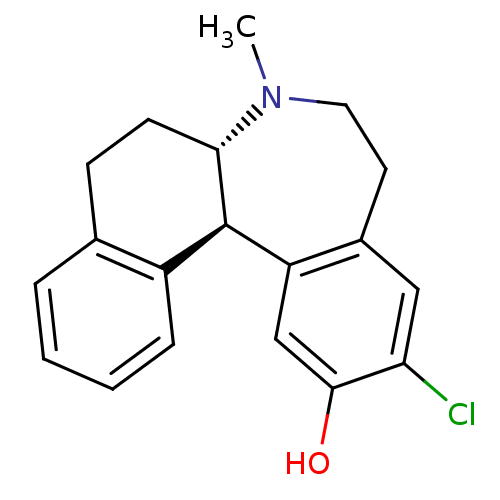 ((6aS,13bR)-11-Chloro-7-methyl-5,6a,7,8,9,13b-hexah...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D1 receptor expressed in CHO-K1 cells by cAMP Hunter assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Adamed Ltd. Curated by ChEMBL | Assay Description Antagonist activity at human recombinant at D1 receptor assessed as inhibition of dopamine-induced cAMP accumulation | J Med Chem 57: 4543-57 (2014) Article DOI: 10.1021/jm401895u BindingDB Entry DOI: 10.7270/Q2N29ZHX | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM86180 (CAS_87075-17-0 | NSC_5018 | SCH 23390 | SCH23390 |...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem Patents Similars | US Patent | 1.24 | -12.3 | 2.52 | n/a | n/a | n/a | n/a | n/a | 30 |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Different concentrations (10^−5 M-10^−11 M) of the compound of the invention and corresponding isotope receptor ligand as well as recepto... | US Patent US9359372 (2016) BindingDB Entry DOI: 10.7270/Q2736PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.52 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Huzhou University Curated by ChEMBL | Assay Description Antagonist activity at D1 receptor (unknown origin) expressed in CHOK1 cells assessed as inhibition of SKF38393-induced cAMP accumulation measured af... | Bioorg Med Chem 27: 2100-2111 (2019) Article DOI: 10.1016/j.bmc.2019.04.014 BindingDB Entry DOI: 10.7270/Q2X06BCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Antagonist activity at C-terminal RLuc8-fused D1R (unknown origin) transfected in human HEK293T cells co-expressing N-terminal Venus-tagged beta-arre... