

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20024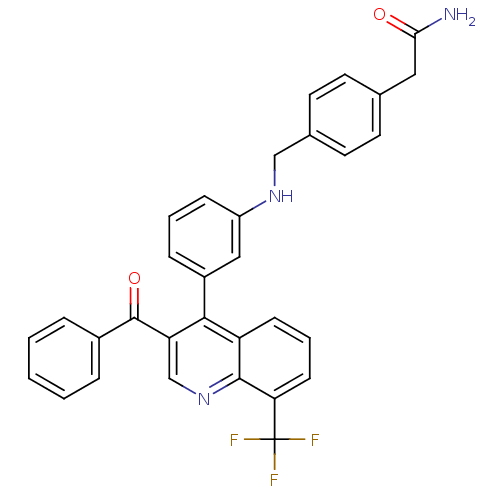 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 316 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20015 (2-[4-({[3-(3-benzyl-8-chloroquinolin-4-yl)phenyl]a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.40 | n/a | 23 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20001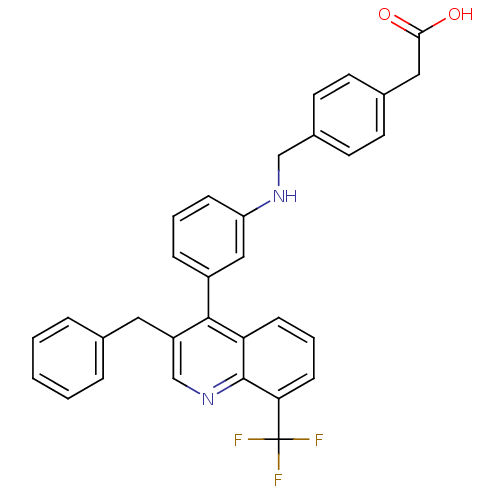 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.90 | n/a | 33 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20004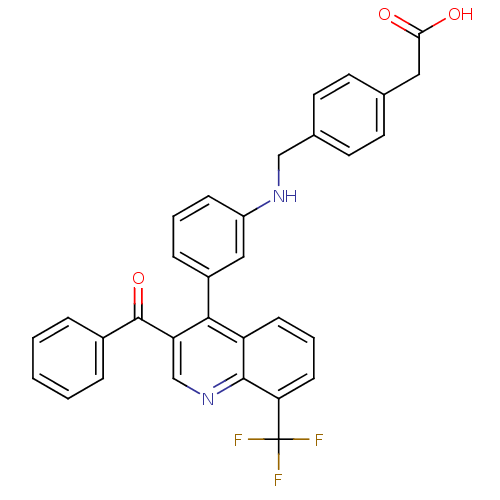 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | 29 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20020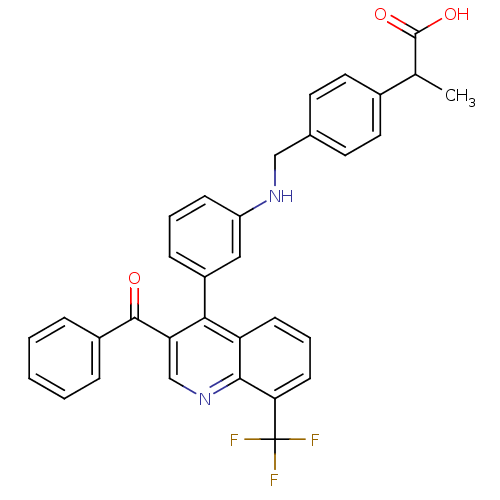 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | 58 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20014 (2-[4-({[3-(3-benzoyl-8-chloroquinolin-4-yl)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.70 | n/a | 39 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20006 (2-{4-[({3-[3-phenyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | 44 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20021 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.80 | n/a | 78 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20005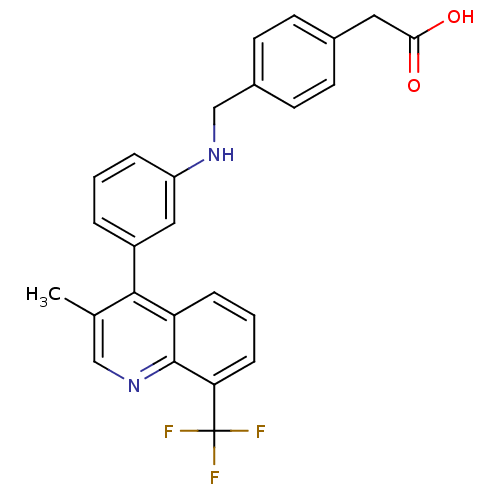 (2-{4-[({3-[3-methyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.30 | n/a | 100 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20011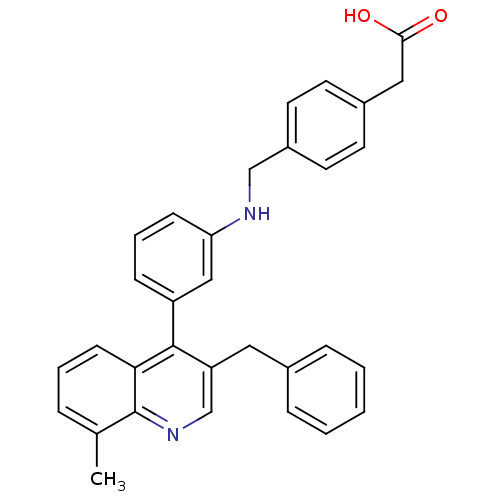 (2-[4-({[3-(3-benzyl-8-methylquinolin-4-yl)phenyl]a...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | 90 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20015 (2-[4-({[3-(3-benzyl-8-chloroquinolin-4-yl)phenyl]a...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20025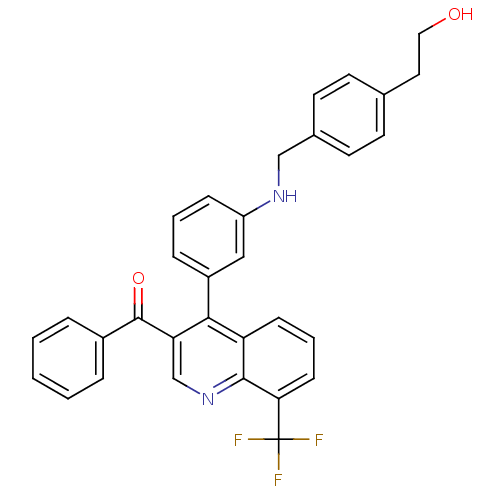 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40 | n/a | 795 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20010 (2-[4-({[3-(3-benzoyl-8-methylquinolin-4-yl)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.5 | n/a | 61 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20016 (2-{3-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6 | n/a | 141 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20004 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20001 (2-{4-[({3-[3-benzyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7.60 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20023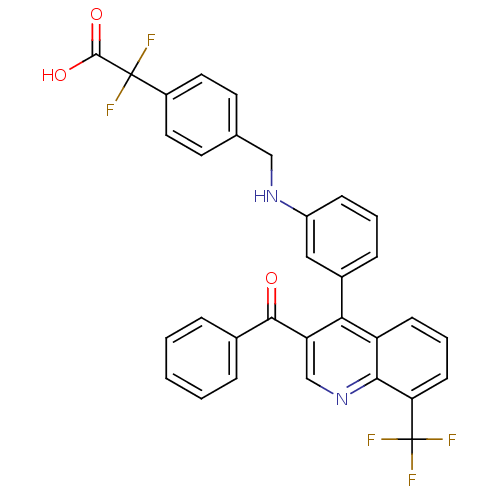 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.80 | n/a | 2.40E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20006 (2-{4-[({3-[3-phenyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20022 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.5 | n/a | 4.00E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20014 (2-[4-({[3-(3-benzoyl-8-chloroquinolin-4-yl)phenyl]...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20024 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20017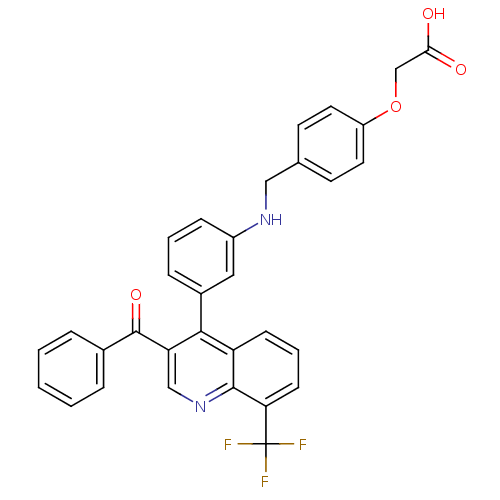 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12 | n/a | 1.27E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20027 (methyl alcohol analogue,39 | {4-[({3-[3-benzoyl-8-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 12.8 | n/a | 2.30E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20020 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 15.2 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20005 (2-{4-[({3-[3-methyl-8-(trifluoromethyl)quinolin-4-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20012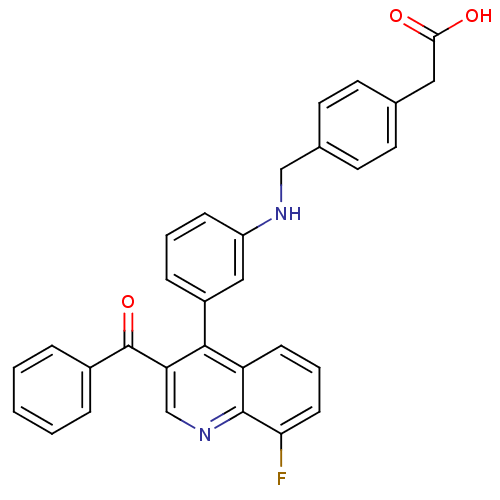 (2-[4-({[3-(3-benzoyl-8-fluoroquinolin-4-yl)phenyl]...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | 700 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20021 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17.2 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20010 (2-[4-({[3-(3-benzoyl-8-methylquinolin-4-yl)phenyl]...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 18 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20011 (2-[4-({[3-(3-benzyl-8-methylquinolin-4-yl)phenyl]a...