 Found 907 hits of ic50 for UniProtKB: P10632
Found 907 hits of ic50 for UniProtKB: P10632 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kcal/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50523585
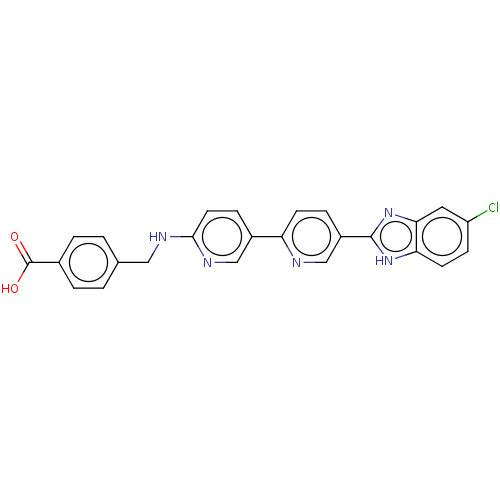
(CHEMBL4577468)Show SMILES OC(=O)c1ccc(CNc2ccc(cn2)-c2ccc(cn2)-c2nc3cc(Cl)ccc3[nH]2)cc1 Show InChI InChI=1S/C25H18ClN5O2/c26-19-7-9-21-22(11-19)31-24(30-21)18-5-8-20(27-14-18)17-6-10-23(29-13-17)28-12-15-1-3-16(4-2-15)25(32)33/h1-11,13-14H,12H2,(H,28,29)(H,30,31)(H,32,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck & Co. Inc
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 29: 1380-1385 (2019)
Article DOI: 10.1016/j.bmcl.2019.03.039
BindingDB Entry DOI: 10.7270/Q2348PSJ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239250

(CHEMBL4086080)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1ccncc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-3-4-22(30(37)36-32(13-14-32)23-11-15-35-16-12-23)18-25(19)21-7-10-27-26(17-21)28(31(38)34-2)29(39-27)20-5-8-24(33)9-6-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384467
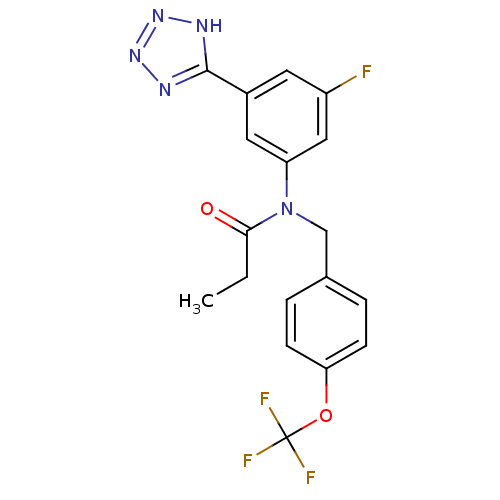
(CHEMBL2036213)Show SMILES CCC(=O)N(Cc1ccc(OC(F)(F)F)cc1)c1cc(F)cc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C18H15F4N5O2/c1-2-16(28)27(10-11-3-5-15(6-4-11)29-18(20,21)22)14-8-12(7-13(19)9-14)17-23-25-26-24-17/h3-9H,2,10H2,1H3,(H,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM155255
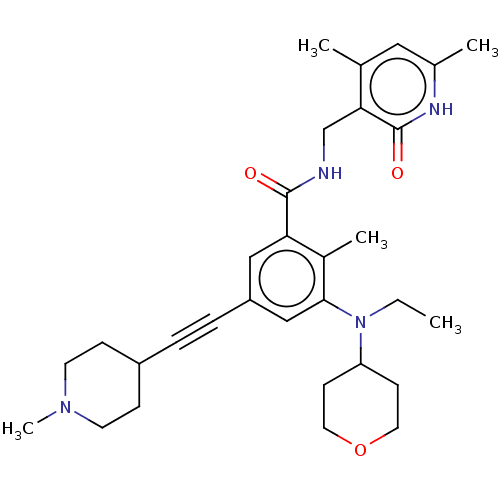
(US10098888, Compound 105 | US11642348, Compound 10...)Show SMILES CCN(C1CCOCC1)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)C#CC1CCN(C)CC1 Show InChI InChI=1S/C31H42N4O3/c1-6-35(26-11-15-38-16-12-26)29-19-25(8-7-24-9-13-34(5)14-10-24)18-27(23(29)4)30(36)32-20-28-21(2)17-22(3)33-31(28)37/h17-19,24,26H,6,9-16,20H2,1-5H3,(H,32,36)(H,33,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 47.4 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc.
US Patent
| Assay Description
The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... |
US Patent US10098888 (2018)
BindingDB Entry DOI: 10.7270/Q2BK1FDQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50123453
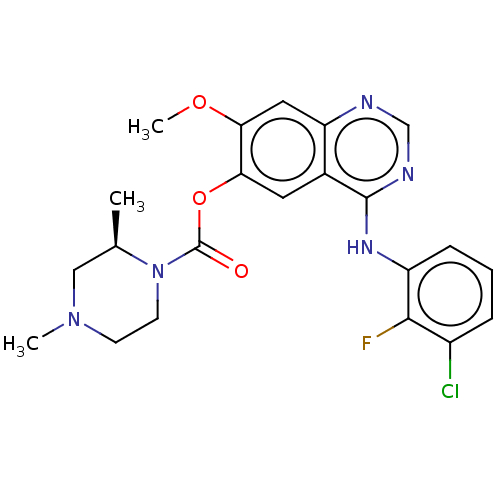
(CHEMBL3623290)Show SMILES COc1cc2ncnc(Nc3cccc(Cl)c3F)c2cc1OC(=O)N1CCN(C)C[C@H]1C |r| Show InChI InChI=1S/C22H23ClFN5O3/c1-13-11-28(2)7-8-29(13)22(30)32-19-9-14-17(10-18(19)31-3)25-12-26-21(14)27-16-6-4-5-15(23)20(16)24/h4-6,9-10,12-13H,7-8,11H2,1-3H3,(H,25,26,27)/t13-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | >50 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
J Med Chem 58: 8200-15 (2015)
Article DOI: 10.1021/acs.jmedchem.5b01073
BindingDB Entry DOI: 10.7270/Q29P33FH |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384475
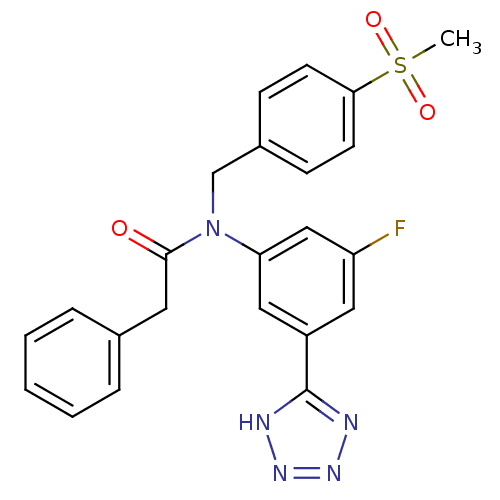
