 Found 39 hits Enz. Inhib. hit(s) with all data for entry = 50035178
Found 39 hits Enz. Inhib. hit(s) with all data for entry = 50035178 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 0.220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105774
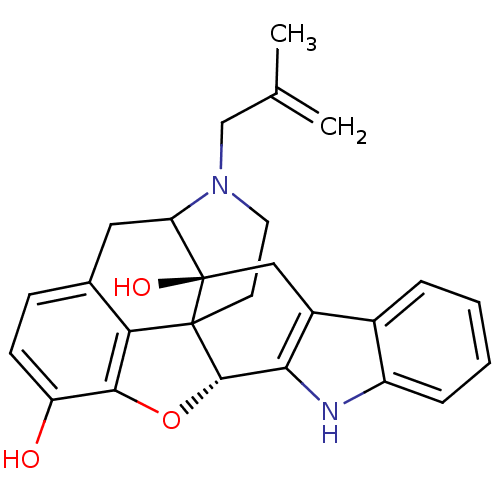
(22-(2-methylallyl)-(2S,13R)-14-oxa-11,22-diazahept...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1c4[nH]c2ccccc12)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C26H26N2O3/c1-14(2)13-28-10-9-25-21-15-7-8-19(29)23(21)31-24(25)22-17(12-26(25,30)20(28)11-15)16-5-3-4-6-18(16)27-22/h3-8,20,24,27,29-30H,1,9-13H2,2H3/t20?,24-,25?,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105776
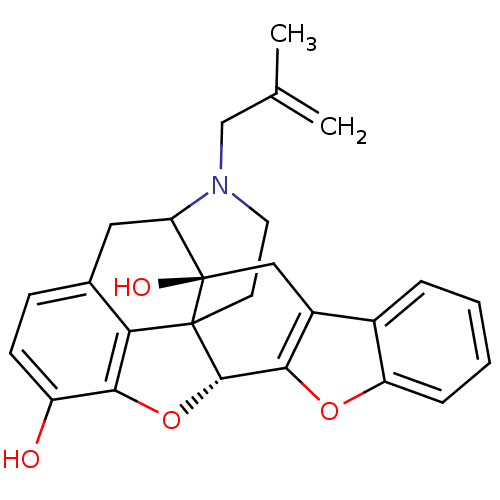
(22-(2-methylallyl)-(2S,13R)-11,14-dioxa-22-azahept...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1c4oc2ccccc12)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C26H25NO4/c1-14(2)13-27-10-9-25-21-15-7-8-18(28)23(21)31-24(25)22-17(12-26(25,29)20(27)11-15)16-5-3-4-6-19(16)30-22/h3-8,20,24,28-29H,1,9-13H2,2H3/t20?,24-,25?,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 8.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105772
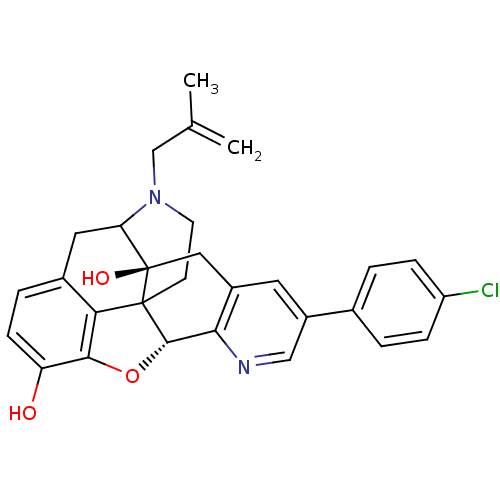
(6-(4-chlorophenyl)-19-(2-methylallyl)-(2S,10R)-11-...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1cc(cnc41)-c1ccc(Cl)cc1)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C29H27ClN2O3/c1-16(2)15-32-10-9-28-24-18-5-8-22(33)26(24)35-27(28)25-19(13-29(28,34)23(32)12-18)11-20(14-31-25)17-3-6-21(30)7-4-17/h3-8,11,14,23,27,33-34H,1,9-10,12-13,15H2,2H3/t23?,27-,28?,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM21864