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Antagonist activity at C-terminal RLuc8-fused D1R (unknown origin) transfected in human HEK293T cells co-expressing N-terminal Venus-tagged beta-arre... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50387561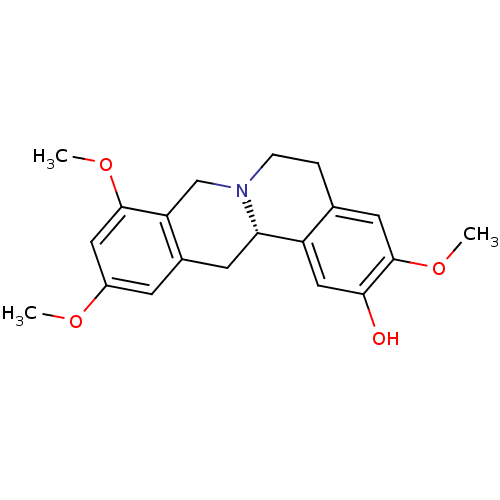 (CHEMBL2057455 | US9359372, DC037079) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.86 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Homo sapiens (Human)) | BDBM21398 (4-[4-(4-Chloro-phenyl)-4-hydroxy-piperidin-1-yl]-1...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG PC cid PC sid PDB UniChem Patents Similars | DrugBank PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The IC50 value was reported as apparent, since [3H]NCA was purported to be irreversible. Result indicates the mean of two separate experiments, each ... | J Med Chem 27: 806-10 (1984) BindingDB Entry DOI: 10.7270/Q26111HB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Antagonist activity at D1R (unknown origin) transfected in human HEK293T cells assessed as increase in cAMP accumulation incubated for 2 hrs by cAMP ... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM82247 (8-Chloro-3-methyl-5-phenyl-2,3,4,5-tetrahydro-1H-b...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Duke University Curated by ChEMBL | Assay Description Antagonist activity at D1R (unknown origin) transfected in human HEK293T cells assessed as increase in cAMP accumulation incubated for 2 hrs by cAMP ... | ACS Med Chem Lett 11: 385-392 (2020) Article DOI: 