 Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50014795
Found 46 hits Enz. Inhib. hit(s) with all data for entry = 50014795 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Heparanase
(Homo sapiens (Human)) | BDBM50147513

(2-[3-[5-(4-Chloro-phenyl)-benzooxazol-2-yl]-4-(2-m...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(Cl)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22ClN3O6/c1-40-13-12-33-25-10-8-21(35-29(36)22-9-4-19(31(38)39)14-23(22)30(35)37)16-24(25)28-34-26-15-18(5-11-27(26)41-28)17-2-6-20(32)7-3-17/h2-11,14-16,33H,12-13H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147546
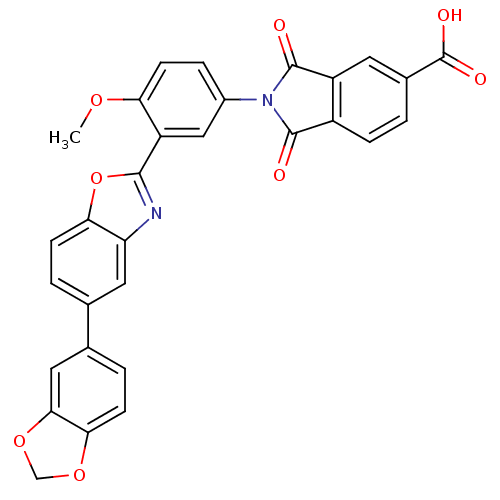
(2-(3-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H18N2O8/c1-37-23-9-5-18(32-28(33)19-6-2-17(30(35)36)10-20(19)29(32)34)13-21(23)27-31-22-11-15(3-7-24(22)40-27)16-4-8-25-26(12-16)39-14-38-25/h2-13H,14H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 200 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147534

(1,3-Dioxo-2-[3-(5-phenyl-benzooxazol-2-yl)-4-propy...)Show SMILES CCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H23N3O5/c1-2-14-32-25-12-10-21(34-29(35)22-11-8-20(31(37)38)15-23(22)30(34)36)17-24(25)28-33-26-16-19(9-13-27(26)39-28)18-6-4-3-5-7-18/h3-13,15-17,32H,2,14H2,1H3,(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147537
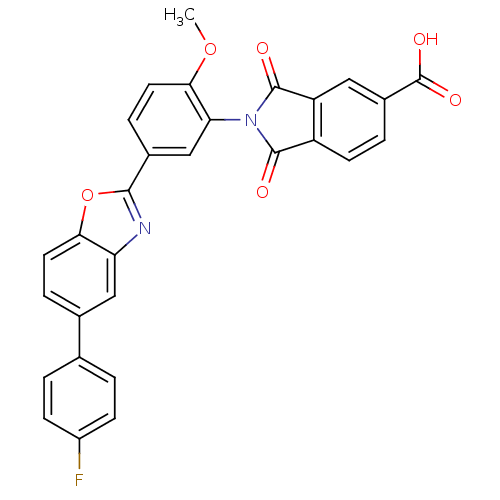
(2-(5-(5-(4-fluorophenyl)benzo[d]oxazol-2-yl)-2-met...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccc(F)cc1 Show InChI InChI=1S/C29H17FN2O6/c1-37-25-11-6-17(14-23(25)32-27(33)20-9-4-18(29(35)36)12-21(20)28(32)34)26-31-22-13-16(5-10-24(22)38-26)15-2-7-19(30)8-3-15/h2-14H,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147522
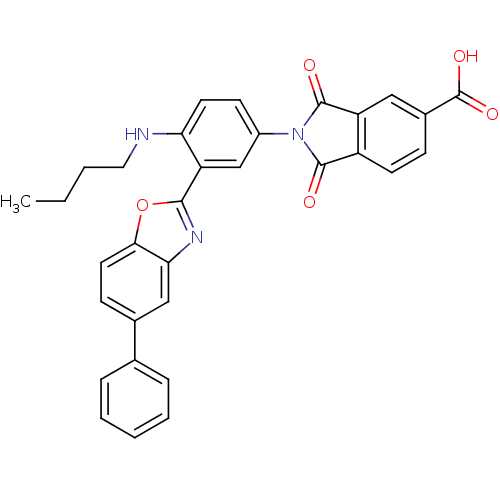
(2-(4-(butylamino)-3-(5-phenylbenzo[d]oxazol-2-yl)p...)Show SMILES CCCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C32H25N3O5/c1-2-3-15-33-26-13-11-22(35-30(36)23-12-9-21(32(38)39)16-24(23)31(35)37)18-25(26)29-34-27-17-20(10-14-28(27)40-29)19-7-5-4-6-8-19/h4-14,16-18,33H,2-3,15H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147544
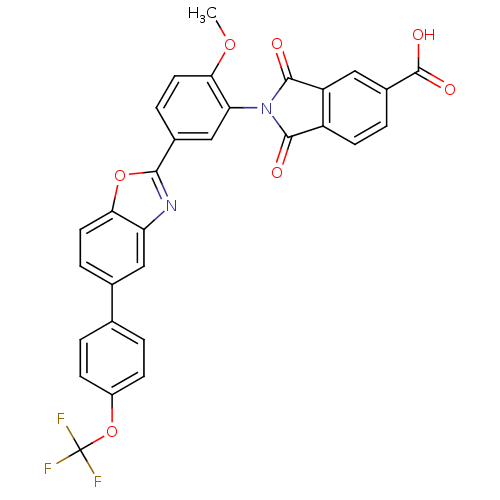
(2-(2-methoxy-5-(5-(4-(trifluoromethoxy)phenyl)benz...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccc(OC(F)(F)F)cc1 Show InChI InChI=1S/C30H17F3N2O7/c1-40-25-11-6-17(14-23(25)35-27(36)20-9-4-18(29(38)39)12-21(20)28(35)37)26-34-22-13-16(5-10-24(22)41-26)15-2-7-19(8-3-15)42-30(31,32)33/h2-14H,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147533