(CHEMBL2036225)Show SMILES CS(=O)(=O)c1ccc(CN(C(=O)Cc2ccccc2)c2cc(F)cc(c2)-c2nnn[nH]2)cc1 Show InChI InChI=1S/C23H20FN5O3S/c1-33(31,32)21-9-7-17(8-10-21)15-29(22(30)11-16-5-3-2-4-6-16)20-13-18(12-19(24)14-20)23-25-27-28-26-23/h2-10,12-14H,11,15H2,1H3,(H,25,26,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384472
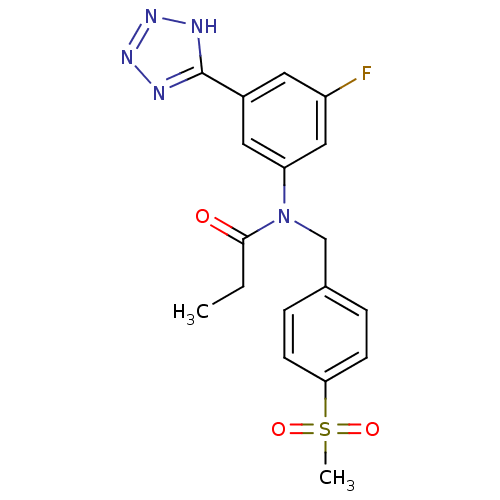
(CHEMBL2036220)Show SMILES CCC(=O)N(Cc1ccc(cc1)S(C)(=O)=O)c1cc(F)cc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C18H18FN5O3S/c1-3-17(25)24(11-12-4-6-16(7-5-12)28(2,26)27)15-9-13(8-14(19)10-15)18-20-22-23-21-18/h4-10H,3,11H2,1-2H3,(H,20,21,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384473
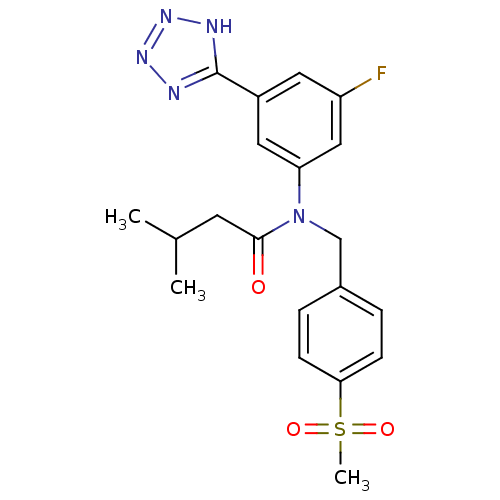
(CHEMBL2036222)Show SMILES CC(C)CC(=O)N(Cc1ccc(cc1)S(C)(=O)=O)c1cc(F)cc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C20H22FN5O3S/c1-13(2)8-19(27)26(12-14-4-6-18(7-5-14)30(3,28)29)17-10-15(9-16(21)11-17)20-22-24-25-23-20/h4-7,9-11,13H,8,12H2,1-3H3,(H,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50533229
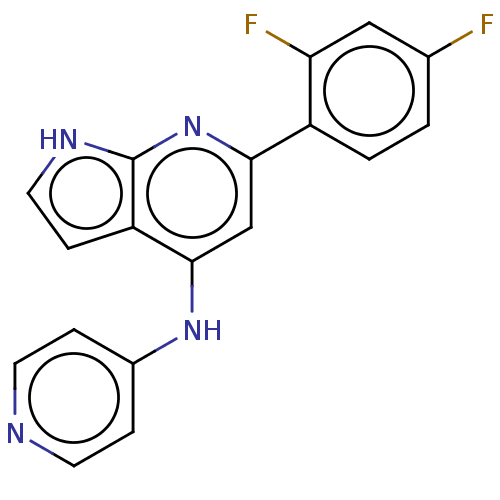
(CHEMBL4446613)Show InChI InChI=1S/C18H12F2N4/c19-11-1-2-13(15(20)9-11)16-10-17(14-5-8-22-18(14)24-16)23-12-3-6-21-7-4-12/h1-10H,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 26: 4334-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.030
BindingDB Entry DOI: 10.7270/Q2NV9NQT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50533229

(CHEMBL4446613)Show InChI InChI=1S/C18H12F2N4/c19-11-1-2-13(15(20)9-11)16-10-17(14-5-8-22-18(14)24-16)23-12-3-6-21-7-4-12/h1-10H,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 79 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 26: 4334-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.030
BindingDB Entry DOI: 10.7270/Q2NV9NQT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384464
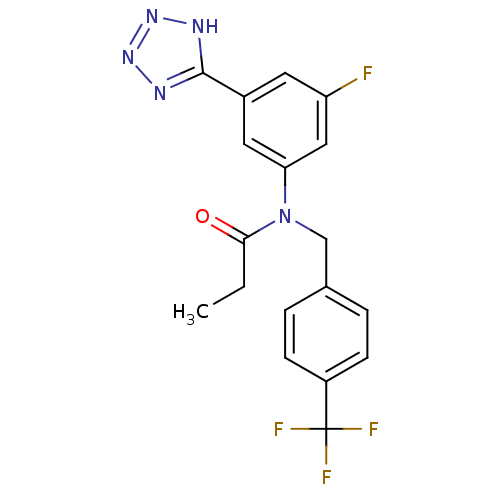
(CHEMBL2036210)Show SMILES CCC(=O)N(Cc1ccc(cc1)C(F)(F)F)c1cc(F)cc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C18H15F4N5O/c1-2-16(28)27(10-11-3-5-13(6-4-11)18(20,21)22)15-8-12(7-14(19)9-15)17-23-25-26-24-17/h3-9H,2,10H2,1H3,(H,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM155253
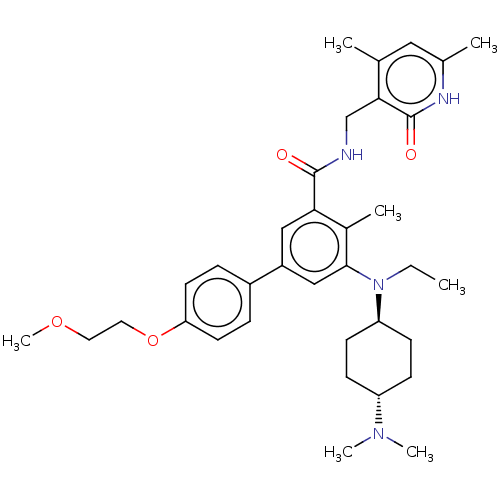
(US10098888, Compound 1 | US9006242, 1)Show SMILES CCN([C@H]1CC[C@@H](CC1)N(C)C)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)-c1ccc(OCCOC)cc1 |r,wU:3.2,wD:6.9,(-6.67,1.54,;-5.33,.77,;-4,1.54,;-4,3.08,;-5.33,3.85,;-5.33,5.39,;-4,6.16,;-2.67,5.39,;-2.67,3.85,;-4,7.7,;-5.33,8.47,;-2.67,8.47,;-2.67,.77,;-1.33,1.54,;,.77,;,-.77,;-1.33,-1.54,;-1.33,-3.08,;,-3.85,;-2.67,-3.85,;-2.67,-5.39,;-4,-6.16,;-4,-7.7,;-2.67,-8.47,;-5.33,-8.47,;-6.67,-7.7,;-8,-8.47,;-6.67,-6.16,;-5.33,-5.39,;-5.33,-3.85,;-2.67,-.77,;-4,-1.54,;1.33,1.54,;2.67,.77,;4,1.54,;4,3.08,;5.33,3.85,;6.67,3.08,;6.67,1.54,;8,.77,;8,-.77,;2.67,3.85,;1.33,3.08,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc.