10.1021/acsmedchemlett.9b00575 BindingDB Entry DOI: 10.7270/Q25X2D8S | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM235778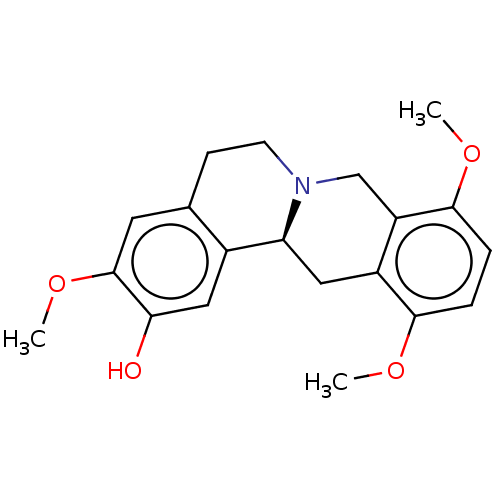 (US9359372, DC037030) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 3.70 | -11.7 | 7.22 | n/a | n/a | n/a | n/a | n/a | 30 |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Different concentrations (10^−5 M-10^−11 M) of the compound of the invention and corresponding isotope receptor ligand as well as recepto... | US Patent US9359372 (2016) BindingDB Entry DOI: 10.7270/Q2736PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50172414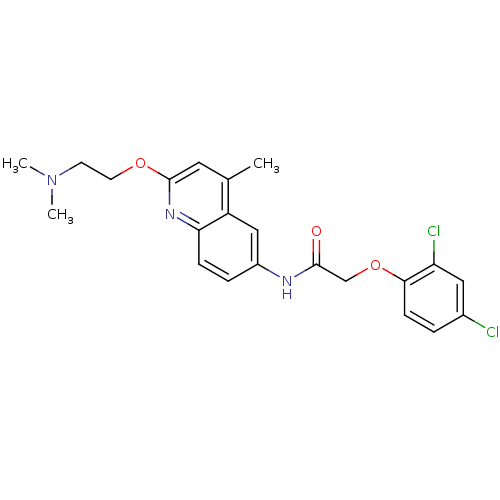 (2-(2,4-Dichloro-phenoxy)-N-[2-(2-dimethylamino-eth...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 7.5 | n/a | n/a | n/a | n/a | n/a | n/a |
7TM Pharma A/S Curated by ChEMBL | Assay Description Inhibitory concentration against dopamine receptor D1 | J Med Chem 48: 5684-97 (2005) Article DOI: 10.1021/jm050103y BindingDB Entry DOI: 10.7270/Q2H41QZ8 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429044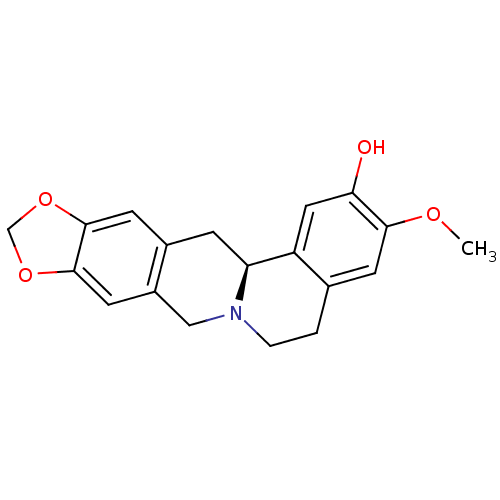 (CHEMBL2334893 | US9359372, DC037029) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 4.20 | -11.6 | 8.19 | n/a | n/a | n/a | n/a | n/a | 30 |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Different concentrations (10^−5 M-10^−11 M) of the compound of the invention and corresponding isotope receptor ligand as well as recepto... | US Patent US9359372 (2016) BindingDB Entry DOI: 10.7270/Q2736PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50544077 (CHEMBL4643069) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 9.40 | n/a | n/a | n/a | n/a | n/a | n/a |