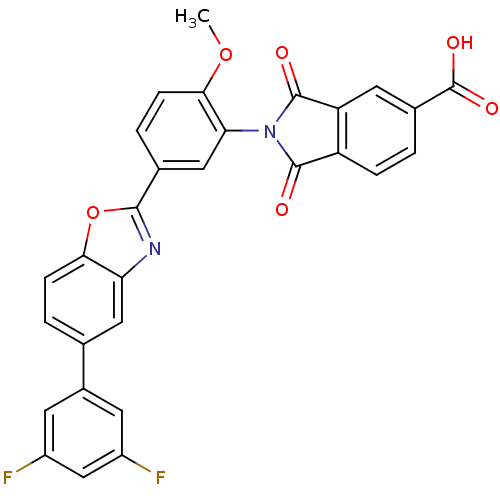
(2-(5-(5-(3,5-difluorophenyl)benzo[d]oxazol-2-yl)-2...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1cc(F)cc(F)c1 Show InChI InChI=1S/C29H16F2N2O6/c1-38-25-7-4-15(12-23(25)33-27(34)20-5-2-16(29(36)37)10-21(20)28(33)35)26-32-22-11-14(3-6-24(22)39-26)17-8-18(30)13-19(31)9-17/h2-13H,1H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147539
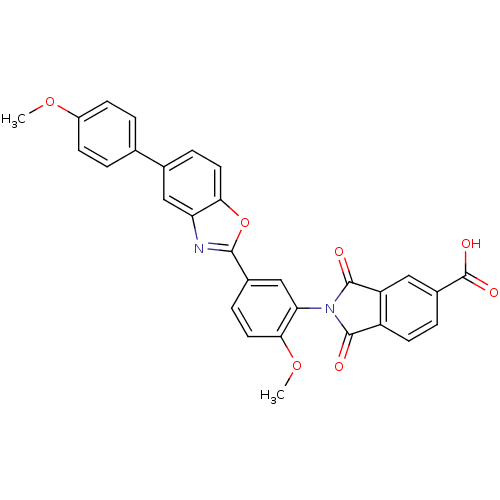
(2-(2-methoxy-5-(5-(4-methoxyphenyl)benzo[d]oxazol-...)Show SMILES COc1ccc(cc1)-c1ccc2oc(nc2c1)-c1ccc(OC)c(c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H20N2O7/c1-37-20-8-3-16(4-9-20)17-6-11-25-23(14-17)31-27(39-25)18-7-12-26(38-2)24(15-18)32-28(33)21-10-5-19(30(35)36)13-22(21)29(32)34/h3-15H,1-2H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147517
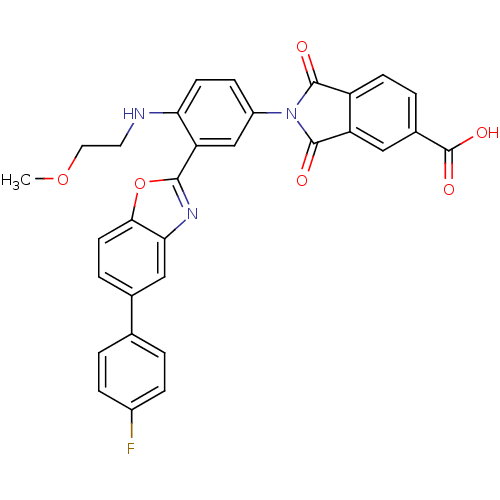
(2-[3-[5-(4-Fluoro-phenyl)-benzooxazol-2-yl]-4-(2-m...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(F)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22FN3O6/c1-40-13-12-33-25-10-8-21(35-29(36)22-9-4-19(31(38)39)14-23(22)30(35)37)16-24(25)28-34-26-15-18(5-11-27(26)41-28)17-2-6-20(32)7-3-17/h2-11,14-16,33H,12-13H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147528
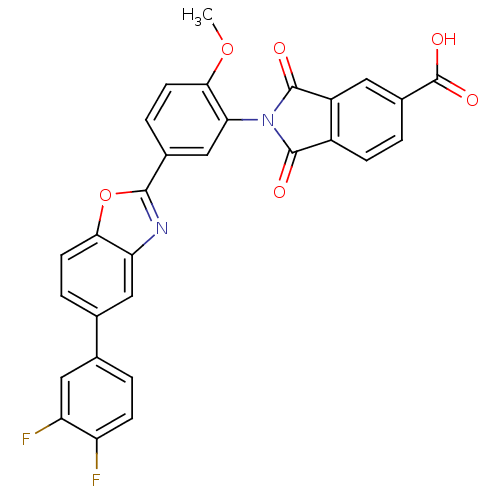
(2-(5-(5-(3,4-difluorophenyl)benzo[d]oxazol-2-yl)-2...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccc(F)c(F)c1 Show InChI InChI=1S/C29H16F2N2O6/c1-38-25-9-5-16(13-23(25)33-27(34)18-6-2-17(29(36)37)10-19(18)28(33)35)26-32-22-12-15(4-8-24(22)39-26)14-3-7-20(30)21(31)11-14/h2-13H,1H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147536
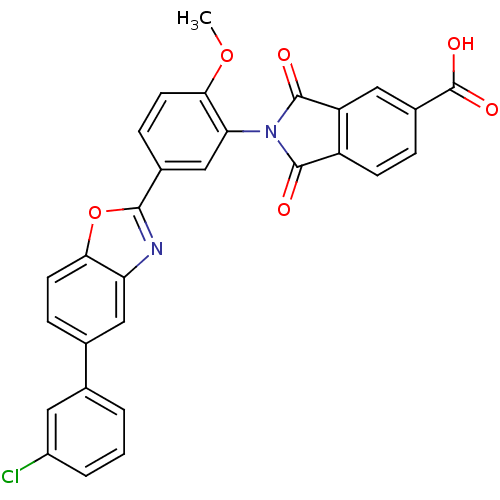
(2-(5-(5-(3-chlorophenyl)benzo[d]oxazol-2-yl)-2-met...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1cccc(Cl)c1 Show InChI InChI=1S/C29H17ClN2O6/c1-37-25-10-7-17(14-23(25)32-27(33)20-8-5-18(29(35)36)12-21(20)28(32)34)26-31-22-13-16(6-9-24(22)38-26)15-3-2-4-19(30)11-15/h2-14H,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147535
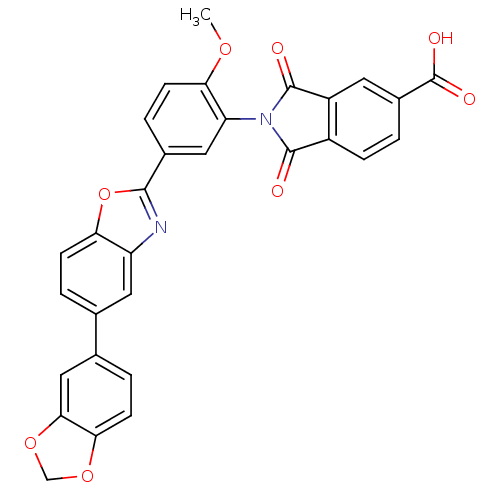
