 Found 164 hits Enz. Inhib. hit(s) with all data for entry = 50028827
Found 164 hits Enz. Inhib. hit(s) with all data for entry = 50028827 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268434

((+/-)-6-(1-methyl-1H-pyrazol-5-yl)-4-oxo-N-((tans)...)Show SMILES Cn1nccc1-c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H28N4O3/c1-30-23(9-12-28-30)19-7-8-25-21(15-19)24(32)17-27(34-25)10-13-31(14-11-27)26(33)29-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22H,10-11,13-14,16-17H2,1H3,(H,29,33)/t20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268321
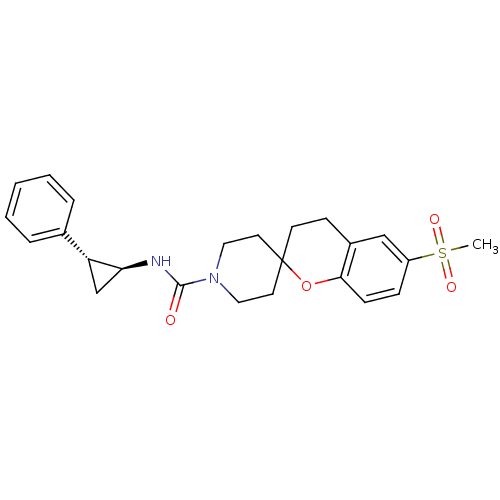
(6-(methylsulfonyl)-N-((trans)-2-phenylcyclopropyl)...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C24H28N2O4S/c1-31(28,29)19-7-8-22-18(15-19)9-10-24(30-22)11-13-26(14-12-24)23(27)25-21-16-20(21)17-5-3-2-4-6-17/h2-8,15,20-21H,9-14,16H2,1H3,(H,25,27)/t20-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268320

((+/-)-6-fluoro-N-((trans)-2-phenylcyclopropyl)spir...)Show SMILES Fc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C23H25FN2O2/c24-18-6-7-21-17(14-18)8-9-23(28-21)10-12-26(13-11-23)22(27)25-20-15-19(20)16-4-2-1-3-5-16/h1-7,14,19-20H,8-13,15H2,(H,25,27)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268435
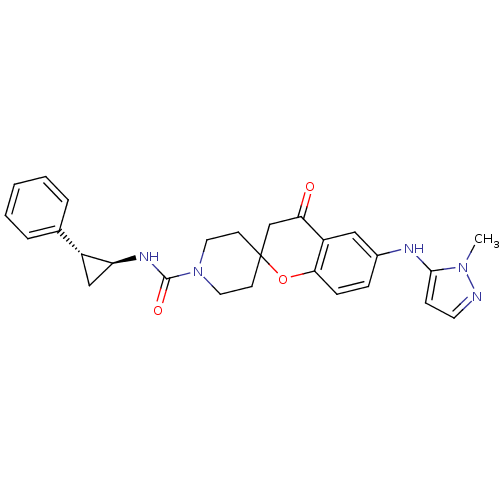
((+/-)-6-(1-methyl-1H-pyrazol-5-ylamino)-4-oxo-N-((...)Show SMILES Cn1nccc1Nc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H29N5O3/c1-31-25(9-12-28-31)29-19-7-8-24-21(15-19)23(33)17-27(35-24)10-13-32(14-11-27)26(34)30-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22,29H,10-11,13-14,16-17H2,1H3,(H,30,34)/t20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268440

(2-(2,4-dichlorophenyl)-1-(6-(methylsulfonyl)spiro[...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)Cc3ccc(Cl)cc3Cl)CCc2c1 Show InChI InChI=1S/C22H23Cl2NO4S/c1-30(27,28)18-4-5-20-16(12-18)6-7-22(29-20)8-10-25(11-9-22)21(26)13-15-2-3-17(23)14-19(15)24/h2-5,12,14H,6-11,13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268439
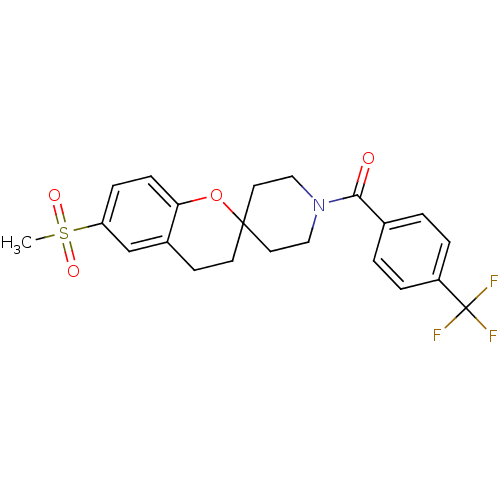
((6-(methylsulfonyl)spiro[chroman-2,4'-piperidine]-...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)c3ccc(cc3)C(F)(F)F)CCc2c1 Show InChI InChI=1S/C22H22F3NO4S/c1-31(28,29)18-6-7-19-16(14-18)8-9-21(30-19)10-12-26(13-11-21)20(27)15-2-4-17(5-3-15)22(23,24)25/h2-7,14H,8-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268438