City University of New York Curated by ChEMBL | Assay Description Antagonist activity at human dopamine D1 receptor expressed in CHO-K1 cells by cAMP Hunter assay | Bioorg Med Chem Lett 30: (2020) Article DOI: 10.1016/j.bmcl.2020.127305 BindingDB Entry DOI: 10.7270/Q2377D85 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50387561 (CHEMBL2057455 | US9359372, DC037079) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 5.18 | -11.5 | 9.85 | n/a | n/a | n/a | n/a | n/a | 30 |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Different concentrations (10^−5 M-10^−11 M) of the compound of the invention and corresponding isotope receptor ligand as well as recepto... | US Patent US9359372 (2016) BindingDB Entry DOI: 10.7270/Q2736PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371829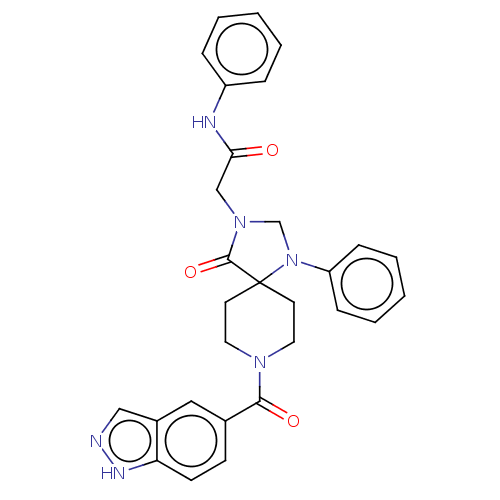 (2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1-phenyl-1,3,8...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.6 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371835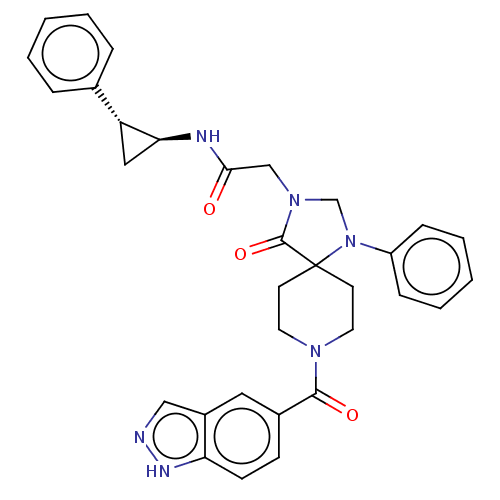 ((rac, trans)-2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 10.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50378584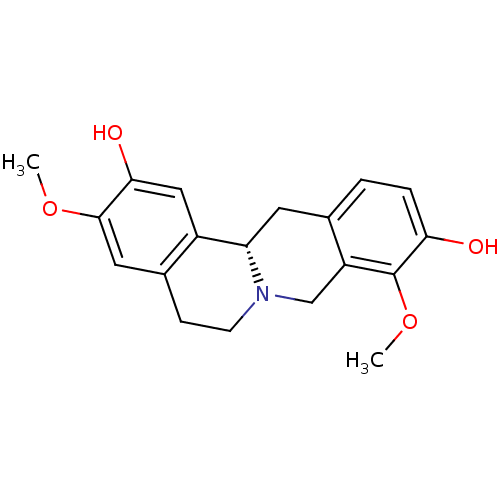 (STEPHOLIDINE) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372088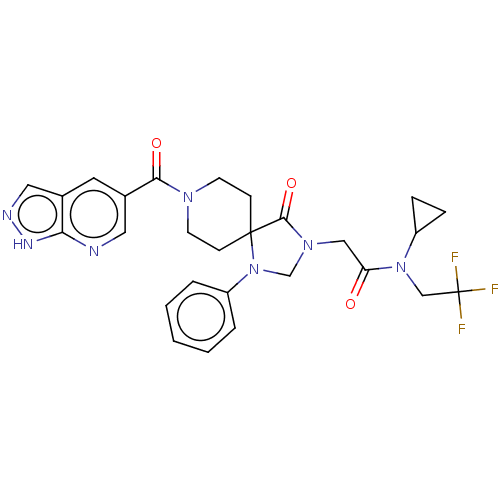 (N-Cyclopropyl-2-[4-oxo-1-phenyl-8-(1H-pyrazolo[3,4...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371821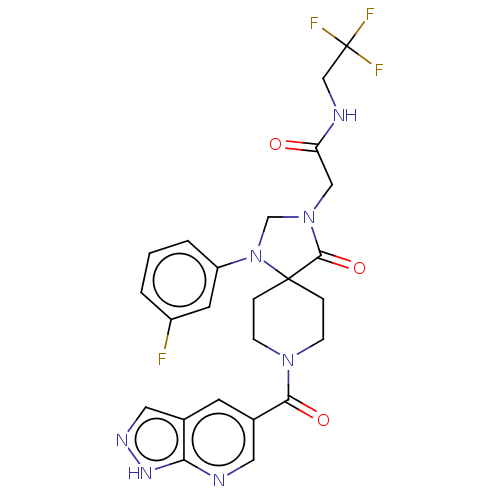 (2-[1-(3-Fluoro-phenyl)-4-oxo-8-(1H-pyrazolo[3,4-b]...