(2-(5-(5-(benzo[d][1,3]dioxol-5-yl)benzo[d]oxazol-2...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccc2OCOc2c1 Show InChI InChI=1S/C30H18N2O8/c1-37-24-8-5-17(12-22(24)32-28(33)19-6-2-18(30(35)36)10-20(19)29(32)34)27-31-21-11-15(3-7-23(21)40-27)16-4-9-25-26(13-16)39-14-38-25/h2-13H,14H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 750 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147519
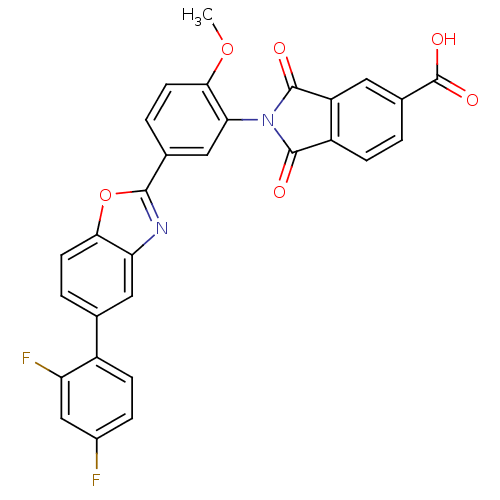
(2-(5-(5-(2,4-difluorophenyl)benzo[d]oxazol-2-yl)-2...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccc(F)cc1F Show InChI InChI=1S/C29H16F2N2O6/c1-38-25-9-4-15(12-23(25)33-27(34)19-6-2-16(29(36)37)10-20(19)28(33)35)26-32-22-11-14(3-8-24(22)39-26)18-7-5-17(30)13-21(18)31/h2-13H,1H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147518
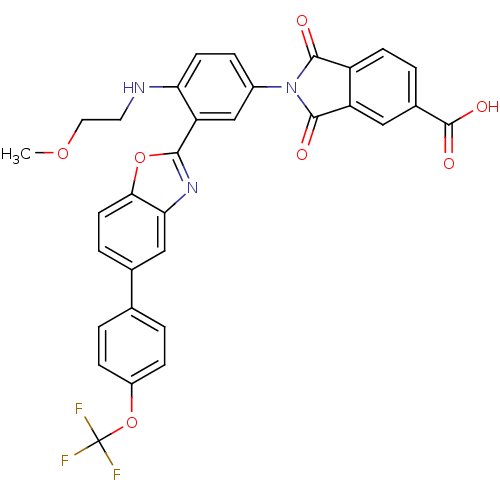
(2-{4-(2-Methoxy-ethylamino)-3-[5-(4-trifluorometho...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(OC(F)(F)F)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C32H22F3N3O7/c1-43-13-12-36-25-10-6-20(38-29(39)22-9-4-19(31(41)42)14-23(22)30(38)40)16-24(25)28-37-26-15-18(5-11-27(26)44-28)17-2-7-21(8-3-17)45-32(33,34)35/h2-11,14-16,36H,12-13H2,1H3,(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147530
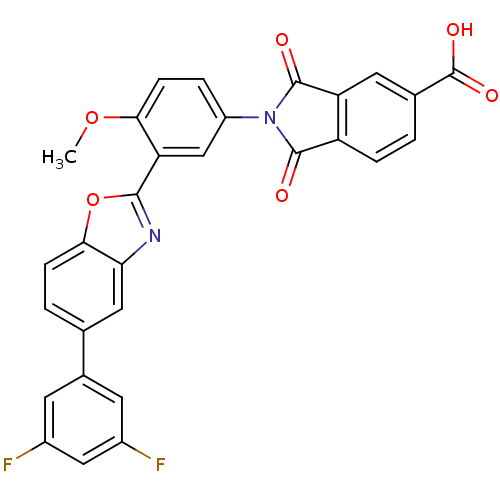
(2-(3-(5-(3,5-difluorophenyl)benzo[d]oxazol-2-yl)-4...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1cc(F)cc(F)c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C29H16F2N2O6/c1-38-24-7-4-19(33-27(34)20-5-2-15(29(36)37)10-21(20)28(33)35)13-22(24)26-32-23-11-14(3-6-25(23)39-26)16-8-17(30)12-18(31)9-16/h2-13H,1H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147531
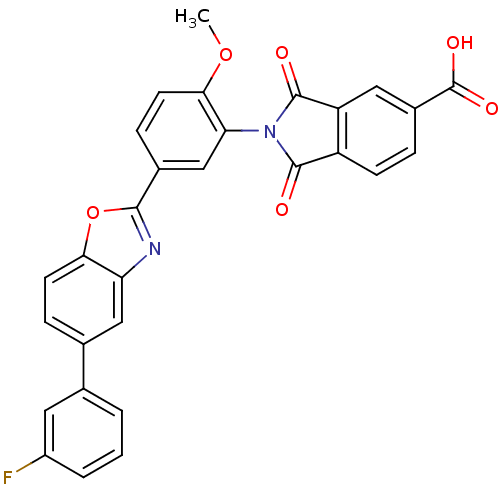
(2-(5-(5-(3-fluorophenyl)benzo[d]oxazol-2-yl)-2-met...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1cccc(F)c1 Show InChI InChI=1S/C29H17FN2O6/c1-37-25-10-7-17(14-23(25)32-27(33)20-8-5-18(29(35)36)12-21(20)28(32)34)26-31-22-13-16(6-9-24(22)38-26)15-3-2-4-19(30)11-15/h2-14H,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147510
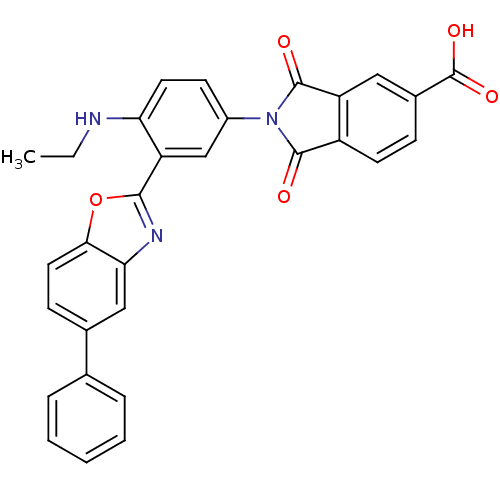
(2-(4-(ethylamino)-3-(5-phenylbenzo[d]oxazol-2-yl)p...)Show SMILES CCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H21N3O5/c1-2-31-24-12-10-20(33-28(34)21-11-8-19(30(36)37)14-22(21)29(33)35)16-23(24)27-32-25-15-18(9-13-26(25)38-27)17-6-4-3-5-7-17/h3-16,31H,2H2,1H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147538
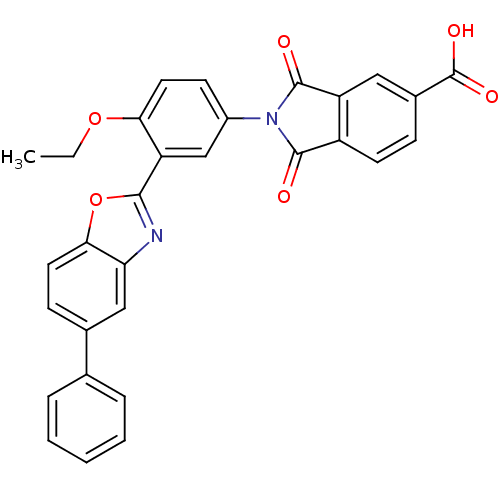