US Patent
| Assay Description
The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... |
US Patent US10098888 (2018)
BindingDB Entry DOI: 10.7270/Q2BK1FDQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50090462
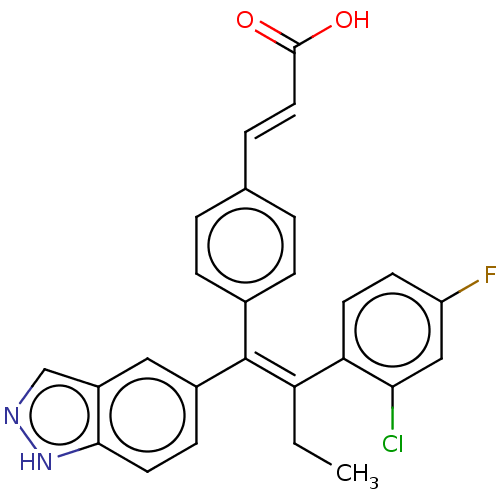
(CHEMBL3581693 | US20240043442, Example GDC-0810)Show SMILES CC\C(=C(\c1ccc(\C=C\C(O)=O)cc1)c1ccc2[nH]ncc2c1)c1ccc(F)cc1Cl Show InChI InChI=1S/C26H20ClFN2O2/c1-2-21(22-10-9-20(28)14-23(22)27)26(18-8-11-24-19(13-18)15-29-30-24)17-6-3-16(4-7-17)5-12-25(31)32/h3-15H,2H2,1H3,(H,29,30)(H,31,32)/b12-5+,26-21+ | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <100 | n/a | n/a | n/a | n/a | n/a | n/a |
Seragon Pharmaceuticals
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
J Med Chem 58: 4888-904 (2015)
Article DOI: 10.1021/acs.jmedchem.5b00054
BindingDB Entry DOI: 10.7270/Q29W0H7Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM155254
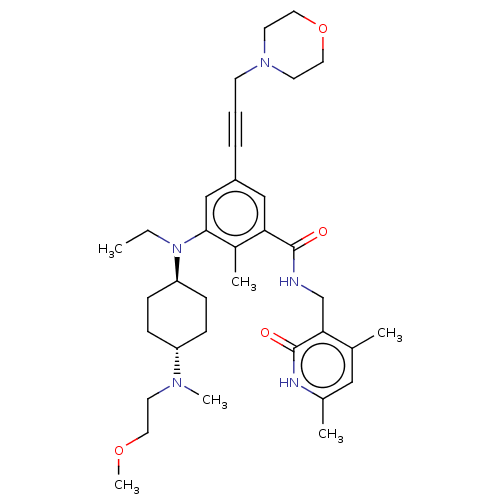
(US10098888, Compound 2 | US9006242, 2)Show SMILES CCN([C@H]1CC[C@@H](CC1)N(C)CCOC)c1cc(cc(C(=O)NCc2c(C)cc(C)[nH]c2=O)c1C)C#CCN1CCOCC1 |r,wU:3.2,wD:6.9,(-6,1.54,;-4.67,.77,;-3.33,1.54,;-3.33,3.08,;-4.67,3.85,;-4.67,5.39,;-3.33,6.16,;-2,5.39,;-2,3.85,;-3.33,7.7,;-2,8.47,;-4.67,8.47,;-6,7.7,;-7.34,8.47,;-8.67,7.7,;-2,.77,;-.67,1.54,;.67,.77,;.67,-.77,;-.67,-1.54,;-.67,-3.08,;.67,-3.85,;-2,-3.85,;-2,-5.39,;-3.33,-6.16,;-3.33,-7.7,;-2,-8.47,;-4.67,-8.47,;-6,-7.7,;-7.34,-8.47,;-6,-6.16,;-4.67,-5.39,;-4.67,-3.85,;-2,-.77,;-3.33,-1.54,;2,1.54,;3.33,2.31,;4.67,3.08,;6,2.31,;6,.77,;7.34,,;8.67,.77,;8.67,2.31,;7.34,3.08,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | >100 | n/a | n/a | n/a | n/a | n/a | n/a |
Epizyme, Inc.
US Patent
| Assay Description
The potential inhibition of enzyme activities of human cytochromes P450 (CYP) of Compound 1, 2, or 105 was evaluated using pooled human liver microso... |
US Patent US10098888 (2018)
BindingDB Entry DOI: 10.7270/Q2BK1FDQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50463725

(CHEMBL4242598)Show SMILES [H][C@]12CN(c3ccc(C(N)=O)c(Oc4ccc(Oc5ccccc5)cc4)n3)[C@]([H])(CN1C(=O)C=C)C2 |r| Show InChI InChI=1S/C26H24N4O4/c1-2-24(31)30-16-17-14-18(30)15-29(17)23-13-12-22(25(27)32)26(28-23)34-21-10-8-20(9-11-21)33-19-6-4-3-5-7-19/h2-13,17-18H,1,14-16H2,(H2,27,32)/t17-,18-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
EMD Serono Research & Development Institute, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 28: 2939-2944 (2018)
Article DOI: 10.1016/j.bmcl.2018.07.008
BindingDB Entry DOI: 10.7270/Q2QR50TC |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50533234
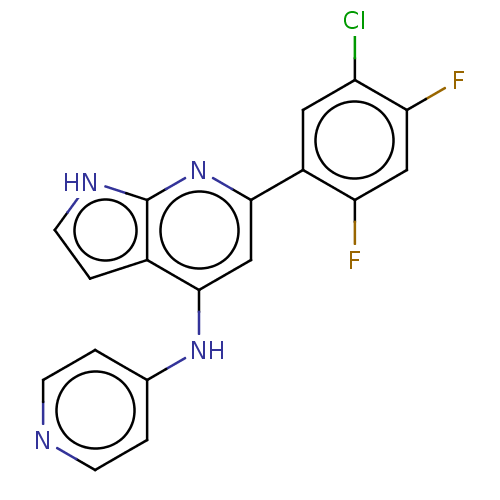
(CHEMBL4437930)Show SMILES Fc1cc(F)c(cc1Cl)-c1cc(Nc2ccncc2)c2cc[nH]c2n1 Show InChI InChI=1S/C18H11ClF2N4/c19-13-7-12(14(20)8-15(13)21)17-9-16(11-3-6-23-18(11)25-17)24-10-1-4-22-5-2-10/h1-9H,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 26: 4334-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.030
BindingDB Entry DOI: 10.7270/Q2NV9NQT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50533234

(CHEMBL4437930)Show SMILES Fc1cc(F)c(cc1Cl)-c1cc(Nc2ccncc2)c2cc[nH]c2n1 Show InChI InChI=1S/C18H11ClF2N4/c19-13-7-12(14(20)8-15(13)21)17-9-16(11-3-6-23-18(11)25-17)24-10-1-4-22-5-2-10/h1-9H,(H2,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 26: 4334-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.030
BindingDB Entry DOI: 10.7270/Q2NV9NQT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50438845
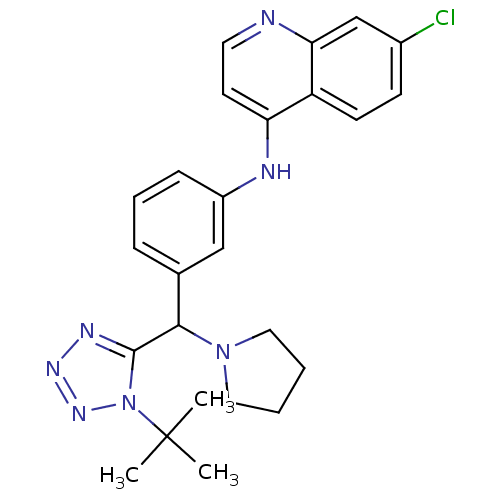
(CHEMBL2413882)Show SMILES CC(C)(C)n1nnnc1C(N1CCCC1)c1cccc(Nc2ccnc3cc(Cl)ccc23)c1 Show InChI InChI=1S/C25H28ClN7/c1-25(2,3)33-24(29-30-31-33)23(32-13-4-5-14-32)17-7-6-8-19(15-17)28-21-11-12-27-22-16-18(26)9-10-20(21)22/h6-12,15-16,23H,4-5,13-14H2,1-3H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 120 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 20 mins by LC-MS analysis |
Bioorg Med Chem 21: 4904-13 (2013)