((21R)-22-(cyclopropylmethyl)-14-oxa-11,22-diazahep...)Show SMILES [H][C@@]12Cc3ccc(O)c4OC5c6[nH]c7ccccc7c6CC1(O)C5(CCN2CC1CC1)c34 |THB:27:26:21:31.2.3| Show InChI InChI=1S/C26H26N2O3/c29-19-8-7-15-11-20-26(30)12-17-16-3-1-2-4-18(16)27-22(17)24-25(26,21(15)23(19)31-24)9-10-28(20)13-14-5-6-14/h1-4,7-8,14,20,24,27,29-30H,5-6,9-13H2/t20-,24?,25?,26?/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| PubMed
| 30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105782
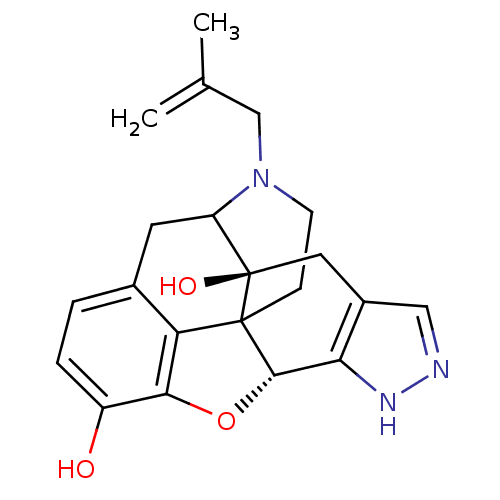
(18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahex...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1cn[nH]c41)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C21H23N3O3/c1-11(2)10-24-6-5-20-16-12-3-4-14(25)18(16)27-19(20)17-13(9-22-23-17)8-21(20,26)15(24)7-12/h3-4,9,15,19,25-26H,1,5-8,10H2,2H3,(H,22,23)/t15?,19-,20?,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 38 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105782

(18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahex...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1cn[nH]c41)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C21H23N3O3/c1-11(2)10-24-6-5-20-16-12-3-4-14(25)18(16)27-19(20)17-13(9-22-23-17)8-21(20,26)15(24)7-12/h3-4,9,15,19,25-26H,1,5-8,10H2,2H3,(H,22,23)/t15?,19-,20?,21+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105777
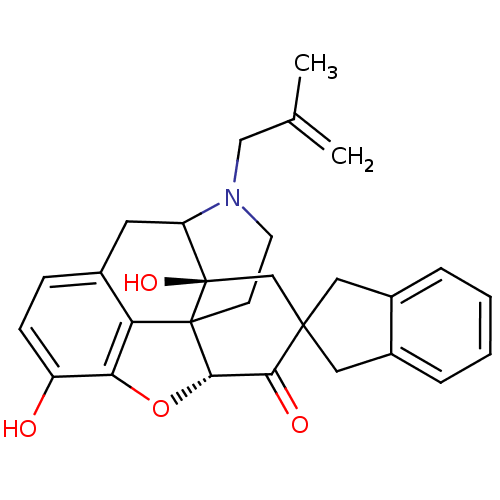
(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)CC1(Cc2ccccc2C1)C4=O)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C28H29NO4/c1-16(2)14-29-10-9-27-22-17-7-8-20(30)23(22)33-25(27)24(31)26(15-28(27,32)21(29)11-17)12-18-5-3-4-6-19(18)13-26/h3-8,21,25,30,32H,1,9-15H2,2H3/t21?,25-,27?,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 41 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105773
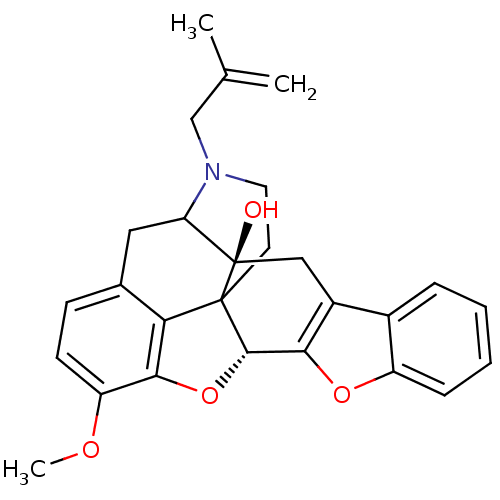
(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1oc2ccccc2c1C[C@@]35O |TLB:31:30:19.5.6:8.14.13,THB:9:8:30:19.5.6,18:19:30:8.14.13| Show InChI InChI=1S/C27H27NO4/c1-15(2)14-28-11-10-26-22-16-8-9-20(30-3)24(22)32-25(26)23-18(13-27(26,29)21(28)12-16)17-6-4-5-7-19(17)31-23/h4-9,21,25,29H,1,10-14H2,2-3H3/t21?,25-,26?,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 44 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105782