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 21 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20023 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 30.2 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20022 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33.6 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20016 (2-{3-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33.6 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20008 (2-{4-[({3-[3-cyano-8-(trifluoromethyl)quinolin-4-y...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 33.8 | n/a | 450 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20025 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20026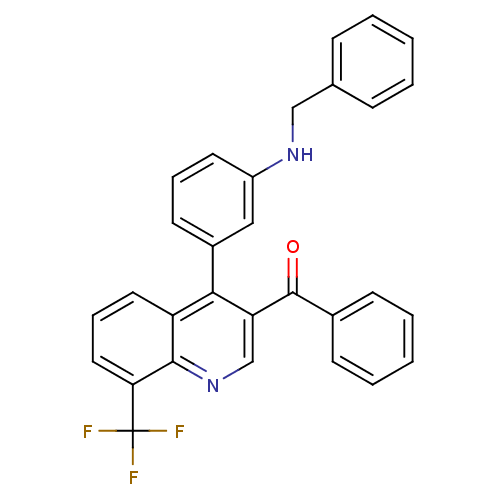 (3-[3-benzoyl-8-(trifluoromethyl)quinolin-4-yl]-N-b...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 45 | n/a | 770 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20007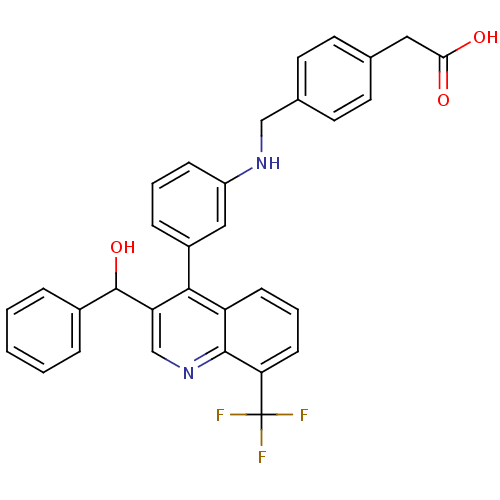 (2-(4-{[(3-{3-[hydroxy(phenyl)methyl]-8-(trifluorom...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 56.4 | n/a | 812 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20017 (2-{4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 63.9 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20027 (methyl alcohol analogue,39 | {4-[({3-[3-benzoyl-8-...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 90.5 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20012 (2-[4-({[3-(3-benzoyl-8-fluoroquinolin-4-yl)phenyl]...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 104 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20019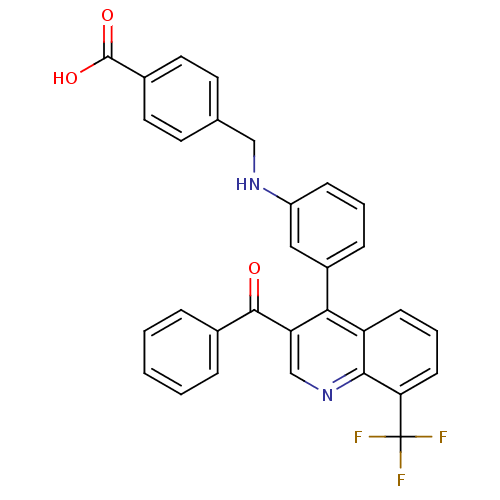 (4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 138 | n/a | 365 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20008 (2-{4-[({3-[3-cyano-8-(trifluoromethyl)quinolin-4-y...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 150 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20013 (2-[4-({[3-(3-benzoylquinolin-4-yl)phenyl]amino}met...