Article DOI: 10.1016/j.bmc.2013.06.067
BindingDB Entry DOI: 10.7270/Q2WH2RD3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50052024

(CHEMBL787 | montelukast)Show SMILES CC(C)(O)c1ccccc1CC[C@@H](SCC1(CC(O)=O)CC1)c1cccc(\C=C\c2ccc3ccc(Cl)cc3n2)c1 |r| Show InChI InChI=1S/C35H36ClNO3S/c1-34(2,40)30-9-4-3-7-25(30)13-17-32(41-23-35(18-19-35)22-33(38)39)27-8-5-6-24(20-27)10-15-29-16-12-26-11-14-28(36)21-31(26)37-29/h3-12,14-16,20-21,32,40H,13,17-19,22-23H2,1-2H3,(H,38,39)/b15-10+/t32-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
PDB
Article
PubMed
| n/a | n/a | 130 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c02071
BindingDB Entry DOI: 10.7270/Q2833X3S |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50175303
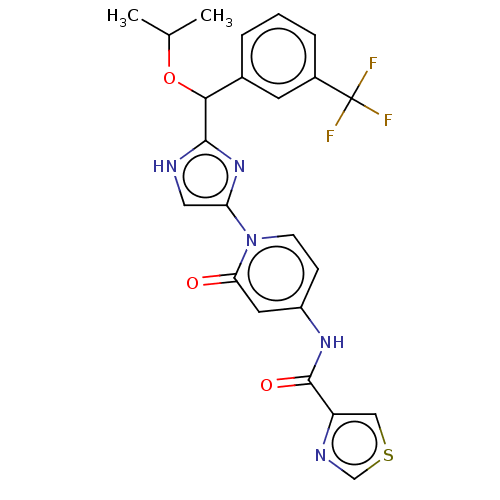
(CHEMBL3810042)Show SMILES CC(C)OC(c1nc(c[nH]1)-n1ccc(NC(=O)c2cscn2)cc1=O)c1cccc(c1)C(F)(F)F Show InChI InChI=1S/C23H20F3N5O3S/c1-13(2)34-20(14-4-3-5-15(8-14)23(24,25)26)21-27-10-18(30-21)31-7-6-16(9-19(31)32)29-22(33)17-11-35-12-28-17/h3-13,20H,1-2H3,(H,27,30)(H,29,33) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer Ingelheim (Canada) Ltd.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
ACS Med Chem Lett 7: 525-30 (2016)
Article DOI: 10.1021/acsmedchemlett.6b00064
BindingDB Entry DOI: 10.7270/Q24B337T |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50265673
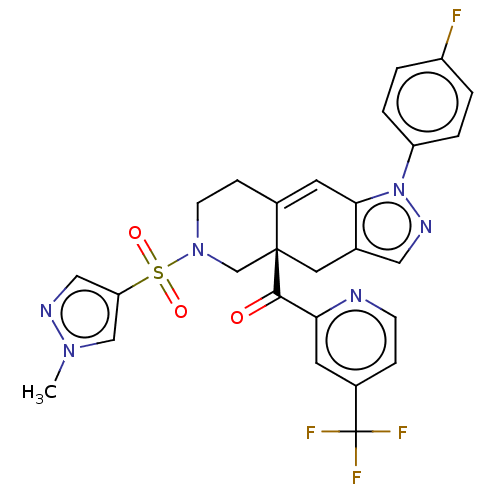
(CHEMBL4068611)Show SMILES Cn1cc(cn1)S(=O)(=O)N1CCC2=Cc3c(C[C@@]2(C1)C(=O)c1cc(ccn1)C(F)(F)F)cnn3-c1ccc(F)cc1 |r,t:13| Show InChI InChI=1S/C27H22F4N6O3S/c1-35-15-22(14-33-35)41(39,40)36-9-7-18-11-24-17(13-34-37(24)21-4-2-20(28)3-5-21)12-26(18,16-36)25(38)23-10-19(6-8-32-23)27(29,30)31/h2-6,8,10-11,13-15H,7,9,12,16H2,1H3/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 210 | n/a | n/a | n/a | n/a | n/a | n/a |
Corcept Therapeutics
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
J Med Chem 60: 3405-3421 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00162
BindingDB Entry DOI: 10.7270/Q27083X5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384474
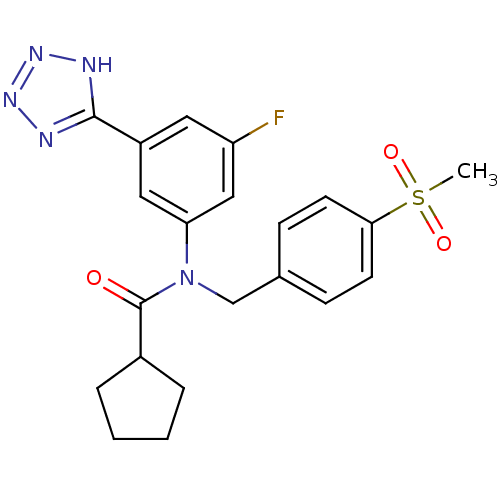
(CHEMBL2036223)Show SMILES CS(=O)(=O)c1ccc(CN(C(=O)C2CCCC2)c2cc(F)cc(c2)-c2nnn[nH]2)cc1 Show InChI InChI=1S/C21H22FN5O3S/c1-31(29,30)19-8-6-14(7-9-19)13-27(21(28)15-4-2-3-5-15)18-11-16(10-17(22)12-18)20-23-25-26-24-20/h6-12,15H,2-5,13H2,1H3,(H,23,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50253810
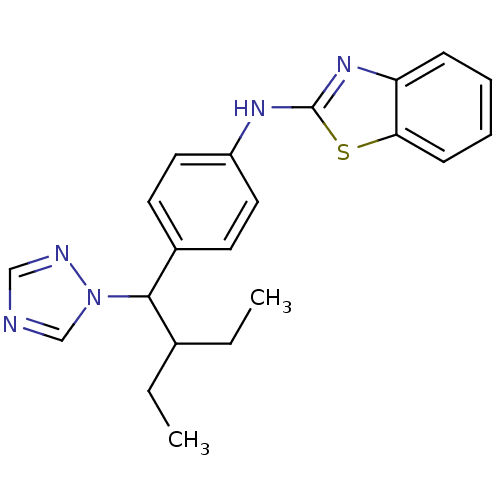
(CHEMBL459505 | N-(4-(2-ethyl-1-(1H-1,2,4-triazol-1...)Show InChI InChI=1S/C21H23N5S/c1-3-15(4-2)20(26-14-22-13-23-26)16-9-11-17(12-10-16)24-21-25-18-7-5-6-8-19(18)27-21/h5-15,20H,3-4H2,1-2H3,(H,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle
| Assay Description
Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... |
J Med Chem 52: 1864-72 (2009)
BindingDB Entry DOI: 10.7270/Q2FN18J2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50438846
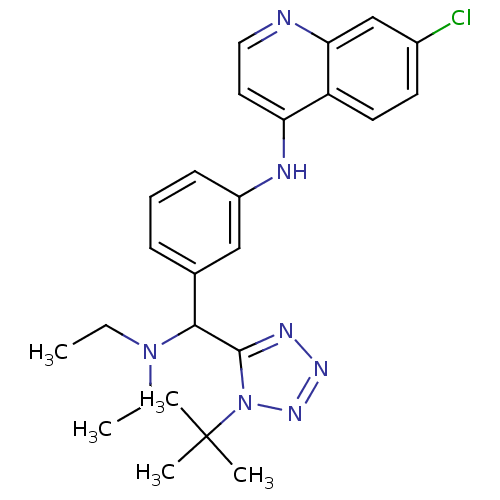
(CHEMBL2413881)Show SMILES CCN(CC)C(c1nnnn1C(C)(C)C)c1cccc(Nc2ccnc3cc(Cl)ccc23)c1 Show InChI InChI=1S/C25H30ClN7/c1-6-32(7-2)23(24-29-30-31-33(24)25(3,4)5)17-9-8-10-19(15-17)28-21-13-14-27-22-16-18(26)11-12-20(21)22/h8-16,23H,6-7H2,1-5H3,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Cape Town
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes assessed as paclitaxel 6alpha-hydroxylation after 20 mins by LC-MS analysis |
Bioorg Med Chem 21: 4904-13 (2013)
Article DOI: 10.1016/j.bmc.2013.06.067
BindingDB Entry DOI: 10.7270/Q2WH2RD3 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50380508

(CHEMBL2018923)Show InChI InChI=1S/C21H18N2OS/c1-24-18-10-11-19-20(13-18)23-21(22-19)25-14-15-6-5-9-17(12-15)16-7-3-2-4-8-16/h2-13H,14H2,1H3,(H,22,23) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 5 mins by LC-MS/MS analysis |
J Med Chem 55: 1205-14 (2012)
Article DOI: 10.1021/jm201346g