(18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,18-triazahex...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1cn[nH]c41)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C21H23N3O3/c1-11(2)10-24-6-5-20-16-12-3-4-14(25)18(16)27-19(20)17-13(9-22-23-17)8-21(20,26)15(24)7-12/h3-4,9,15,19,25-26H,1,5-8,10H2,2H3,(H,22,23)/t15?,19-,20?,21+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105780
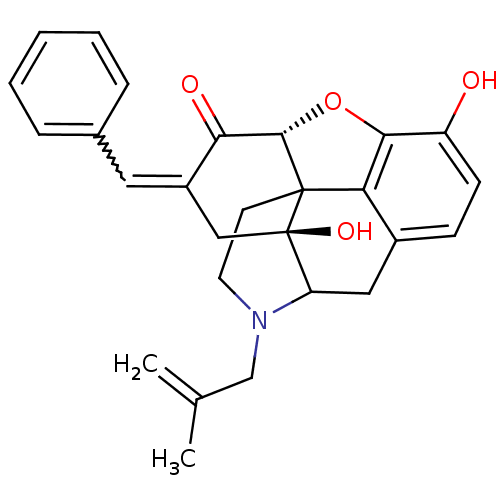
(10,17-dihydroxy-4-(2-methylallyl)-15-[1-phenyl-(E)...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)CC(=Cc1ccccc1)C4=O)ccc5O |w:19.22,TLB:16:15:11.12.13:4.6.5,THB:10:11:15:4.6.5,3:4:15:11.12.13| Show InChI InChI=1S/C27H27NO4/c1-16(2)15-28-11-10-26-22-18-8-9-20(29)24(22)32-25(26)23(30)19(12-17-6-4-3-5-7-17)14-27(26,31)21(28)13-18/h3-9,12,21,25,29,31H,1,10-11,13-15H2,2H3/t21?,25-,26?,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 210 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105771
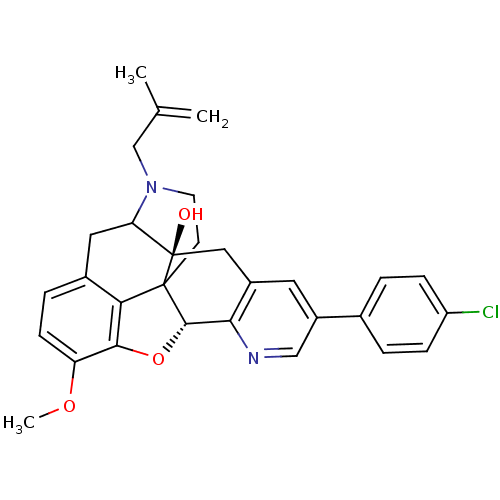
(6-(4-chlorophenyl)-13-methoxy-19-(2-methylallyl)-(...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1ncc(cc1C[C@@]35O)-c1ccc(Cl)cc1 |TLB:28:27:19.5.6:8.14.13,THB:9:8:27:19.5.6,18:19:27:8.14.13| Show InChI InChI=1S/C30H29ClN2O3/c1-17(2)16-33-11-10-29-25-19-6-9-23(35-3)27(25)36-28(29)26-20(14-30(29,34)24(33)13-19)12-21(15-32-26)18-4-7-22(31)8-5-18/h4-9,12,15,24,28,34H,1,10-11,13-14,16H2,2-3H3/t24?,28-,29?,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 260 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105772