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372102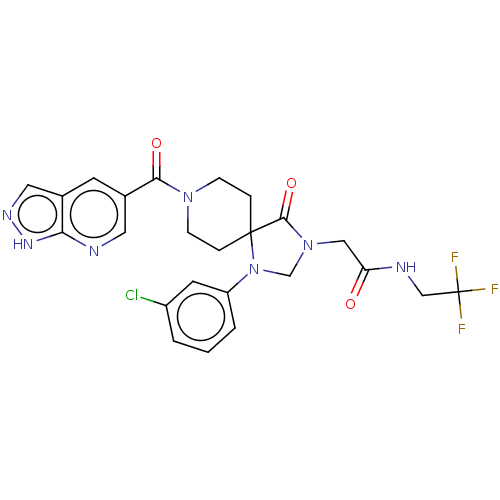 (2-(1-(3-Chlorophenyl)-4-oxo-8-(1H-pyrazolo[3,4-b]p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 12.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM235782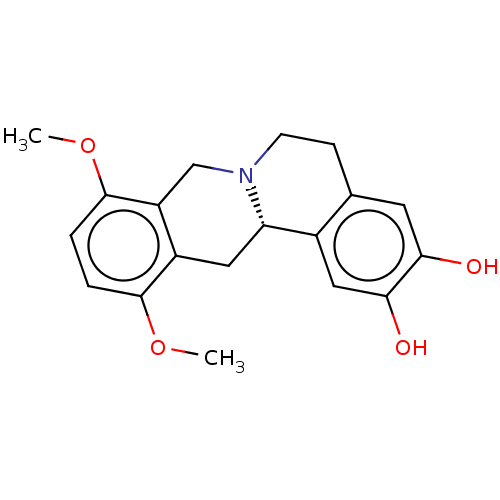 (US9359372, DC037035) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | 6.72 | -11.3 | 13.3 | n/a | n/a | n/a | n/a | n/a | 30 |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Different concentrations (10^−5 M-10^−11 M) of the compound of the invention and corresponding isotope receptor ligand as well as recepto... | US Patent US9359372 (2016) BindingDB Entry DOI: 10.7270/Q2736PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371932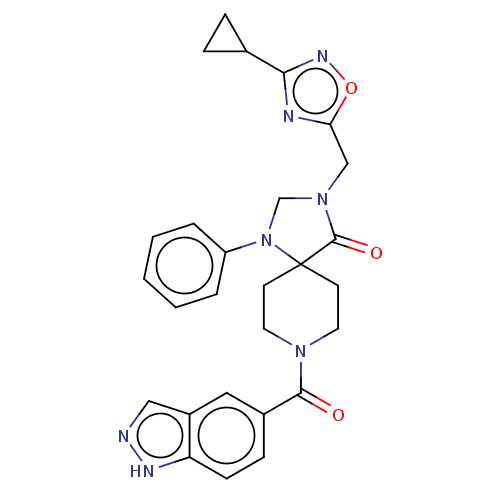 (3-[(3-Cyclopropyl-1,2,4-oxadiazol-5-yl)methyl]-8-(...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | US Patent | n/a | n/a | 13.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50387564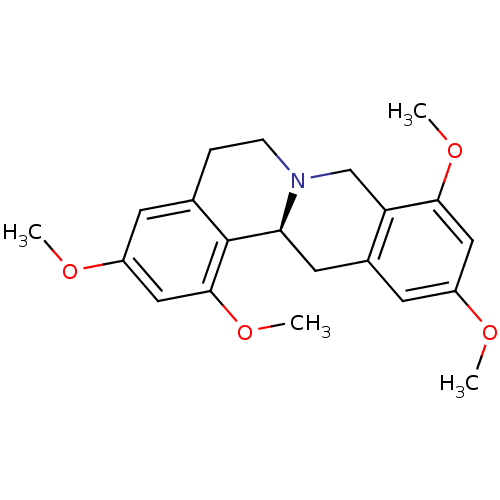 (CHEMBL2057445) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 14.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Shenyang Pharmaceutical University Curated by ChEMBL | Assay Description Displacement of [3H]SCH23390 from human dopamine D1 receptor expressed in HEK293 cells | Bioorg Med Chem 20: 4862-71 (2012) Article DOI: 10.1016/j.bmc.2012.05.057 BindingDB Entry DOI: 10.7270/Q28P61K9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM50429052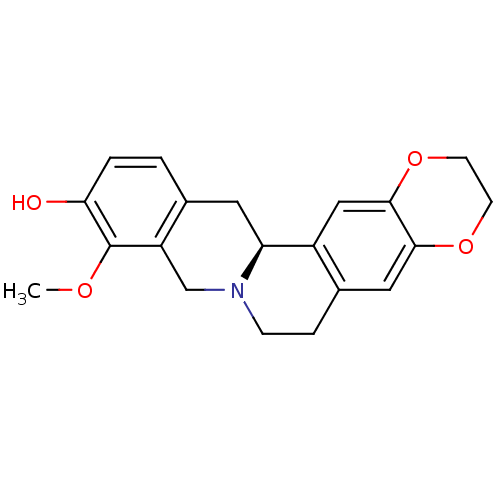 (CHEMBL2334881 | US9359372, DC037082) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | 7.51 | -11.3 | 14.7 | n/a | n/a | n/a | n/a | n/a | 30 |