BindingDB Entry DOI: 10.7270/Q2QV3NHG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50366401

(CHEMBL4169721)Show InChI InChI=1S/C13H16N2O/c1-2-3-10-6-7-15-9-12(10)13-5-4-11(8-14)16-13/h4-7,9H,2-3,8,14H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Washington State University
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate preincubated for 5 mins followed by addition of NADPH-regenerating syst... |
J Med Chem 61: 7065-7086 (2018)
Article DOI: 10.1021/acs.jmedchem.8b00084
BindingDB Entry DOI: 10.7270/Q2QN69B5 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50614704
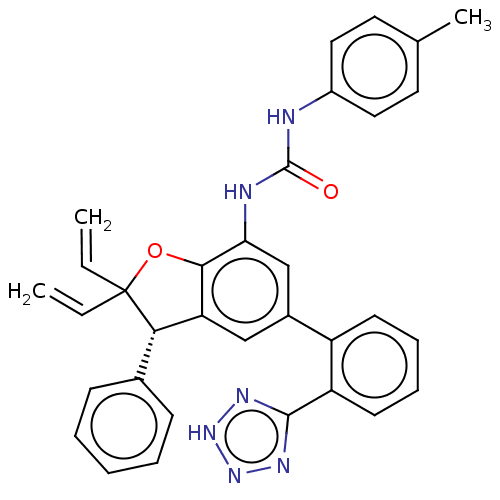
(CHEMBL5279540) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| UniChem
| | n/a | n/a | 270 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM253985
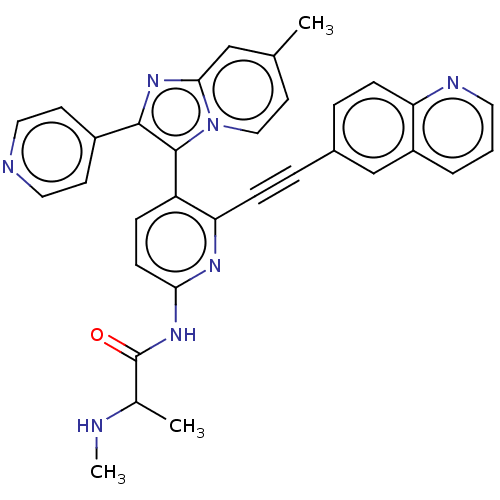
(US9481673, 27)Show SMILES CNC(C)C(=O)Nc1ccc(-c2c(nc3cc(C)ccn23)-c2ccncc2)c(n1)C#Cc1ccc2ncccc2c1 |(-6,-4.53,;-7.34,-3.76,;-7.34,-2.22,;-8.67,-1.45,;-6,-1.45,;-6,.09,;-4.67,-2.22,;-3.33,-1.45,;-3.33,.09,;-2,.86,;-.67,.09,;.67,.86,;.67,2.4,;2.13,2.88,;3.04,1.63,;4.57,1.47,;5.19,.06,;6.73,-.1,;4.29,-1.18,;2.76,-1.02,;2.13,.39,;-.42,3.49,;-.02,4.98,;-1.11,6.07,;-2.6,5.67,;-3,4.18,;-1.91,3.09,;-.67,-1.45,;-2,-2.22,;.67,-2.22,;2,-2.99,;3.33,-3.76,;3.33,-5.3,;4.67,-6.07,;6,-5.3,;7.34,-6.07,;8.67,-5.3,;8.67,-3.76,;7.34,-2.99,;6,-3.76,;4.67,-2.99,)| Show InChI InChI=1S/C33H27N7O/c1-21-14-18-40-30(19-21)39-31(24-12-16-35-17-13-24)32(40)26-8-11-29(38-33(41)22(2)34-3)37-28(26)10-7-23-6-9-27-25(20-23)5-4-15-36-27/h4-6,8-9,11-20,22,34H,1-3H3,(H,37,38,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer ingelheim International GmbH
US Patent
| Assay Description
The final incubation volume contains TRIS buffer (0.1 M), MgCl2 (5 mM), a certain concentration of human liver microsomes dependent on the P450 isoen... |
US Patent US9481673 (2016)
BindingDB Entry DOI: 10.7270/Q24M93G0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM253988
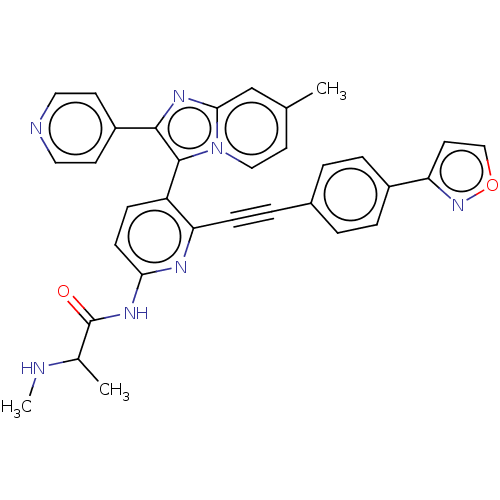
(US9481673, 82)Show SMILES CNC(C)C(=O)Nc1ccc(-c2c(nc3cc(C)ccn23)-c2ccncc2)c(n1)C#Cc1ccc(cc1)-c1ccon1 |(-6.52,-3.52,;-7.85,-2.75,;-7.85,-1.21,;-9.19,-.44,;-6.52,-.44,;-6.52,1.1,;-5.19,-1.21,;-3.85,-.44,;-3.85,1.1,;-2.52,1.87,;-1.18,1.1,;.15,1.87,;.15,3.41,;1.61,3.89,;2.52,2.64,;4.05,2.48,;4.68,1.07,;6.21,.91,;3.77,-.17,;2.24,-.01,;1.61,1.39,;-.94,4.5,;-.54,5.99,;-1.63,7.08,;-3.12,6.68,;-3.52,5.19,;-2.43,4.1,;-1.18,-.44,;-2.52,-1.21,;.15,-1.21,;1.48,-1.98,;2.82,-2.75,;2.82,-4.29,;4.15,-5.06,;5.48,-4.29,;5.48,-2.75,;4.15,-1.98,;6.82,-5.06,;6.82,-6.6,;8.28,-7.08,;9.19,-5.83,;8.28,-4.58,)| Show InChI InChI=1S/C33H27N7O2/c1-21-14-18-40-30(20-21)38-31(25-12-16-35-17-13-25)32(40)26-9-11-29(37-33(41)22(2)34-3)36-28(26)10-6-23-4-7-24(8-5-23)27-15-19-42-39-27/h4-5,7-9,11-20,22,34H,1-3H3,(H,36,37,41) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer ingelheim International GmbH
US Patent
| Assay Description
The final incubation volume contains TRIS buffer (0.1 M), MgCl2 (5 mM), a certain concentration of human liver microsomes dependent on the P450 isoen... |
US Patent US9481673 (2016)
BindingDB Entry DOI: 10.7270/Q24M93G0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50238082
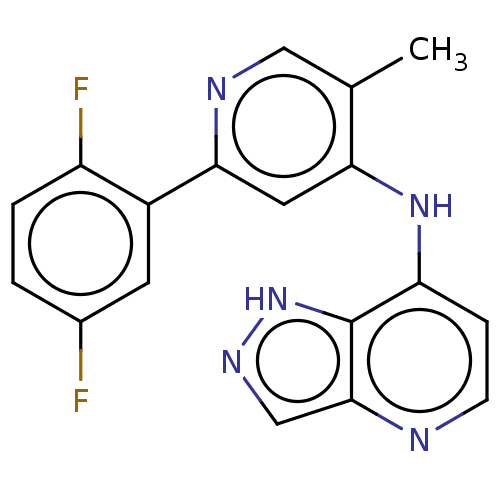
(CHEMBL4102696)Show InChI InChI=1S/C18H13F2N5/c1-10-8-22-16(12-6-11(19)2-3-13(12)20)7-15(10)24-14-4-5-21-17-9-23-25-18(14)17/h2-9H,1H3,(H,23,25)(H,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 316 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 27: 1955-1961 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.026
BindingDB Entry DOI: 10.7270/Q2QJ7KKZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384469
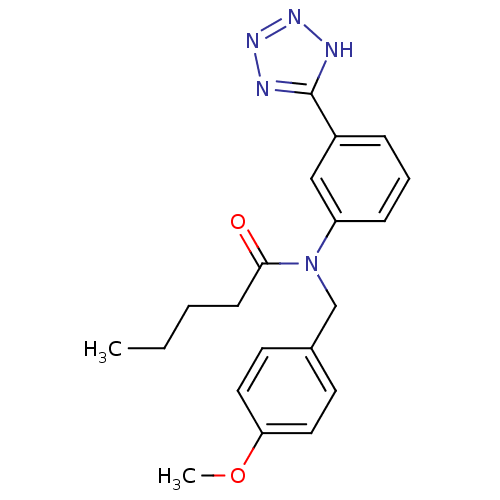
(CHEMBL2036017)Show SMILES CCCCC(=O)N(Cc1ccc(OC)cc1)c1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C20H23N5O2/c1-3-4-8-19(26)25(14-15-9-11-18(27-2)12-10-15)17-7-5-6-16(13-17)20-21-23-24-22-20/h5-7,9-13H,3-4,8,14H2,1-2H3,(H,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM639341