(6-(4-chlorophenyl)-19-(2-methylallyl)-(2S,10R)-11-...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1cc(cnc41)-c1ccc(Cl)cc1)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C29H27ClN2O3/c1-16(2)15-32-10-9-28-24-18-5-8-22(33)26(24)35-27(28)25-19(13-29(28,34)23(32)12-18)11-20(14-31-25)17-3-6-21(30)7-4-17/h3-8,11,14,23,27,33-34H,1,9-10,12-13,15H2,2H3/t23?,27-,28?,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105775
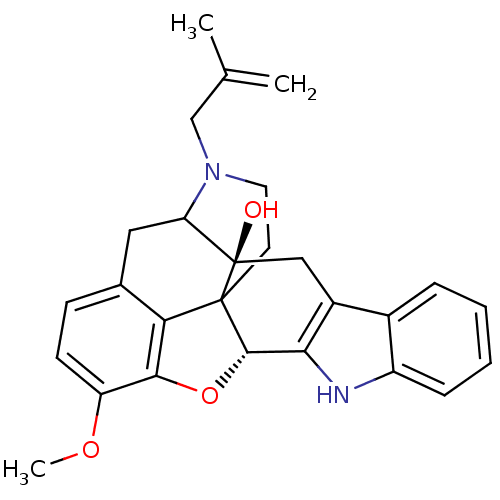
(16-methoxy-22-(2-methylallyl)-(2S,13R)-14-oxa-11,2...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1[nH]c2ccccc2c1C[C@@]35O |TLB:31:30:19.5.6:8.14.13,THB:9:8:30:19.5.6,18:19:30:8.14.13| Show InChI InChI=1S/C27H28N2O3/c1-15(2)14-29-11-10-26-22-16-8-9-20(31-3)24(22)32-25(26)23-18(13-27(26,30)21(29)12-16)17-6-4-5-7-19(17)28-23/h4-9,21,25,28,30H,1,10-14H2,2-3H3/t21?,25-,26?,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 500 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105776

(22-(2-methylallyl)-(2S,13R)-11,14-dioxa-22-azahept...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1c4oc2ccccc12)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C26H25NO4/c1-14(2)13-27-10-9-25-21-15-7-8-18(28)23(21)31-24(25)22-17(12-26(25,29)20(27)11-15)16-5-3-4-6-19(16)30-22/h3-8,20,24,28-29H,1,9-13H2,2H3/t20?,24-,25?,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 580 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105777

(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)CC1(Cc2ccccc2C1)C4=O)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C28H29NO4/c1-16(2)14-29-10-9-27-22-17-7-8-20(30)23(22)33-25(27)24(31)26(15-28(27,32)21(29)11-17)12-18-5-3-4-6-19(18)13-26/h3-8,21,25,30,32H,1,9-15H2,2H3/t21?,25-,27?,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105776

(22-(2-methylallyl)-(2S,13R)-11,14-dioxa-22-azahept...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1c4oc2ccccc12)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C26H25NO4/c1-14(2)13-27-10-9-25-21-15-7-8-18(28)23(21)31-24(25)22-17(12-26(25,29)20(27)11-15)16-5-3-4-6-19(16)30-22/h3-8,20,24,28-29H,1,9-13H2,2H3/t20?,24-,25?,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105777

(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)CC1(Cc2ccccc2C1)C4=O)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C28H29NO4/c1-16(2)14-29-10-9-27-22-17-7-8-20(30)23(22)33-25(27)24(31)26(15-28(27,32)21(29)11-17)12-18-5-3-4-6-19(18)13-26/h3-8,21,25,30,32H,1,9-15H2,2H3/t21?,25-,27?,28+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105778
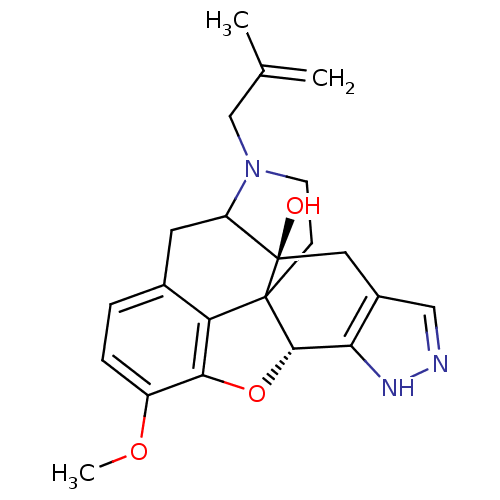
(12-methoxy-18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,1...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1[nH]ncc1C[C@@]35O |TLB:27:26:19.5.6:8.14.13,THB:9:8:26:19.5.6,18:19:26:8.14.13| Show InChI InChI=1S/C22H25N3O3/c1-12(2)11-25-7-6-21-17-13-4-5-15(27-3)19(17)28-20(21)18-14(10-23-24-18)9-22(21,26)16(25)8-13/h4-5,10,16,20,26H,1,6-9,11H2,2-3H3,(H,23,24)/t16?,20-,21?,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105778