SHANGHAI INSTITUTE OF MATERIA MEDICA, CHINESE ACADEMY OF SCIENCES US Patent | Assay Description Different concentrations (10^−5 M-10^−11 M) of the compound of the invention and corresponding isotope receptor ligand as well as recepto... | US Patent US9359372 (2016) BindingDB Entry DOI: 10.7270/Q2736PSP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Homo sapiens (Human)) | BDBM55121 (3-HYDROXYTYRAMINE HYDROCHLORIDE | 4-(2-aminoethyl)...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase DrugBank KEGG PC cid PC sid PDB UniChem Similars | DrugBank PDB PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA Curated by ChEMBL | Assay Description The IC50 value was reported as apparent, since [3H]NCA was purported to be irreversible. Result indicates the mean of two separate experiments, each ... | J Med Chem 27: 806-10 (1984) BindingDB Entry DOI: 10.7270/Q26111HB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371847 (2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1-phenyl-1,3,8...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371830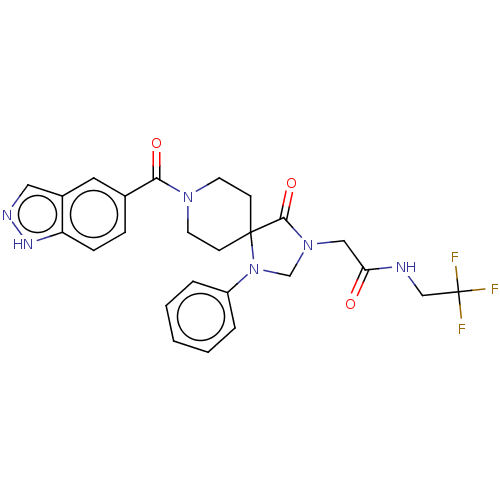 (2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1-phenyl-1,3,8...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 15.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A)/D(1B)/D(2)/D(3)/D(4) dopamine receptor (Homo sapiens (Human)) | BDBM50020221 ((-)-5-hydroxy-2-(dipropylamino)tetralin | (-)-6-(d...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia antibodypedia antibodypedia antibodypedia antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