(2-(4-ethoxy-3-(5-phenylbenzo[d]oxazol-2-yl)phenyl)...)Show SMILES CCOc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H20N2O6/c1-2-37-25-13-10-20(32-28(33)21-11-8-19(30(35)36)14-22(21)29(32)34)16-23(25)27-31-24-15-18(9-12-26(24)38-27)17-6-4-3-5-7-17/h3-16H,2H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147515
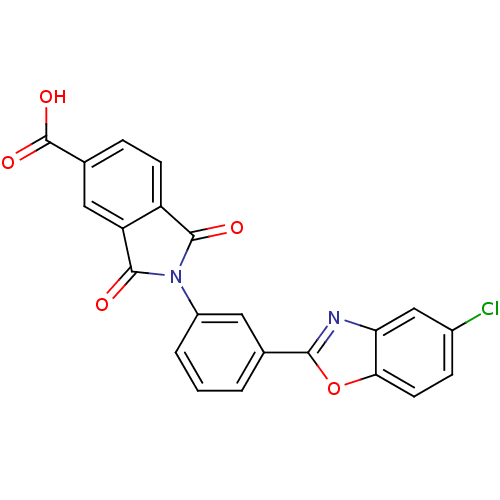
(2-(3-(5-chlorobenzo[d]oxazol-2-yl)phenyl)-1,3-diox...)Show SMILES OC(=O)c1ccc2C(=O)N(C(=O)c2c1)c1cccc(c1)-c1nc2cc(Cl)ccc2o1 Show InChI InChI=1S/C22H11ClN2O5/c23-13-5-7-18-17(10-13)24-19(30-18)11-2-1-3-14(8-11)25-20(26)15-6-4-12(22(28)29)9-16(15)21(25)27/h1-10H,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147511
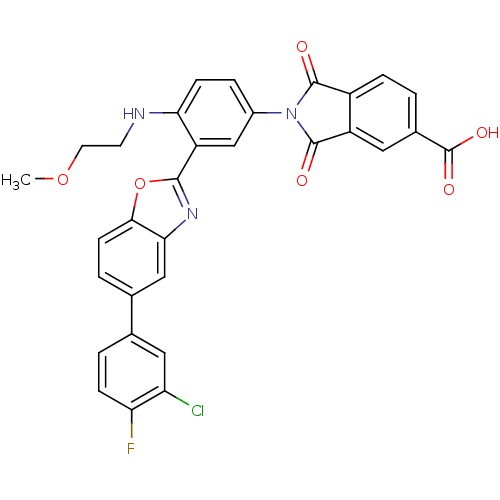
(2-[3-[5-(3-Chloro-4-fluoro-phenyl)-benzooxazol-2-y...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(F)c(Cl)c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H21ClFN3O6/c1-41-11-10-34-25-8-5-19(36-29(37)20-6-2-18(31(39)40)12-21(20)30(36)38)15-22(25)28-35-26-14-17(4-9-27(26)42-28)16-3-7-24(33)23(32)13-16/h2-9,12-15,34H,10-11H2,1H3,(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147524
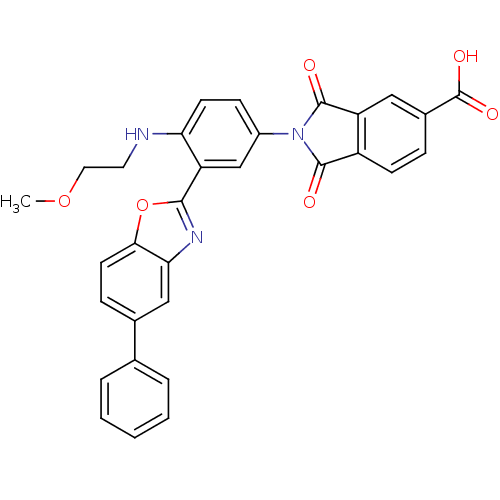
(2-(4-(2-methoxyethylamino)-3-(5-phenylbenzo[d]oxaz...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H23N3O6/c1-39-14-13-32-25-11-9-21(34-29(35)22-10-7-20(31(37)38)15-23(22)30(34)36)17-24(25)28-33-26-16-19(8-12-27(26)40-28)18-5-3-2-4-6-18/h2-12,15-17,32H,13-14H2,1H3,(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147509
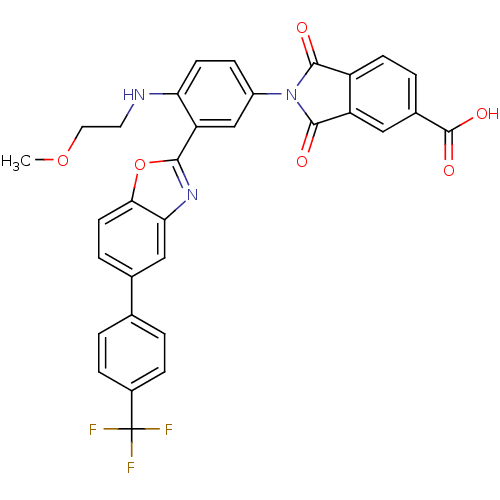
(2-{4-(2-Methoxy-ethylamino)-3-[5-(4-trifluoromethy...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(cc1)C(F)(F)F)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C32H22F3N3O6/c1-43-13-12-36-25-10-8-21(38-29(39)22-9-4-19(31(41)42)14-23(22)30(38)40)16-24(25)28-37-26-15-18(5-11-27(26)44-28)17-2-6-20(7-3-17)32(33,34)35/h2-11,14-16,36H,12-13H2,1H3,(H,41,42) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147548
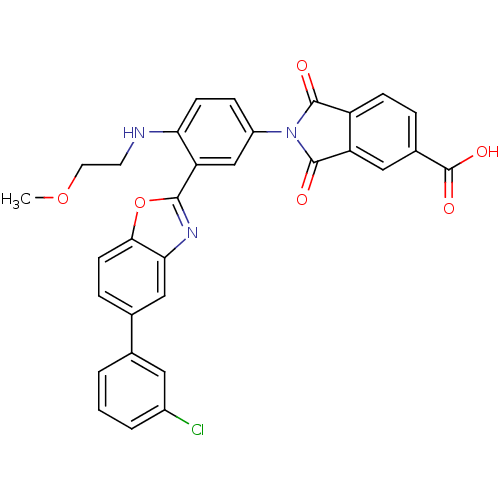
(2-[3-[5-(3-Chloro-phenyl)-benzooxazol-2-yl]-4-(2-m...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1cccc(Cl)c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22ClN3O6/c1-40-12-11-33-25-9-7-21(35-29(36)22-8-5-19(31(38)39)14-23(22)30(35)37)16-24(25)28-34-26-15-18(6-10-27(26)41-28)17-3-2-4-20(32)13-17/h2-10,13-16,33H,11-12H2,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147506