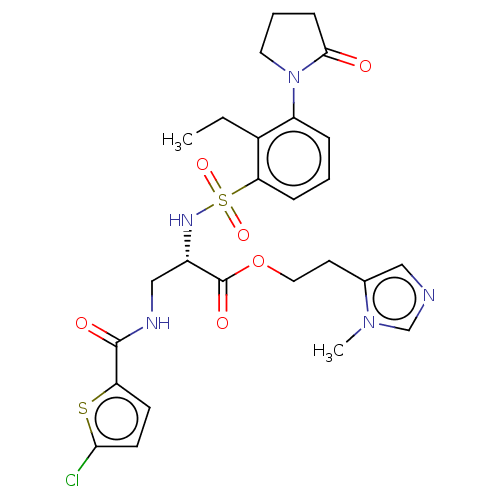
(2-(1-Methyl-1H-imidazol-5-yl)ethyl 3-[(5-chlorothi...)Show SMILES CCc1c(cccc1S(=O)(=O)N[C@@H](CNC(=O)c1ccc(Cl)s1)C(=O)OCCc1cncn1C)N1CCCC1=O |r| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| | n/a | n/a | 340 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
|
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50239251

(CHEMBL4096241)Show SMILES CNC(=O)c1c(oc2ccc(cc12)-c1cc(ccc1C)C(=O)NC1(CC1)c1cccnc1)-c1ccc(F)cc1 Show InChI InChI=1S/C32H26FN3O3/c1-19-5-6-22(30(37)36-32(13-14-32)23-4-3-15-35-18-23)17-25(19)21-9-12-27-26(16-21)28(31(38)34-2)29(39-27)20-7-10-24(33)11-8-20/h3-12,15-18H,13-14H2,1-2H3,(H,34,38)(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 350 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Research and Development
Curated by ChEMBL
| Assay Description
Inhibition of microsomal CYP2C8 (unknown origin) |
J Med Chem 60: 4369-4385 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00328
BindingDB Entry DOI: 10.7270/Q20R9RKB |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50238081
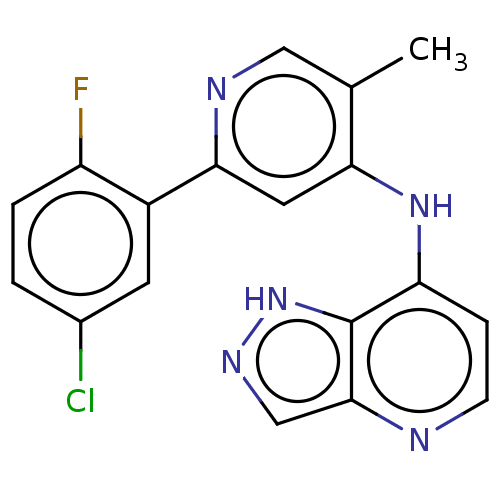
(CHEMBL4084771)Show InChI InChI=1S/C18H13ClFN5/c1-10-8-22-16(12-6-11(19)2-3-13(12)20)7-15(10)24-14-4-5-21-17-9-23-25-18(14)17/h2-9H,1H3,(H,23,25)(H,21,22,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 398 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 27: 1955-1961 (2017)
Article DOI: 10.1016/j.bmcl.2017.03.026
BindingDB Entry DOI: 10.7270/Q2QJ7KKZ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50440368

(CHEMBL2425146)Show SMILES Cc1ccc(cc1)-n1nc(cc1NC(=O)Nc1ccc(cc1)-n1ccnc1)C(C)(C)C Show InChI InChI=1S/C24H26N6O/c1-17-5-9-20(10-6-17)30-22(15-21(28-30)24(2,3)4)27-23(31)26-18-7-11-19(12-8-18)29-14-13-25-16-29/h5-16H,1-4H3,(H2,26,27,31) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck KGaA
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 23: 5401-9 (2013)
Article DOI: 10.1016/j.bmcl.2013.07.050
BindingDB Entry DOI: 10.7270/Q2891782 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50125972
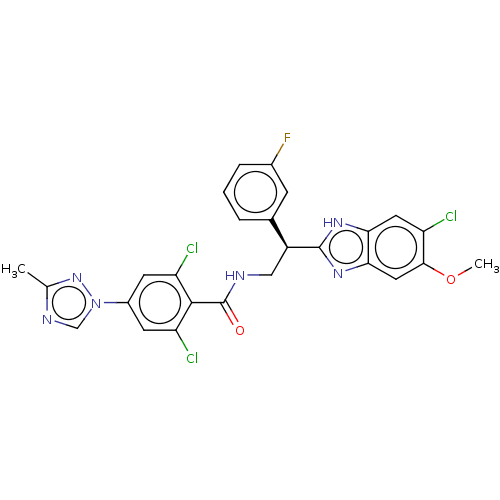
(CHEMBL3627894)Show SMILES COc1cc2nc([nH]c2cc1Cl)[C@@H](CNC(=O)c1c(Cl)cc(cc1Cl)-n1cnc(C)n1)c1cccc(F)c1 |r| Show InChI InChI=1S/C26H20Cl3FN6O2/c1-13-32-12-36(35-13)16-7-19(28)24(20(29)8-16)26(37)31-11-17(14-4-3-5-15(30)6-14)25-33-21-9-18(27)23(38-2)10-22(21)34-25/h3-10,12,17H,11H2,1-2H3,(H,31,37)(H,33,34)/t17-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 456 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 25: 4945-9 (2015)
Article DOI: 10.1016/j.bmcl.2015.04.057
BindingDB Entry DOI: 10.7270/Q20Z753B |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384470
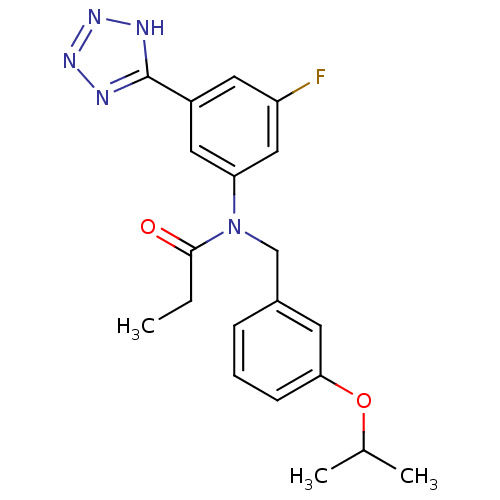
(CHEMBL2036030)Show SMILES CCC(=O)N(Cc1cccc(OC(C)C)c1)c1cc(F)cc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C20H22FN5O2/c1-4-19(27)26(12-14-6-5-7-18(8-14)28-13(2)3)17-10-15(9-16(21)11-17)20-22-24-25-23-20/h5-11,13H,4,12H2,1-3H3,(H,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 470 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50157605
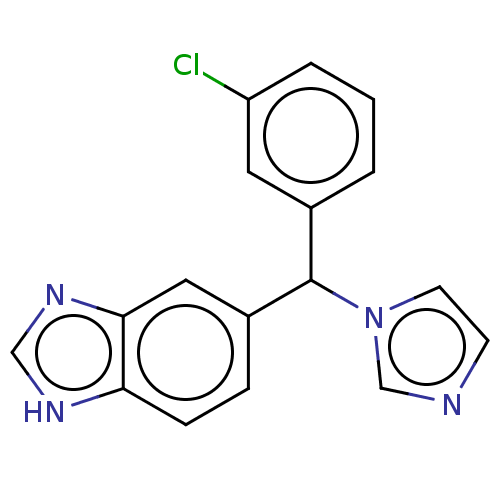
(Liarozole | Liazal | R-75251 | US9963439, Liarozol...)Show InChI InChI=1S/C17H13ClN4/c18-14-3-1-2-12(8-14)17(22-7-6-19-11-22)13-4-5-15-16(9-13)21-10-20-15/h1-11,17H,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| US Patent
| n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Washington at Seattle
| Assay Description
Compounds were assessed for inhibition (IC50, n=2) of CYP2C8, CYP2C9 and CYP3A4 in pooled human liver microsomes using selective probe substrates at ... |
J Med Chem 52: 1864-72 (2009)
BindingDB Entry DOI: 10.7270/Q2FN18J2 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM253986