(12-methoxy-18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,1...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1[nH]ncc1C[C@@]35O |TLB:27:26:19.5.6:8.14.13,THB:9:8:26:19.5.6,18:19:26:8.14.13| Show InChI InChI=1S/C22H25N3O3/c1-12(2)11-25-7-6-21-17-13-4-5-15(27-3)19(17)28-20(21)18-14(10-23-24-18)9-22(21,26)16(25)8-13/h4-5,10,16,20,26H,1,6-9,11H2,2-3H3,(H,23,24)/t16?,20-,21?,22+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105771

(6-(4-chlorophenyl)-13-methoxy-19-(2-methylallyl)-(...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1ncc(cc1C[C@@]35O)-c1ccc(Cl)cc1 |TLB:28:27:19.5.6:8.14.13,THB:9:8:27:19.5.6,18:19:27:8.14.13| Show InChI InChI=1S/C30H29ClN2O3/c1-17(2)16-33-11-10-29-25-19-6-9-23(35-3)27(25)36-28(29)26-20(14-30(29,34)24(33)13-19)12-21(15-32-26)18-4-7-22(31)8-5-18/h4-9,12,15,24,28,34H,1,10-11,13-14,16H2,2-3H3/t24?,28-,29?,30+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105779
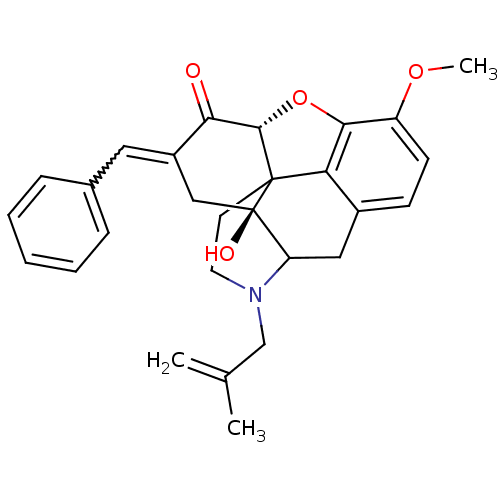
(17-hydroxy-10-methoxy-4-(2-methylallyl)-15-[1-phen...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)C(=O)C(C[C@@]35O)=Cc1ccccc1 |w:26.31,TLB:25:24:19.5.6:8.14.13,THB:18:19:24:8.14.13,9:8:24:19.5.6| Show InChI InChI=1S/C28H29NO4/c1-17(2)16-29-12-11-27-23-19-9-10-21(32-3)25(23)33-26(27)24(30)20(13-18-7-5-4-6-8-18)15-28(27,31)22(29)14-19/h4-10,13,22,26,31H,1,11-12,14-16H2,2-3H3/t22?,26-,27?,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105774

(22-(2-methylallyl)-(2S,13R)-14-oxa-11,22-diazahept...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1c4[nH]c2ccccc12)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C26H26N2O3/c1-14(2)13-28-10-9-25-21-15-7-8-19(29)23(21)31-24(25)22-17(12-26(25,30)20(28)11-15)16-5-3-4-6-18(16)27-22/h3-8,20,24,27,29-30H,1,9-13H2,2H3/t20?,24-,25?,26+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105780

(10,17-dihydroxy-4-(2-methylallyl)-15-[1-phenyl-(E)...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)CC(=Cc1ccccc1)C4=O)ccc5O |w:19.22,TLB:16:15:11.12.13:4.6.5,THB:10:11:15:4.6.5,3:4:15:11.12.13| Show InChI InChI=1S/C27H27NO4/c1-16(2)15-28-11-10-26-22-18-8-9-20(29)24(22)32-25(26)23(30)19(12-17-6-4-3-5-7-17)14-27(26,31)21(28)13-18/h3-9,12,21,25,29,31H,1,10-11,13-15H2,2H3/t21?,25-,26?,27+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105780

(10,17-dihydroxy-4-(2-methylallyl)-15-[1-phenyl-(E)...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)CC(=Cc1ccccc1)C4=O)ccc5O |w:19.22,TLB:16:15:11.12.13:4.6.5,THB:10:11:15:4.6.5,3:4:15:11.12.13| Show InChI InChI=1S/C27H27NO4/c1-16(2)15-28-11-10-26-22-18-8-9-20(29)24(22)32-25(26)23(30)19(12-17-6-4-3-5-7-17)14-27(26,31)21(28)13-18/h3-9,12,21,25,29,31H,1,10-11,13-15H2,2H3/t21?,25-,26?,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105781