State University of Groningen Curated by ChEMBL | Assay Description Potency to displace the specific in vitro binding of [3H]-N-0437 to calf striatal membrane | J Med Chem 31: 2178-82 (1988) BindingDB Entry DOI: 10.7270/Q22R3S82 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372136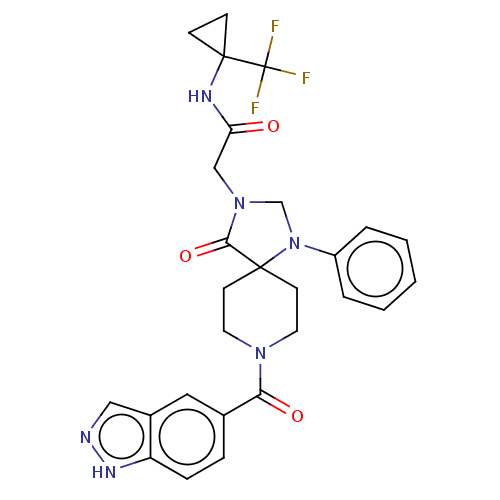 (2-[8-(1H-indazole-5-carbonyl)-4-oxo-1-phenyl-1,3,8...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372052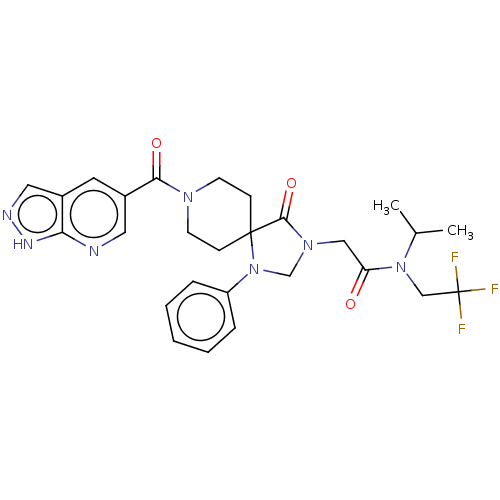 (2-[4-Oxo-1-phenyl-8-(1H-pyrazolo[3,4-b]pyridine-5-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371865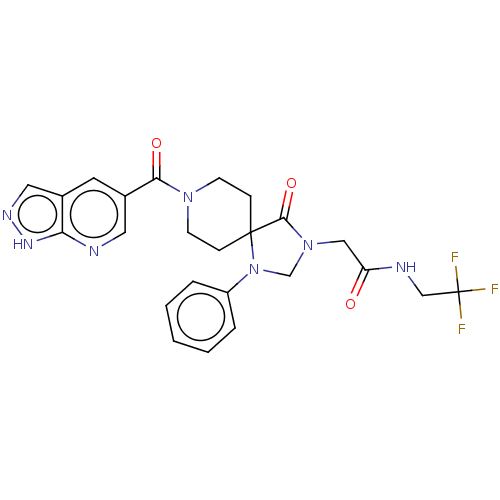 (2-[4-Oxo-1-phenyl-8-(1H-pyrazolo[3,4-b]pyridine-5-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372117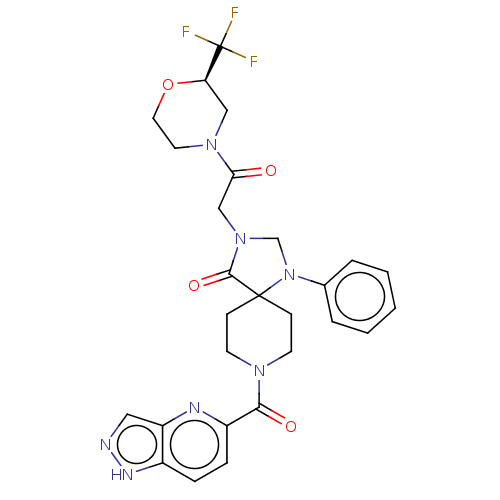 ((R)- or (S)-3-(2-Oxo-2-(2-(trifluoromethyl)morphol...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372092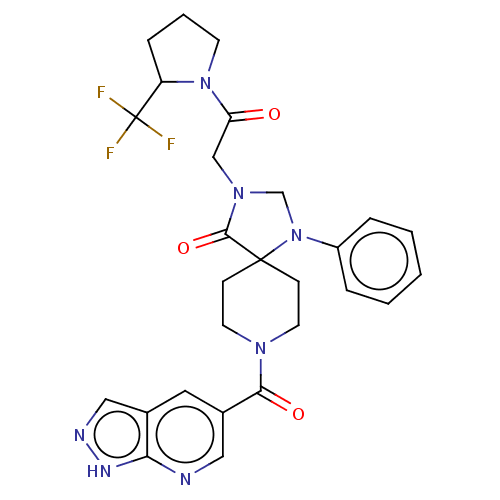 ((S)-3-(2-Oxo-2-(2-(trifluoromethyl)pyrrolidin-1-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372139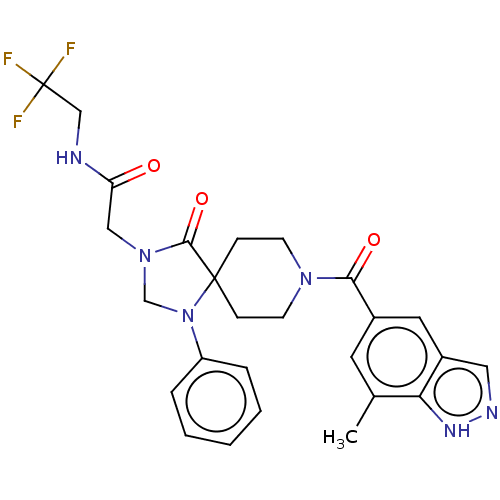 (2-(8-(7-Methyl-1H-indazole-5-carbonyl)-4-oxo-1-phe...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 17.8 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372125 ((S)- or (R)-8-(1H-indazole-5-carbonyl)-3-(2-oxo-2-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372078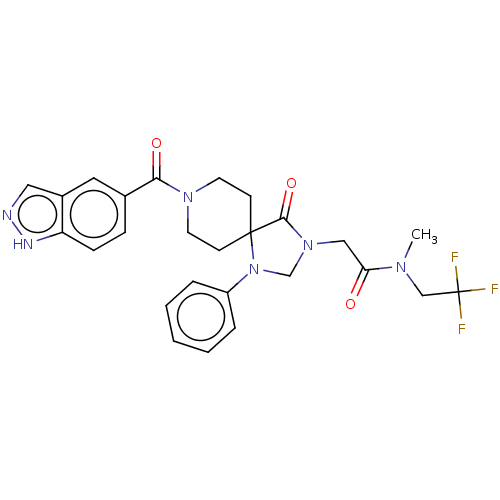 (2-(8-(1H-Indazole-5-carbonyl)-4-oxo-1-phenyl-1,3,8...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 18.