(US9481673, 64)Show SMILES CNC(C)C(=O)Nc1ccc(-c2c(nc3cc(C)ccn23)-c2ccncc2)c(n1)C#Cc1ccc2nc(C)ccc2c1 |(-6.67,-4.53,;-8,-3.76,;-8,-2.22,;-9.34,-1.45,;-6.67,-1.45,;-6.67,.09,;-5.33,-2.22,;-4,-1.45,;-4,.09,;-2.67,.86,;-1.33,.09,;,.86,;,2.4,;1.46,2.88,;2.37,1.63,;3.9,1.47,;4.53,.06,;6.06,-.1,;3.62,-1.18,;2.09,-1.02,;1.46,.39,;-1.09,3.49,;-.69,4.98,;-1.78,6.07,;-3.27,5.67,;-3.67,4.18,;-2.58,3.09,;-1.33,-1.45,;-2.67,-2.22,;,-2.22,;1.33,-2.99,;2.67,-3.76,;2.67,-5.3,;4,-6.07,;5.33,-5.3,;6.67,-6.07,;8,-5.3,;9.34,-6.07,;8,-3.76,;6.67,-2.99,;5.33,-3.76,;4,-2.99,)| Show InChI InChI=1S/C34H29N7O/c1-21-15-18-41-31(19-21)40-32(25-13-16-36-17-14-25)33(41)27-9-12-30(39-34(42)23(3)35-4)38-29(27)11-7-24-6-10-28-26(20-24)8-5-22(2)37-28/h5-6,8-10,12-20,23,35H,1-4H3,(H,38,39,42) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Boehringer ingelheim International GmbH
US Patent
| Assay Description
The final incubation volume contains TRIS buffer (0.1 M), MgCl2 (5 mM), a certain concentration of human liver microsomes dependent on the P450 isoen... |
US Patent US9481673 (2016)
BindingDB Entry DOI: 10.7270/Q24M93G0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50275736
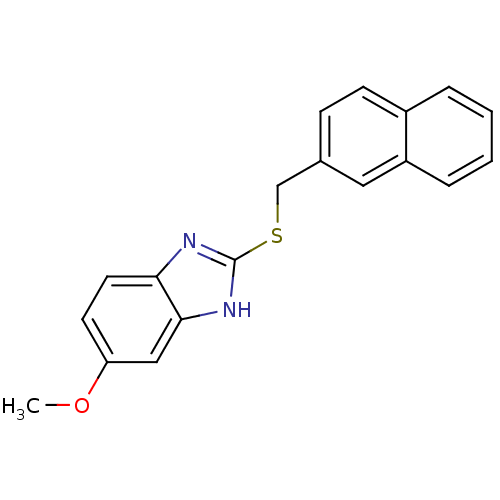
(6-methoxy-2-(naphthalen-2-ylmethylthio)-1H-benzo[d...)Show InChI InChI=1S/C19H16N2OS/c1-22-16-8-9-17-18(11-16)21-19(20-17)23-12-13-6-7-14-4-2-3-5-15(14)10-13/h2-11H,12H2,1H3,(H,20,21) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 510 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 5 mins by LC-MS/MS analysis |
J Med Chem 55: 1205-14 (2012)
Article DOI: 10.1021/jm201346g
BindingDB Entry DOI: 10.7270/Q2QV3NHG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50384471
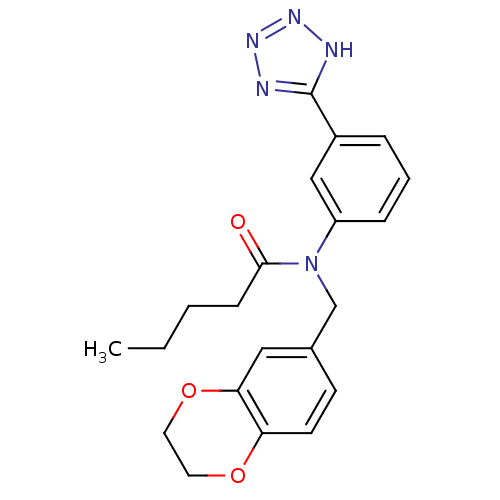
(CHEMBL2036203)Show SMILES CCCCC(=O)N(Cc1ccc2OCCOc2c1)c1cccc(c1)-c1nnn[nH]1 Show InChI InChI=1S/C21H23N5O3/c1-2-3-7-20(27)26(14-15-8-9-18-19(12-15)29-11-10-28-18)17-6-4-5-16(13-17)21-22-24-25-23-21/h4-6,8-9,12-13H,2-3,7,10-11,14H2,1H3,(H,22,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
Curated by ChEMBL
| Assay Description
Inhibition of human recombinant CYP2C8 incubated for 15 mins prior to substrate addition measured after 30 mins by spectrophotometry |
ACS Med Chem Lett 2: 938-942 (2011)
Article DOI: 10.1021/ml200223s
BindingDB Entry DOI: 10.7270/Q2Z89DGQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50586189

(CHEMBL5086239)Show SMILES C(N1CCC(CC1)c1nc(cs1)-c1cc2ccccc2o1)c1nc2cnccc2[nH]1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis |
Citation and Details
Article DOI: 10.1021/acsmedchemlett.1c00666
BindingDB Entry DOI: 10.7270/Q28G8QMQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50533237

(CHEMBL4526347)Show InChI InChI=1S/C18H12F2N4/c19-14-2-1-11(9-15(14)20)16-10-17(13-5-8-22-18(13)24-16)23-12-3-6-21-7-4-12/h1-10H,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 26: 4334-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.030
BindingDB Entry DOI: 10.7270/Q2NV9NQT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50533237

(CHEMBL4526347)Show InChI InChI=1S/C18H12F2N4/c19-14-2-1-11(9-15(14)20)16-10-17(13-5-8-22-18(13)24-16)23-12-3-6-21-7-4-12/h1-10H,(H2,21,22,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 631 | n/a | n/a | n/a | n/a | n/a | n/a |
Takeda California
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Bioorg Med Chem Lett 26: 4334-9 (2016)
Article DOI: 10.1016/j.bmcl.2016.07.030
BindingDB Entry DOI: 10.7270/Q2NV9NQT |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50380511
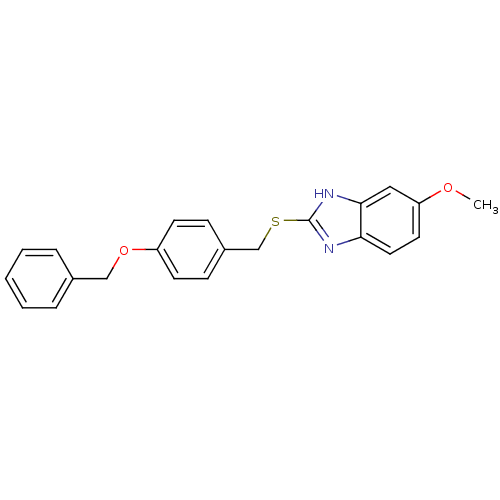
(CHEMBL2018926)Show InChI InChI=1S/C22H20N2O2S/c1-25-19-11-12-20-21(13-19)24-22(23-20)27-15-17-7-9-18(10-8-17)26-14-16-5-3-2-4-6-16/h2-13H,14-15H2,1H3,(H,23,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate preincubated for 5 mins by LC-MS/MS analysis |
J Med Chem 55: 1205-14 (2012)
Article DOI: 10.1021/jm201346g
BindingDB Entry DOI: 10.7270/Q2QV3NHG |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM253135
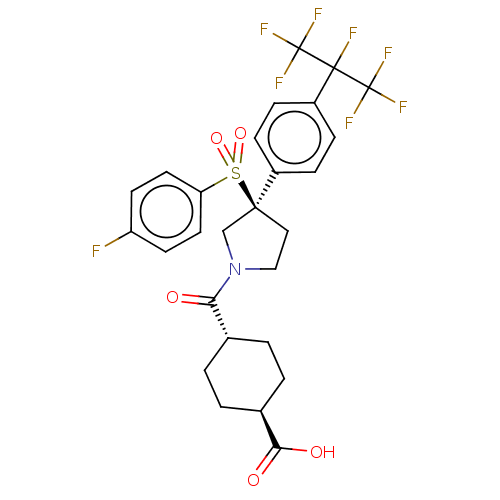
