

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081444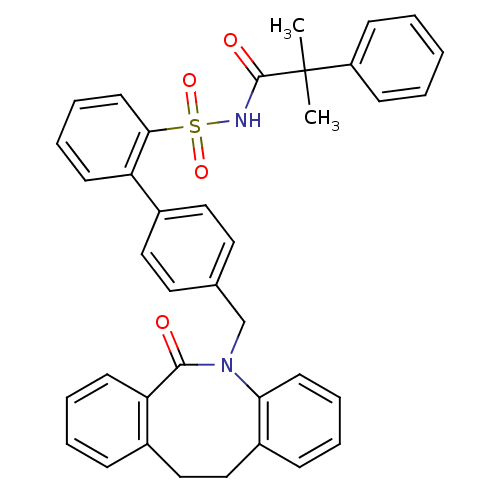 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081440 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081453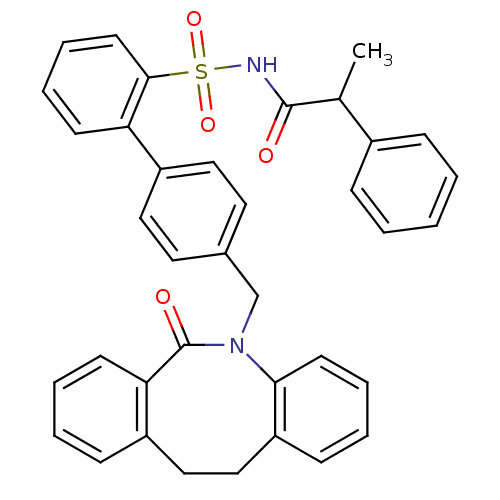 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081439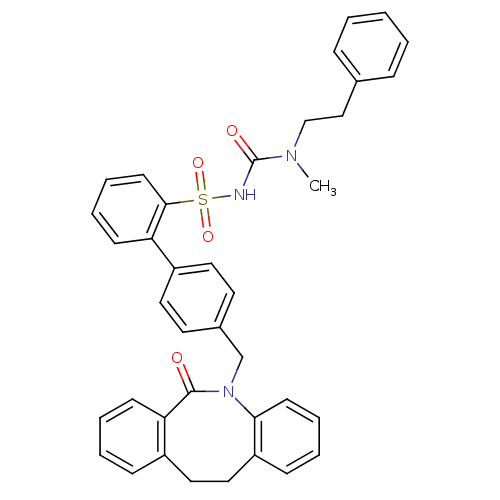 (CHEMBL92539 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081445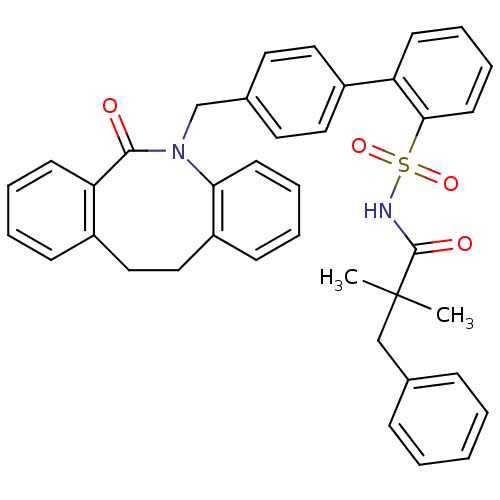 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 18 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081436 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 47 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081438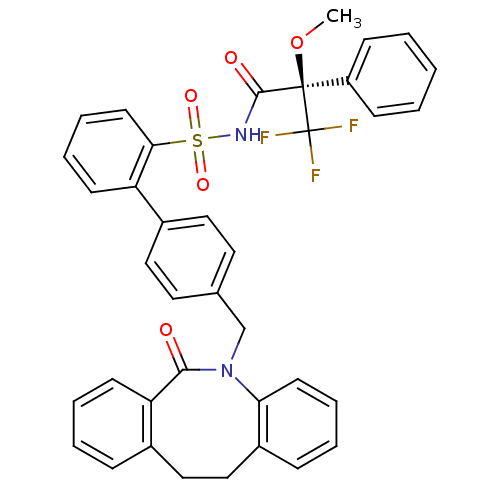 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 50 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081452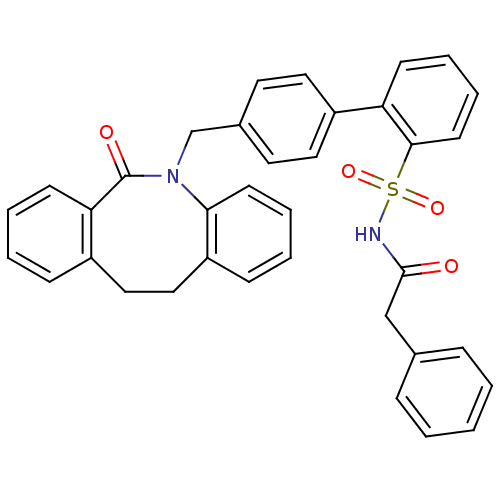 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 57 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081435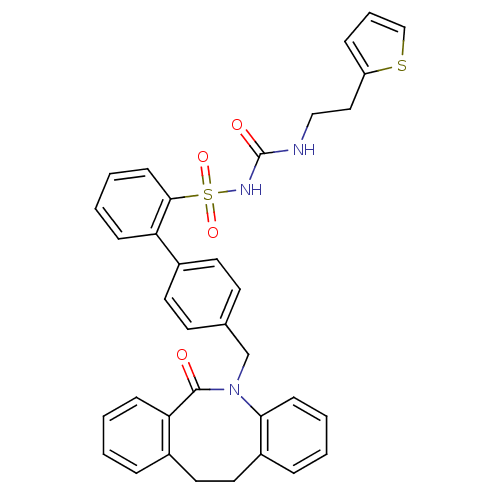 (CHEMBL328697 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 100 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081450 (CHEMBL90269 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 120 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081448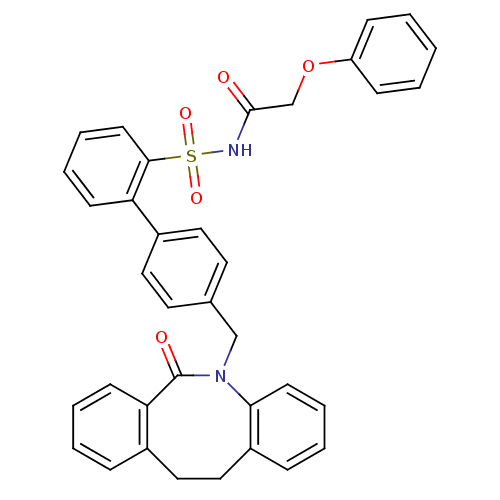 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081434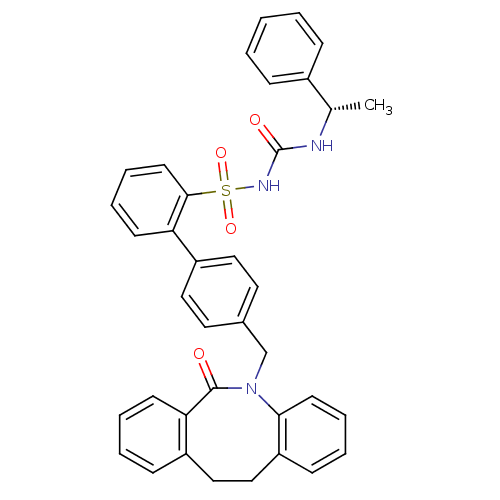 (CHEMBL327597 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 140 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081447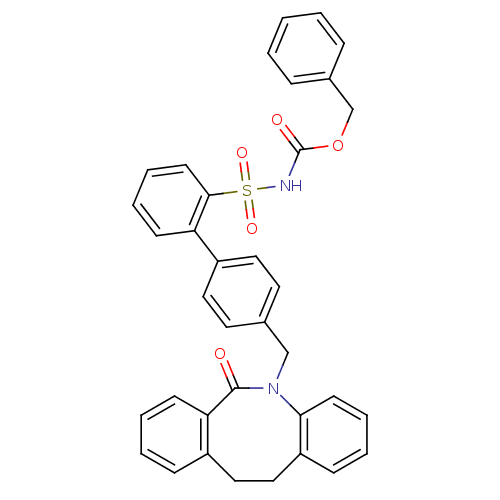 (CHEMBL91063 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 150 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081442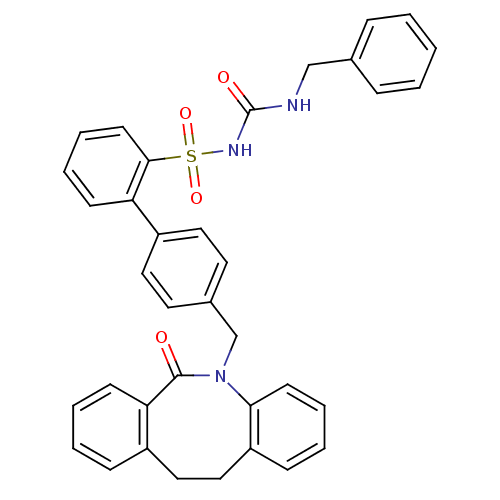 (CHEMBL90226 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081446 (CHEMBL91270 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 220 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081437 (CHEMBL327596 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 360 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081443 (CHEMBL90935 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 400 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081456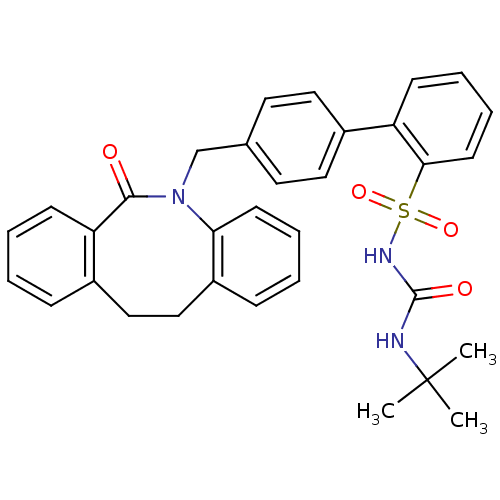 (CHEMBL88154 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 770 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50081439 (CHEMBL92539 | sulfonylurea analogue) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP4 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50081445 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human TP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081456 (CHEMBL88154 | sulfonylurea analogue) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081451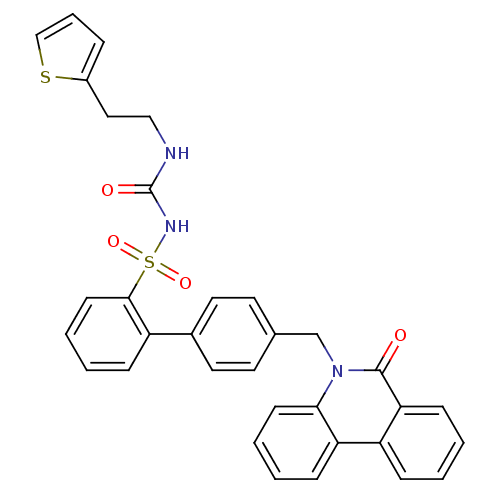 (CHEMBL314097 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081440 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50081445 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human FP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50081440 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human TP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081438 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50081440 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human DP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50081439 (CHEMBL92539 | sulfonylurea analogue) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human TP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081435 (CHEMBL328697 | sulfonylurea analogue) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081447 (CHEMBL91063 | sulfonylurea analogue) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50081445 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP4 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50081444 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human DP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081452 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50081453 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human DP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50081453 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human TP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP1 subtype (Homo sapiens (Human)) | BDBM50081455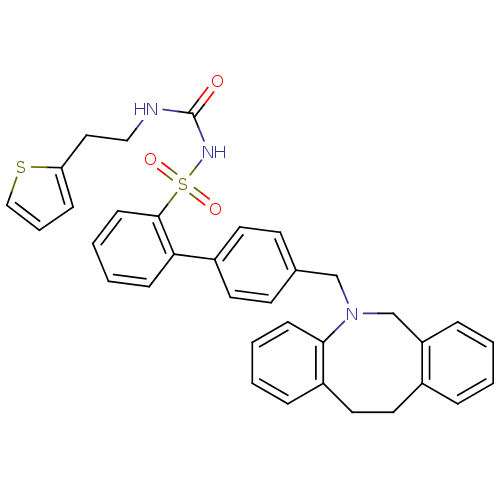 (CHEMBL316278 | sulfonylurea analogue) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP1 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50081439 (CHEMBL92539 | sulfonylurea