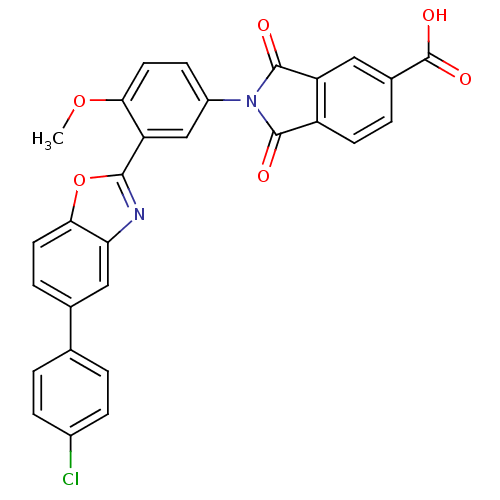
(2-(3-(5-(4-chlorophenyl)benzo[d]oxazol-2-yl)-4-met...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(Cl)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C29H17ClN2O6/c1-37-24-11-8-19(32-27(33)20-9-4-17(29(35)36)12-21(20)28(32)34)14-22(24)26-31-23-13-16(5-10-25(23)38-26)15-2-6-18(30)7-3-15/h2-14H,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147527
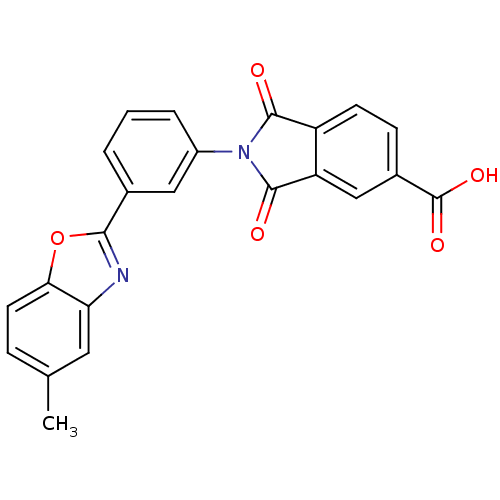
(2-(3-(5-methylbenzo[d]oxazol-2-yl)phenyl)-1,3-diox...)Show SMILES Cc1ccc2oc(nc2c1)-c1cccc(c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C23H14N2O5/c1-12-5-8-19-18(9-12)24-20(30-19)13-3-2-4-15(10-13)25-21(26)16-7-6-14(23(28)29)11-17(16)22(25)27/h2-11H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147525
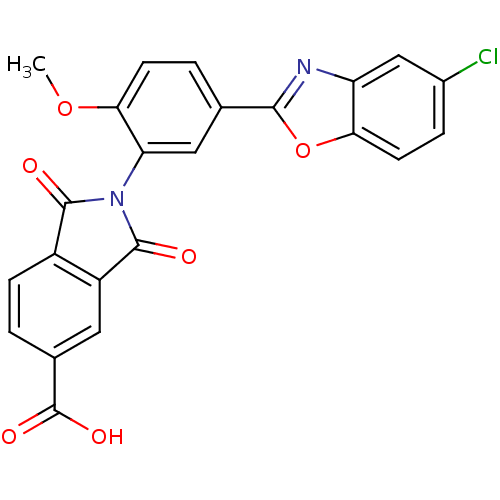
(2-(5-(5-chlorobenzo[d]oxazol-2-yl)-2-methoxyphenyl...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(Cl)ccc2o1 Show InChI InChI=1S/C23H13ClN2O6/c1-31-19-6-3-11(20-25-16-10-13(24)4-7-18(16)32-20)9-17(19)26-21(27)14-5-2-12(23(29)30)8-15(14)22(26)28/h2-10H,1H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147523
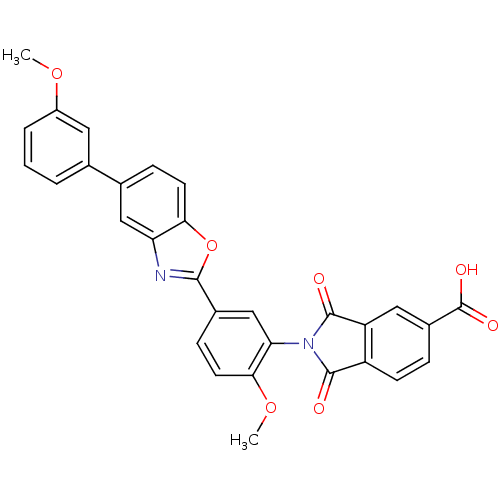
(2-(2-methoxy-5-(5-(3-methoxyphenyl)benzo[d]oxazol-...)Show SMILES COc1cccc(c1)-c1ccc2oc(nc2c1)-c1ccc(OC)c(c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H20N2O7/c1-37-20-5-3-4-16(12-20)17-7-10-25-23(14-17)31-27(39-25)18-8-11-26(38-2)24(15-18)32-28(33)21-9-6-19(30(35)36)13-22(21)29(32)34/h3-15H,1-2H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147514
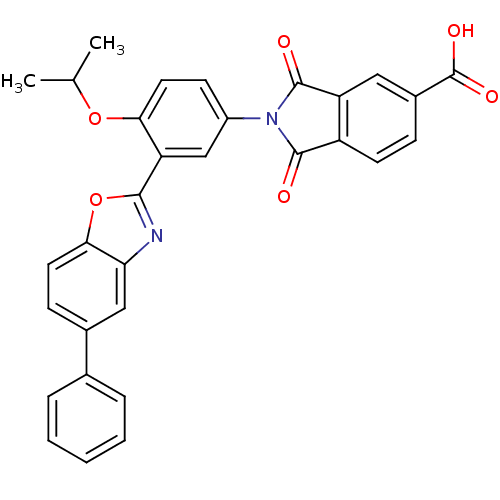
(2-(4-isopropoxy-3-(5-phenylbenzo[d]oxazol-2-yl)phe...)Show SMILES CC(C)Oc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22N2O6/c1-17(2)38-26-13-10-21(33-29(34)22-11-8-20(31(36)37)14-23(22)30(33)35)16-24(26)28-32-25-15-19(9-12-27(25)39-28)18-6-4-3-5-7-18/h3-17H,1-2H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147516
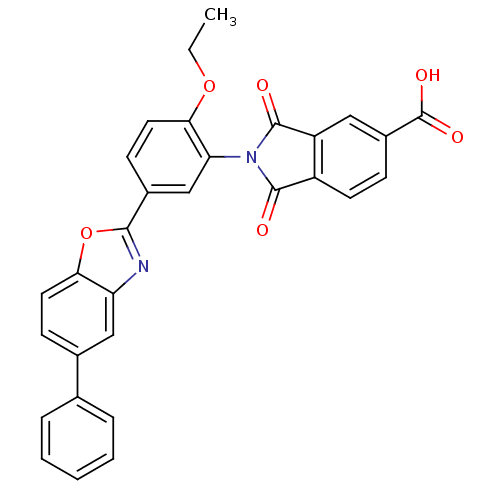
(2-(2-ethoxy-5-(5-phenylbenzo[d]oxazol-2-yl)phenyl)...)Show SMILES CCOc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccccc1 Show InChI InChI=1S/C30H20N2O6/c1-2-37-26-13-10-19(27-31-23-15-18(9-12-25(23)38-27)17-6-4-3-5-7-17)16-24(26)32-28(33)21-11-8-20(30(35)36)14-22(21)29(32)34/h3-16H,2H2,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147551
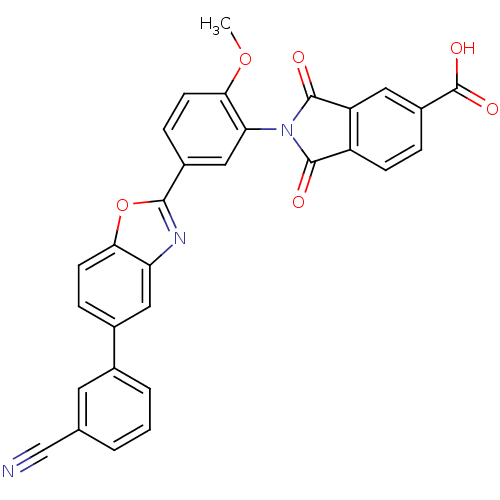