(US9458171, 95)Show SMILES OC(=O)[C@H]1CC[C@@H](CC1)C(=O)N1CC[C@](C1)(c1ccc(cc1)C(F)(C(F)(F)F)C(F)(F)F)S(=O)(=O)c1ccc(F)cc1 |r,wU:14.17,3.2,wD:6.9,(.2,-10.82,;-.7,-9.58,;-2.23,-9.74,;-.07,-8.17,;-.98,-6.92,;-.35,-5.52,;1.18,-5.36,;2.08,-6.6,;1.46,-8.01,;1.8,-3.95,;3.34,-3.79,;.9,-2.7,;-.64,-2.7,;-1.12,-1.24,;.13,-.33,;1.38,-1.24,;-.9,.81,;-.43,2.28,;-1.46,3.42,;-2.96,3.1,;-3.44,1.64,;-2.41,.49,;-3.99,4.24,;-5.02,5.39,;-5.14,3.21,;-6.28,2.18,;-6.17,4.36,;-4.11,2.07,;-2.85,5.28,;-1.7,6.31,;-1.82,4.13,;-3.88,6.42,;1.16,.81,;2.3,-.22,;.02,1.84,;2.19,1.96,;1.71,3.42,;2.74,4.56,;4.25,4.24,;5.28,5.39,;4.73,2.78,;3.7,1.64,)| Show InChI InChI=1S/C27H25F8NO5S/c28-20-9-11-21(12-10-20)42(40,41)24(13-14-36(15-24)22(37)16-1-3-17(4-2-16)23(38)39)18-5-7-19(8-6-18)25(29,26(30,31)32)27(33,34)35/h5-12,16-17H,1-4,13-15H2,(H,38,39)/t16-,17-,24-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | 680 | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
ACS Med Chem Lett 10: 367-373 (2019)
Article DOI: 10.1021/acsmedchemlett.9b00010
BindingDB Entry DOI: 10.7270/Q2T72MS0 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50505787
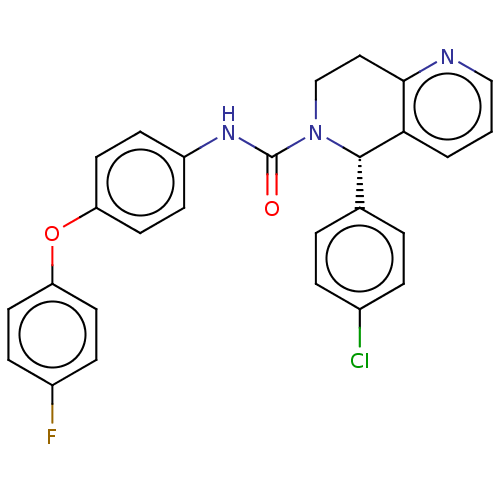
(CHEMBL4537998)Show SMILES Fc1ccc(Oc2ccc(NC(=O)N3CCc4ncccc4[C@@H]3c3ccc(Cl)cc3)cc2)cc1 |r| Show InChI InChI=1S/C27H21ClFN3O2/c28-19-5-3-18(4-6-19)26-24-2-1-16-30-25(24)15-17-32(26)27(33)31-21-9-13-23(14-10-21)34-22-11-7-20(29)8-12-22/h1-14,16,26H,15,17H2,(H,31,33)/t26-/m0/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using amodiaquine as substrate by LC-MS/MS analysis |
J Med Chem 62: 10321-10341 (2019)
Article DOI: 10.1021/acs.jmedchem.9b01382
BindingDB Entry DOI: 10.7270/Q29P34X6 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM135112
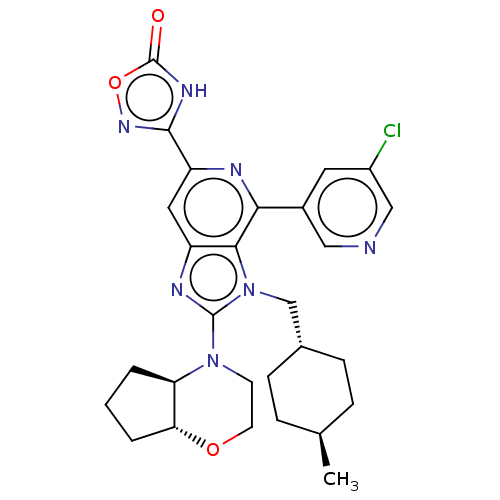
(US8846657, 3.2)Show SMILES C[C@H]1CC[C@H](Cn2c(nc3cc(nc(-c4cncc(Cl)c4)c23)-c2noc(=O)[nH]2)N2CCO[C@@H]3CCC[C@@H]23)CC1 |r,wU:4.4,32.36,wD:1.0,36.39,(7.09,-3.69,;5.6,-3.29,;5.21,-1.81,;3.72,-1.41,;2.63,-2.5,;1.14,-2.1,;.74,-.61,;1.65,.64,;.74,1.88,;-.72,1.41,;-2.05,2.18,;-3.39,1.41,;-3.39,-.13,;-2.05,-.9,;-2.05,-2.44,;-.72,-3.21,;-.72,-4.75,;-2.05,-5.52,;-3.39,-4.75,;-4.72,-5.52,;-3.39,-3.21,;-.72,-.13,;-4.72,2.18,;-6.19,1.7,;-7.09,2.95,;-6.19,4.19,;-6.96,5.52,;-4.72,3.72,;3.19,.64,;3.96,-.7,;5.5,-.7,;6.27,.64,;5.5,1.97,;5.97,3.43,;4.73,4.34,;3.48,3.43,;3.96,1.97,;3.03,-3.98,;4.52,-4.38,)| | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| Assay Description
Inhibition of CYP2C8 (unknown origin) |
Citation and Details
Article DOI: 10.1021/acs.jmedchem.1c01524
BindingDB Entry DOI: 10.7270/Q2Z03D1V |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM50446755
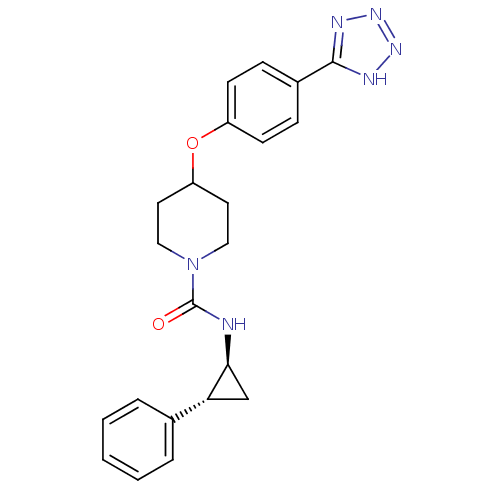
(CHEMBL3114608)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC(CC1)Oc1ccc(cc1)-c1nnn[nH]1 |r| Show InChI InChI=1S/C22H24N6O2/c29-22(23-20-14-19(20)15-4-2-1-3-5-15)28-12-10-18(11-13-28)30-17-8-6-16(7-9-17)21-24-26-27-25-21/h1-9,18-20H,10-14H2,(H,23,29)(H,24,25,26,27)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Dainippon Sumitomo Pharma Co., Ltd
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes after 10 mins by LC/MS/MS analysis |
Bioorg Med Chem 22: 1548-57 (2014)
Article DOI: 10.1016/j.bmc.2014.01.040
BindingDB Entry DOI: 10.7270/Q22F7PW9 |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C8
(Homo sapiens (Human)) | BDBM31774
(CHEMBL104 | Canesten | Clotrimazole | Lotrimin | M...)Show InChI InChI=1S/C22H17ClN2/c23-21-14-8-7-13-20(21)22(25-16-15-24-17-25,18-9-3-1-4-10-18)19-11-5-2-6-12-19/h1-17H | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 803 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc.
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C8 in human liver microsomes using paclitaxel as substrate after 8 mins by LC-MS/MS analysis |
Drug Metab Dispos 40: 943-51 (2012)
Article DOI: 10.1124/dmd.111.043505
BindingDB Entry DOI: 10.7270/Q2PN97D2 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data