(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)C(=O)C1(Cc2ccccc2C1)C[C@@]35O |TLB:33:32:19.5.6:8.14.13,THB:9:8:32:19.5.6,18:19:32:8.14.13| Show InChI InChI=1S/C29H31NO4/c1-17(2)15-30-11-10-28-23-18-8-9-21(33-3)24(23)34-26(28)25(31)27(16-29(28,32)22(30)12-18)13-19-6-4-5-7-20(19)14-27/h4-9,22,26,32H,1,10-16H2,2-3H3/t22?,26-,28?,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105771

(6-(4-chlorophenyl)-13-methoxy-19-(2-methylallyl)-(...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1ncc(cc1C[C@@]35O)-c1ccc(Cl)cc1 |TLB:28:27:19.5.6:8.14.13,THB:9:8:27:19.5.6,18:19:27:8.14.13| Show InChI InChI=1S/C30H29ClN2O3/c1-17(2)16-33-11-10-29-25-19-6-9-23(35-3)27(25)36-28(29)26-20(14-30(29,34)24(33)13-19)12-21(15-32-26)18-4-7-22(31)8-5-18/h4-9,12,15,24,28,34H,1,10-11,13-14,16H2,2-3H3/t24?,28-,29?,30+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105775

(16-methoxy-22-(2-methylallyl)-(2S,13R)-14-oxa-11,2...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1[nH]c2ccccc2c1C[C@@]35O |TLB:31:30:19.5.6:8.14.13,THB:9:8:30:19.5.6,18:19:30:8.14.13| Show InChI InChI=1S/C27H28N2O3/c1-15(2)14-29-11-10-26-22-16-8-9-20(31-3)24(22)32-25(26)23-18(13-27(26,30)21(29)12-16)17-6-4-5-7-19(17)28-23/h4-9,21,25,28,30H,1,10-14H2,2-3H3/t21?,25-,26?,27+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105773

(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1oc2ccccc2c1C[C@@]35O |TLB:31:30:19.5.6:8.14.13,THB:9:8:30:19.5.6,18:19:30:8.14.13| Show InChI InChI=1S/C27H27NO4/c1-15(2)14-28-11-10-26-22-16-8-9-20(30-3)24(22)32-25(26)23-18(13-27(26,29)21(28)12-16)17-6-4-5-7-19(17)31-23/h4-9,21,25,29H,1,10-14H2,2-3H3/t21?,25-,26?,27+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105772

(6-(4-chlorophenyl)-19-(2-methylallyl)-(2S,10R)-11-...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1cc(cnc41)-c1ccc(Cl)cc1)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C29H27ClN2O3/c1-16(2)15-32-10-9-28-24-18-5-8-22(33)26(24)35-27(28)25-19(13-29(28,34)23(32)12-18)11-20(14-31-25)17-3-6-21(30)7-4-17/h3-8,11,14,23,27,33-34H,1,9-10,12-13,15H2,2H3/t23?,27-,28?,29+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105778

(12-methoxy-18-(2-methylallyl)-(2S,9R)-10-oxa-6,7,1...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1[nH]ncc1C[C@@]35O |TLB:27:26:19.5.6:8.14.13,THB:9:8:26:19.5.6,18:19:26:8.14.13| Show InChI InChI=1S/C22H25N3O3/c1-12(2)11-25-7-6-21-17-13-4-5-15(27-3)19(17)28-20(21)18-14(10-23-24-18)9-22(21,26)16(25)8-13/h4-5,10,16,20,26H,1,6-9,11H2,2-3H3,(H,23,24)/t16?,20-,21?,22+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105779

(17-hydroxy-10-methoxy-4-(2-methylallyl)-15-[1-phen...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)C(=O)C(C[C@@]35O)=Cc1ccccc1 |w:26.31,TLB:25:24:19.5.6:8.14.13,THB:18:19:24:8.14.13,9:8:24:19.5.6| Show InChI InChI=1S/C28H29NO4/c1-17(2)16-29-12-11-27-23-19-9-10-21(32-3)25(23)33-26(27)24(30)20(13-18-7-5-4-6-8-18)15-28(27,31)22(29)14-19/h4-10,13,22,26,31H,1,11-12,14-16H2,2-3H3/t22?,26-,27?,28+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105779