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 154 | n/a | 1.65E+3 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20026 (3-[3-benzoyl-8-(trifluoromethyl)quinolin-4-yl]-N-b...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 241 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20028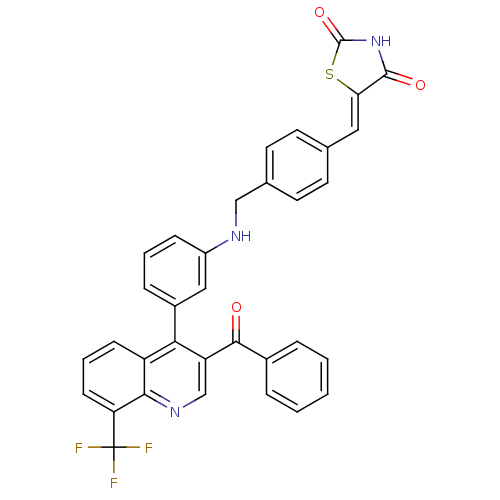 ((5Z)-5-({4-[({3-[3-benzoyl-8-(trifluoromethyl)quin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 276 | n/a | 369 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-beta [154-461] (Homo sapiens (Human)) | BDBM20029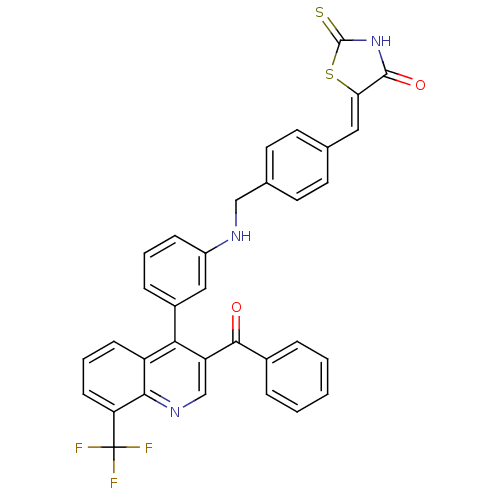 ((5Z)-5-({4-[({3-[3-benzoyl-8-(trifluoromethyl)quin...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | 286 | n/a | n/a | n/a | n/a |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20007 (2-(4-{[(3-{3-[hydroxy(phenyl)methyl]-8-(trifluorom...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 384 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20019 (4-[({3-[3-benzoyl-8-(trifluoromethyl)quinolin-4-yl...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 420 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20028 ((5Z)-5-({4-[({3-[3-benzoyl-8-(trifluoromethyl)quin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 456 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20029 ((5Z)-5-({4-[({3-[3-benzoyl-8-(trifluoromethyl)quin...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 479 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Oxysterols receptor LXR-alpha [197-447] (Homo sapiens (Human)) | BDBM20013 (2-[4-({[3-(3-benzoylquinolin-4-yl)phenyl]amino}met...) | PDB MMDB Reactome pathway UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 650 | n/a | n/a | n/a | n/a | 7.4 | 4 |
Wyeth Research | Assay Description Binding reaction was initiated by adding a dilution series of test compound in tracer solution to LXR-coated flash plates. Each concentration of test... | Bioorg Med Chem 15: 3321-33 (2007) Article DOI: 10.1016/j.bmc.2007.03.013 BindingDB Entry DOI: 10.7270/Q2VH5M37 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 54 total ) | Next | Last >> |