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372045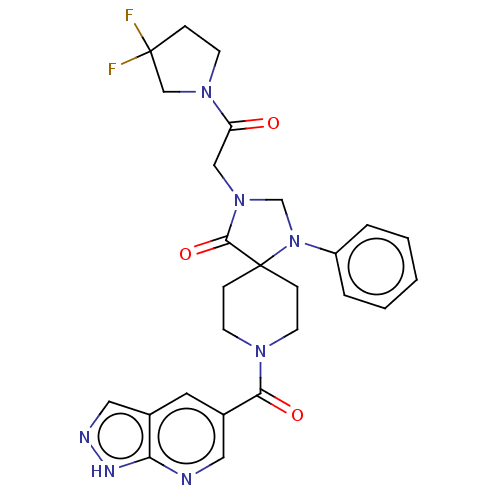 (3-[2-(3,3-Difluoropyrrolidin-1-yl)-2-oxoethyl]-1-p...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372110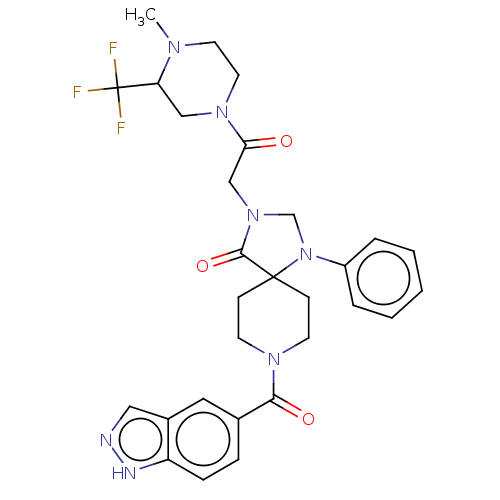 (8-(1H-Indazole-5-carbonyl)-3-[2-[4-methyl-3-(trifl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372046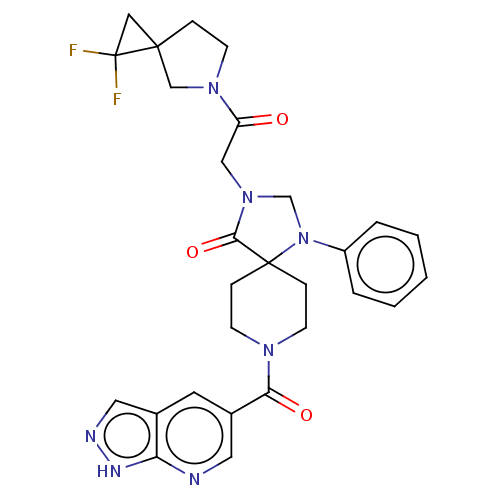 ((RS)-3-[2-(2,2-Difluoro-5-azaspiro[2.4]heptan-5-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.3 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372068 (2-(4-Oxo-1-phenyl-8-(1H-pyrazolo[4,3-b]pyridine-5-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM371875 (2-(4-Oxo-1-phenyl-8-(1H-pyrazolo[3,4-b]pyridine-5-...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 19.7 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372081 (2-(8-(3-Amino-1H-indazole-5-carbonyl)-4-oxo-1-phen...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372090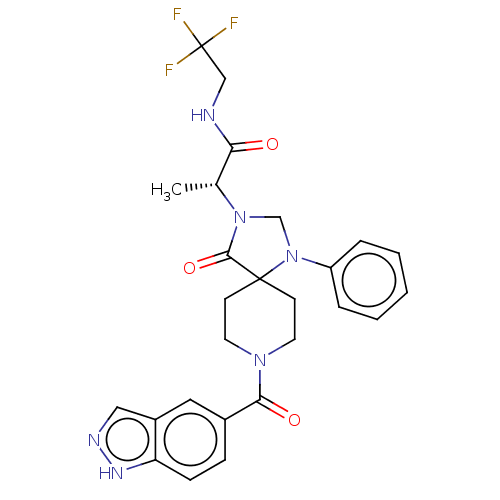 ((−)-2-[8-(1H-Indazole-5-carbonyl)-4-oxo-1-ph...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| D(1A) dopamine receptor (Homo sapiens (Human)) | BDBM372114 (N-(2-(Morpholinomethyl)phenyl)-2-(4-oxo-1-phenyl-8...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 23.1 | n/a | n/a | n/a | n/a | n/a | n/a |
Georgia Institute of Technology | Assay Description This assay is based on the intracellular domain of the DDR1 protein which contains the kinase active site. The recombinant protein additionally carri... | J Med Chem 51: 2816-32 (2008) BindingDB Entry DOI: 10.7270/Q2D220ZB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 369 total ) | Next | Last >> |