(2-(5-(5-(3-cyanophenyl)benzo[d]oxazol-2-yl)-2-meth...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1cccc(c1)C#N Show InChI InChI=1S/C30H17N3O6/c1-38-26-10-7-19(14-24(26)33-28(34)21-8-5-20(30(36)37)12-22(21)29(33)35)27-32-23-13-18(6-9-25(23)39-27)17-4-2-3-16(11-17)15-31/h2-14H,1H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147529
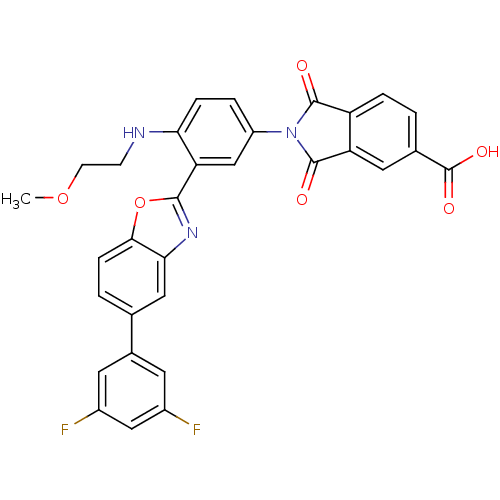
(2-[3-[5-(3,5-Difluoro-phenyl)-benzooxazol-2-yl]-4-...)Show SMILES COCCNc1ccc(cc1-c1nc2cc(ccc2o1)-c1cc(F)cc(F)c1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H21F2N3O6/c1-41-9-8-34-25-6-4-21(36-29(37)22-5-2-17(31(39)40)12-23(22)30(36)38)15-24(25)28-35-26-13-16(3-7-27(26)42-28)18-10-19(32)14-20(33)11-18/h2-7,10-15,34H,8-9H2,1H3,(H,39,40) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147521
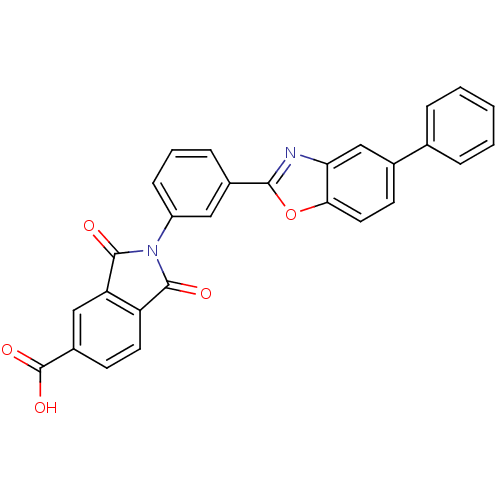
(1,3-Dioxo-2-[3-(5-phenyl-benzooxazol-2-yl)-phenyl]...)Show SMILES OC(=O)c1ccc2C(=O)N(C(=O)c2c1)c1cccc(c1)-c1nc2cc(ccc2o1)-c1ccccc1 Show InChI InChI=1S/C28H16N2O5/c31-26-21-11-9-19(28(33)34)14-22(21)27(32)30(26)20-8-4-7-18(13-20)25-29-23-15-17(10-12-24(23)35-25)16-5-2-1-3-6-16/h1-15H,(H,33,34) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147549
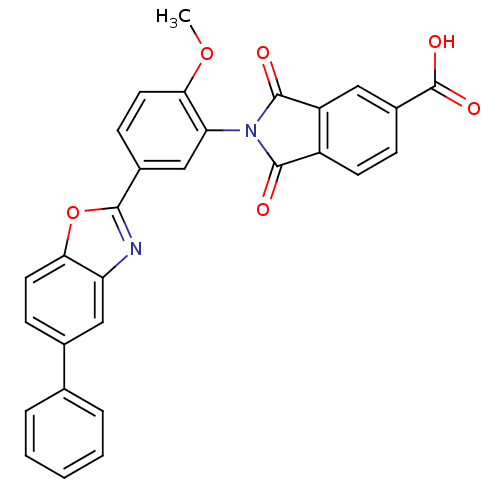
(2-(2-methoxy-5-(5-phenylbenzo[d]oxazol-2-yl)phenyl...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccccc1 Show InChI InChI=1S/C29H18N2O6/c1-36-25-12-9-18(26-30-22-14-17(8-11-24(22)37-26)16-5-3-2-4-6-16)15-23(25)31-27(32)20-10-7-19(29(34)35)13-21(20)28(31)33/h2-15H,1H3,(H,34,35) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147545
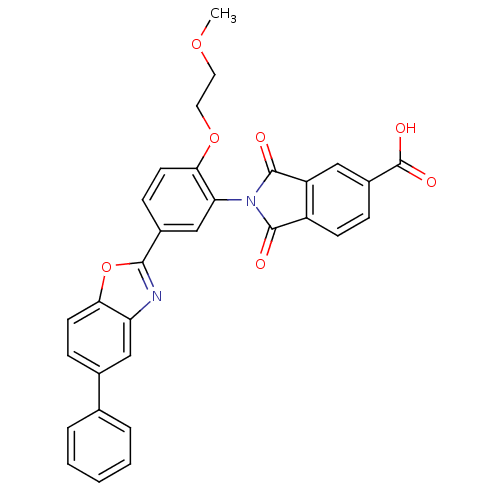
(2-(2-(2-methoxyethoxy)-5-(5-phenylbenzo[d]oxazol-2...)Show SMILES COCCOc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccccc1 Show InChI InChI=1S/C31H22N2O7/c1-38-13-14-39-27-12-9-20(28-32-24-16-19(8-11-26(24)40-28)18-5-3-2-4-6-18)17-25(27)33-29(34)22-10-7-21(31(36)37)15-23(22)30(33)35/h2-12,15-17H,13-14H2,1H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147540
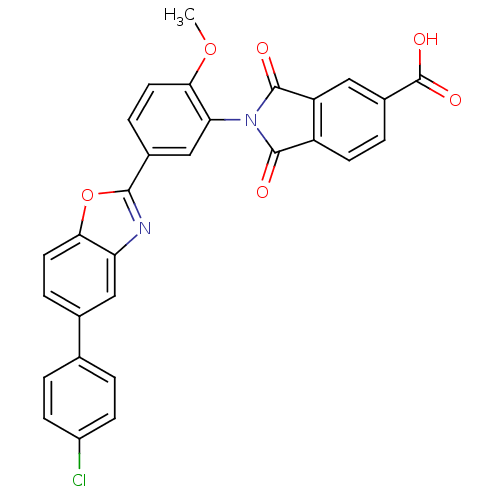
(2-(5-(5-(4-chlorophenyl)benzo[d]oxazol-2-yl)-2-met...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccc(Cl)cc1 Show InChI InChI=1S/C29H17ClN2O6/c1-37-25-11-6-17(14-23(25)32-27(33)20-9-4-18(29(35)36)12-21(20)28(32)34)26-31-22-13-16(5-10-24(22)38-26)15-2-7-19(30)8-3-15/h2-14H,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147520