(17-hydroxy-10-methoxy-4-(2-methylallyl)-15-[1-phen...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)C(=O)C(C[C@@]35O)=Cc1ccccc1 |w:26.31,TLB:25:24:19.5.6:8.14.13,THB:18:19:24:8.14.13,9:8:24:19.5.6| Show InChI InChI=1S/C28H29NO4/c1-17(2)16-29-12-11-27-23-19-9-10-21(32-3)25(23)33-26(27)24(30)20(13-18-7-5-4-6-8-18)15-28(27,31)22(29)14-19/h4-10,13,22,26,31H,1,11-12,14-16H2,2-3H3/t22?,26-,27?,28+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105781

(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)C(=O)C1(Cc2ccccc2C1)C[C@@]35O |TLB:33:32:19.5.6:8.14.13,THB:9:8:32:19.5.6,18:19:32:8.14.13| Show InChI InChI=1S/C29H31NO4/c1-17(2)15-30-11-10-28-23-18-8-9-21(33-3)24(23)34-26(28)25(31)27(16-29(28,32)22(30)12-18)13-19-6-4-5-7-20(19)14-27/h4-9,22,26,32H,1,10-16H2,2-3H3/t22?,26-,28?,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >7.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Delta-type opioid receptor
(Homo sapiens (Human)) | BDBM50105781

(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)C(=O)C1(Cc2ccccc2C1)C[C@@]35O |TLB:33:32:19.5.6:8.14.13,THB:9:8:32:19.5.6,18:19:32:8.14.13| Show InChI InChI=1S/C29H31NO4/c1-17(2)15-30-11-10-28-23-18-8-9-21(33-3)24(23)34-26(28)25(31)27(16-29(28,32)22(30)12-18)13-19-6-4-5-7-20(19)14-27/h4-9,22,26,32H,1,10-16H2,2-3H3/t22?,26-,28?,29+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >7.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DADLE from Opioid receptor delta 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Mu-type opioid receptor
(Homo sapiens (Human)) | BDBM50105774

(22-(2-methylallyl)-(2S,13R)-14-oxa-11,22-diazahept...)Show SMILES CC(=C)CN1CCC23[C@H]4Oc5c2c(CC1[C@]3(O)Cc1c4[nH]c2ccccc12)ccc5O |TLB:16:15:11.12.13:4.6.5,THB:3:4:15:11.12.13,10:11:15:4.6.5| Show InChI InChI=1S/C26H26N2O3/c1-14(2)13-28-10-9-25-21-15-7-8-19(29)23(21)31-24(25)22-17(12-26(25,30)20(28)11-15)16-5-3-4-6-18(16)27-22/h3-8,20,24,27,29-30H,1,9-13H2,2H3/t20?,24-,25?,26+/m0/s1 | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]DAMGO from Opioid receptor mu 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105773

(17-(2-methylallyl)-substituted noroxymorphone deri...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1oc2ccccc2c1C[C@@]35O |TLB:31:30:19.5.6:8.14.13,THB:9:8:30:19.5.6,18:19:30:8.14.13| Show InChI InChI=1S/C27H27NO4/c1-15(2)14-28-11-10-26-22-16-8-9-20(30-3)24(22)32-25(26)23-18(13-27(26,29)21(28)12-16)17-6-4-5-7-19(17)31-23/h4-9,21,25,29H,1,10-14H2,2-3H3/t21?,25-,26?,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |
Kappa-type opioid receptor
(Homo sapiens (Human)) | BDBM50105775

(16-methoxy-22-(2-methylallyl)-(2S,13R)-14-oxa-11,2...)Show SMILES COc1ccc2CC3N(CC(C)=C)CCC45[C@@H](Oc1c24)c1[nH]c2ccccc2c1C[C@@]35O |TLB:31:30:19.5.6:8.14.13,THB:9:8:30:19.5.6,18:19:30:8.14.13| Show InChI InChI=1S/C27H28N2O3/c1-15(2)14-29-11-10-26-22-16-8-9-20(31-3)24(22)32-25(26)23-18(13-27(26,30)21(29)12-16)17-6-4-5-7-19(17)28-23/h4-9,21,25,28,30H,1,10-14H2,2-3H3/t21?,25-,26?,27+/m0/s1 | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| >1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
NIDDK
Curated by ChEMBL
| Assay Description
Displacement of [3H]U-69593 from Opioid receptor kappa 1 |
Bioorg Med Chem Lett 11: 2883-5 (2001)
BindingDB Entry DOI: 10.7270/Q2D79BZV |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data