analogue) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 2.87E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human DP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50081438 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP4 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thromboxane A2 receptor (Homo sapiens (Human)) | BDBM50081444 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human TP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081436 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50081440 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human FP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081444 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin D2 receptor (Homo sapiens (Human)) | BDBM50081445 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human DP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50081440 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP4 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081441 (CHEMBL262690 | sulfonylurea analogue) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP4 subtype (Homo sapiens (Human)) | BDBM50081456 (CHEMBL88154 | sulfonylurea analogue) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP4 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081434 (CHEMBL327597 | sulfonylurea analogue) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081448 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin F2-alpha receptor (Homo sapiens (Human)) | BDBM50081444 (4'-(6-Oxo-11,12-dihydro-6H-dibenzo[b,f]azocin-5-yl...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Compound was evaluated for its secondary binding affinity to human FP receptors by using Aequorin luminescence-based functional calcium assay | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E2 receptor EP3 subtype (Homo sapiens (Human)) | BDBM50081439 (CHEMBL92539 | sulfonylurea analogue) | PDB KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | 5.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Frosst Centre for Therapeutic Research Curated by ChEMBL | Assay Description Affinity at human EP3 receptor. | Bioorg Med Chem Lett 9: 2699-704 (1999) BindingDB Entry DOI: 10.7270/Q2HX1BV9 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 103 total ) | Next | Last >> |