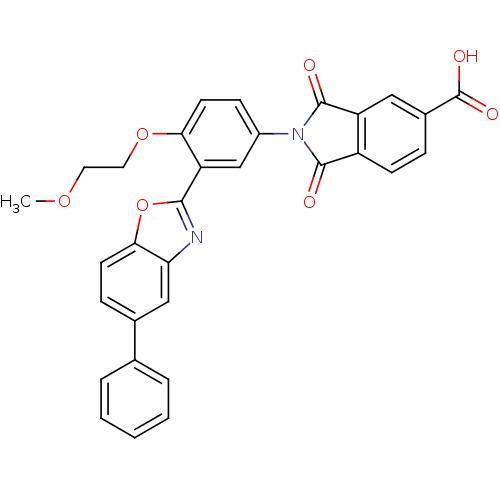
(2-(4-(2-methoxyethoxy)-3-(5-phenylbenzo[d]oxazol-2...)Show SMILES COCCOc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C31H22N2O7/c1-38-13-14-39-26-12-9-21(33-29(34)22-10-7-20(31(36)37)15-23(22)30(33)35)17-24(26)28-32-25-16-19(8-11-27(25)40-28)18-5-3-2-4-6-18/h2-12,15-17H,13-14H2,1H3,(H,36,37) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147512
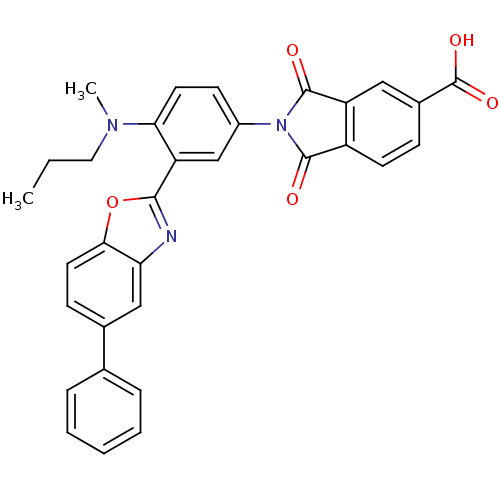
(2-(4-(methyl(propyl)amino)-3-(5-phenylbenzo[d]oxaz...)Show SMILES CCCN(C)c1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C32H25N3O5/c1-3-15-34(2)27-13-11-22(35-30(36)23-12-9-21(32(38)39)16-24(23)31(35)37)18-25(27)29-33-26-17-20(10-14-28(26)40-29)19-7-5-4-6-8-19/h4-14,16-18H,3,15H2,1-2H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147526
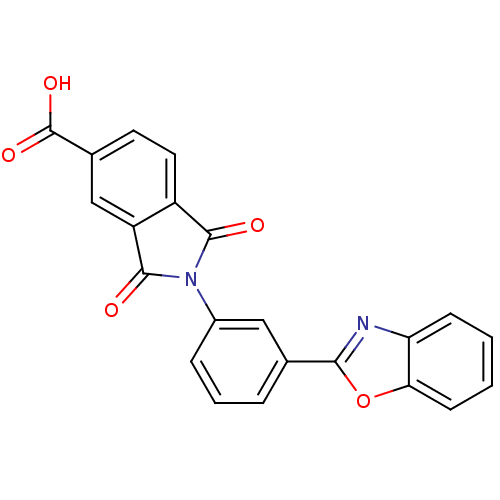
(2-(3-(benzo[d]oxazol-2-yl)phenyl)-1,3-dioxoisoindo...)Show SMILES OC(=O)c1ccc2C(=O)N(C(=O)c2c1)c1cccc(c1)-c1nc2ccccc2o1 Show InChI InChI=1S/C22H12N2O5/c25-20-15-9-8-13(22(27)28)11-16(15)21(26)24(20)14-5-3-4-12(10-14)19-23-17-6-1-2-7-18(17)29-19/h1-11H,(H,27,28) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147547
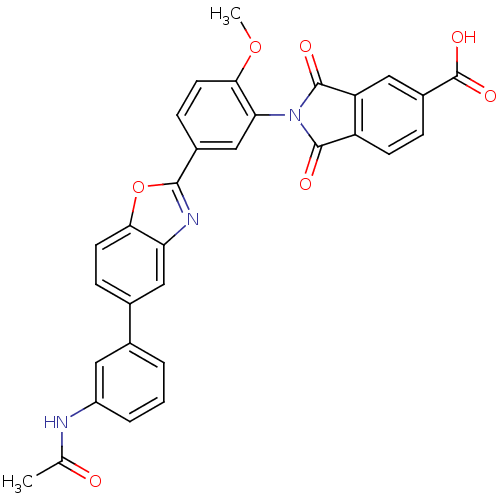
(2-(5-(5-(3-acetamidophenyl)benzo[d]oxazol-2-yl)-2-...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1cccc(NC(C)=O)c1 Show InChI InChI=1S/C31H21N3O7/c1-16(35)32-21-5-3-4-17(12-21)18-7-10-26-24(14-18)33-28(41-26)19-8-11-27(40-2)25(15-19)34-29(36)22-9-6-20(31(38)39)13-23(22)30(34)37/h3-15H,1-2H3,(H,32,35)(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147508
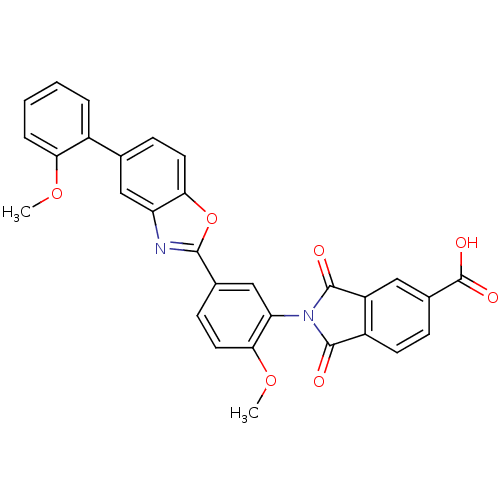
(2-(2-methoxy-5-(5-(2-methoxyphenyl)benzo[d]oxazol-...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(ccc2o1)-c1ccccc1OC Show InChI InChI=1S/C30H20N2O7/c1-37-24-6-4-3-5-19(24)16-8-11-25-22(14-16)31-27(39-25)17-9-12-26(38-2)23(15-17)32-28(33)20-10-7-18(30(35)36)13-21(20)29(32)34/h3-15H,1-2H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147541
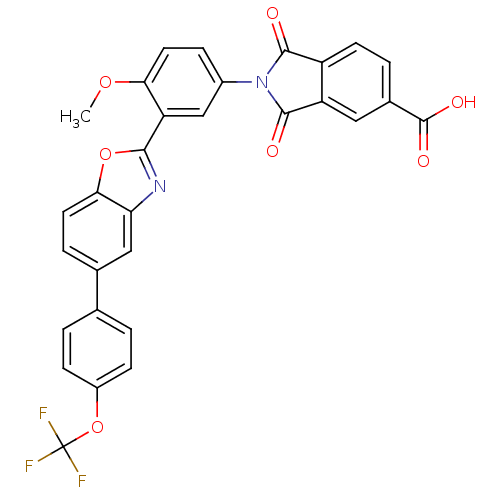
(2-{4-Methoxy-3-[5-(4-trifluoromethoxy-phenyl)-benz...)Show SMILES COc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccc(OC(F)(F)F)cc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C30H17F3N2O7/c1-40-24-11-6-18(35-27(36)20-9-4-17(29(38)39)12-21(20)28(35)37)14-22(24)26-34-23-13-16(5-10-25(23)41-26)15-2-7-19(8-3-15)42-30(31,32)33/h2-14H,1H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147543
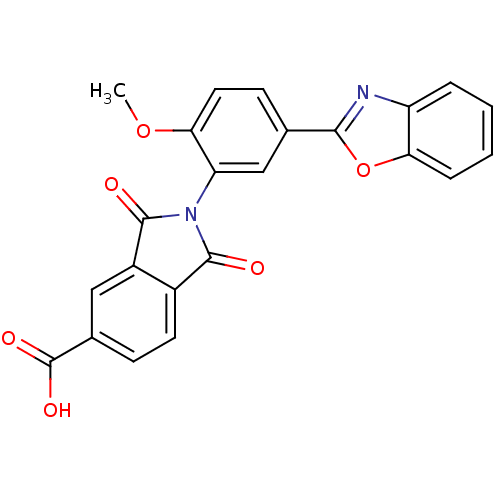
(2-(5-Benzooxazol-2-yl-2-methoxy-phenyl)-1,3-dioxo-...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2ccccc2o1 Show InChI InChI=1S/C23H14N2O6/c1-30-19-9-7-12(20-24-16-4-2-3-5-18(16)31-20)11-17(19)25-21(26)14-8-6-13(23(28)29)10-15(14)22(25)27/h2-11H,1H3,(H,28,29) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147532

(2-[4-Methylamino-3-(5-phenyl-benzooxazol-2-yl)-phe...)Show SMILES CNc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C29H19N3O5/c1-30-23-11-9-19(32-27(33)20-10-7-18(29(35)36)13-21(20)28(32)34)15-22(23)26-31-24-14-17(8-12-25(24)37-26)16-5-3-2-4-6-16/h2-15,30H,1H3,(H,35,36) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147550
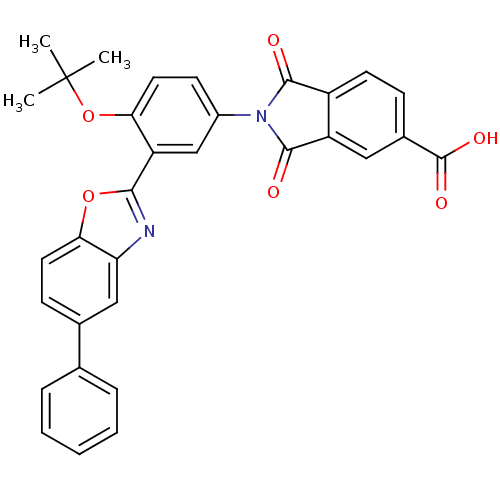
(2-[4-tert-Butoxy-3-(5-phenyl-benzooxazol-2-yl)-phe...)Show SMILES CC(C)(C)Oc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C32H24N2O6/c1-32(2,3)40-26-14-11-21(34-29(35)22-12-9-20(31(37)38)15-23(22)30(34)36)17-24(26)28-33-25-16-19(10-13-27(25)39-28)18-7-5-4-6-8-18/h4-17H,1-3H3,(H,37,38) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147542
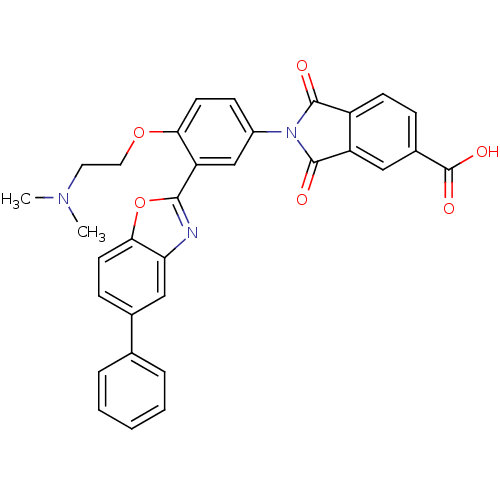
(2-[4-(2-Dimethylamino-ethoxy)-3-(5-phenyl-benzooxa...)Show SMILES CN(C)CCOc1ccc(cc1-c1nc2cc(ccc2o1)-c1ccccc1)N1C(=O)c2ccc(cc2C1=O)C(O)=O Show InChI InChI=1S/C32H25N3O6/c1-34(2)14-15-40-27-13-10-22(35-30(36)23-11-8-21(32(38)39)16-24(23)31(35)37)18-25(27)29-33-26-17-20(9-12-28(26)41-29)19-6-4-3-5-7-19/h3-13,16-18H,14-15H2,1-2H3,(H,38,39) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |
Heparanase
(Homo sapiens (Human)) | BDBM50147507
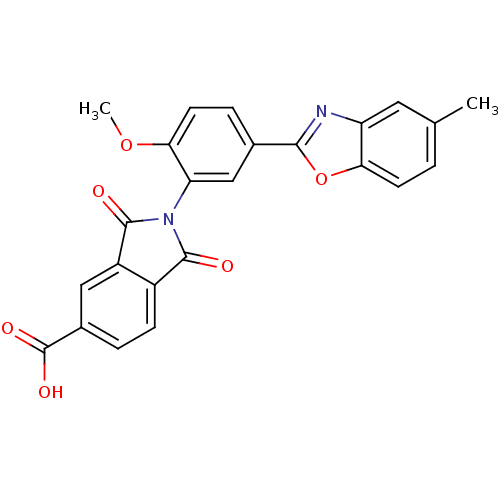
(2-[2-Methoxy-5-(5-methyl-benzooxazol-2-yl)-phenyl]...)Show SMILES COc1ccc(cc1N1C(=O)c2ccc(cc2C1=O)C(O)=O)-c1nc2cc(C)ccc2o1 Show InChI InChI=1S/C24H16N2O6/c1-12-3-7-19-17(9-12)25-21(32-19)13-5-8-20(31-2)18(11-13)26-22(27)15-6-4-14(24(29)30)10-16(15)23(26)28/h3-11H,1-2H3,(H,29,30) | PDB
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Celltech R&D Ltd
Curated by ChEMBL
| Assay Description
In vivo inhibitory activity against human Heparanase |
Bioorg Med Chem Lett 14: 3269-73 (2004)
Article DOI: 10.1016/j.bmcl.2004.03.086
BindingDB Entry DOI: 10.7270/Q2CC1040 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data