(CHEMBL524992 | spiro[chroman-2,4'-piperidine]-1'-y...)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)N1CCC2(CC1)CCc1ccccc1O2 Show InChI InChI=1S/C21H20F3NO2/c22-21(23,24)17-7-5-16(6-8-17)19(26)25-13-11-20(12-14-25)10-9-15-3-1-2-4-18(15)27-20/h1-8H,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268381
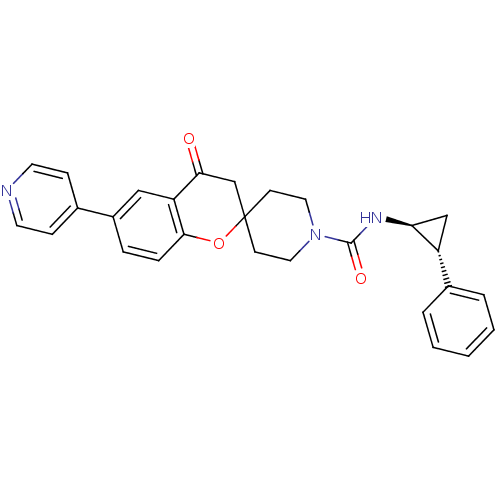
(4-oxo-N-((trans)-2-phenylcyclopropyl)-6-(pyridin-4...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1cc(ccc1O2)-c1ccncc1 |r| Show InChI InChI=1S/C28H27N3O3/c32-25-18-28(34-26-7-6-21(16-23(25)26)19-8-12-29-13-9-19)10-14-31(15-11-28)27(33)30-24-17-22(24)20-4-2-1-3-5-20/h1-9,12-13,16,22,24H,10-11,14-15,17-18H2,(H,30,33)/t22-,24+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268440

(2-(2,4-dichlorophenyl)-1-(6-(methylsulfonyl)spiro[...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)Cc3ccc(Cl)cc3Cl)CCc2c1 Show InChI InChI=1S/C22H23Cl2NO4S/c1-30(27,28)18-4-5-20-16(12-18)6-7-22(29-20)8-10-25(11-9-22)21(26)13-15-2-3-17(23)14-19(15)24/h2-5,12,14H,6-11,13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268384

(CHEMBL522211 | N-((trans)-2-phenylcyclopropyl)-3',...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CCc1ncccc1O2 |r| Show InChI InChI=1S/C22H25N3O2/c26-21(24-19-15-17(19)16-5-2-1-3-6-16)25-13-10-22(11-14-25)9-8-18-20(27-22)7-4-12-23-18/h1-7,12,17,19H,8-11,13-15H2,(H,24,26)/t17-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | <1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268439

((6-(methylsulfonyl)spiro[chroman-2,4'-piperidine]-...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)c3ccc(cc3)C(F)(F)F)CCc2c1 Show InChI InChI=1S/C22H22F3NO4S/c1-31(28,29)18-6-7-19-16(14-18)8-9-21(30-19)10-12-26(13-11-21)20(27)15-2-4-17(5-3-15)22(23,24)25/h2-7,14H,8-13H2,1H3 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268380
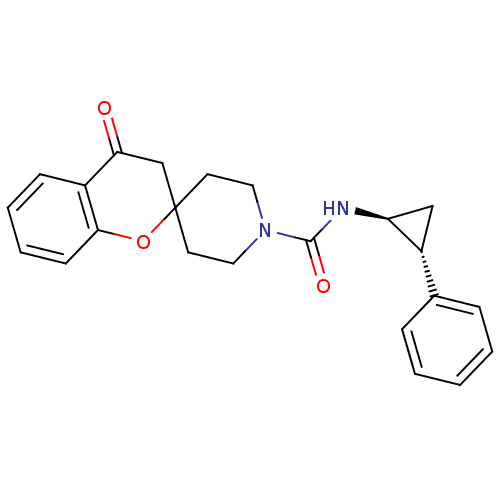
((+/-)-4-oxo-N-((tans)-2-phenylcyclopropyl)spiro[ch...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ccccc1O2 |r| Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-9-5-4-8-17(20)21)10-12-25(13-11-23)22(27)24-19-14-18(19)16-6-2-1-3-7-16/h1-9,18-19H,10-15H2,(H,24,27)/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268321

(6-(methylsulfonyl)-N-((trans)-2-phenylcyclopropyl)...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C24H28N2O4S/c1-31(28,29)19-7-8-22-18(15-19)9-10-24(30-22)11-13-26(14-12-24)23(27)25-21-16-20(21)17-5-3-2-4-6-17/h2-8,15,20-21H,9-14,16H2,1H3,(H,25,27)/t20-,21+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268320

((+/-)-6-fluoro-N-((trans)-2-phenylcyclopropyl)spir...)Show SMILES Fc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C23H25FN2O2/c24-18-6-7-21-17(14-18)8-9-23(28-21)10-12-26(13-11-23)22(27)25-20-15-19(20)16-4-2-1-3-5-16/h1-7,14,19-20H,8-13,15H2,(H,25,27)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268380

((+/-)-4-oxo-N-((tans)-2-phenylcyclopropyl)spiro[ch...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ccccc1O2 |r| Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-9-5-4-8-17(20)21)10-12-25(13-11-23)22(27)24-19-14-18(19)16-6-2-1-3-7-16/h1-9,18-19H,10-15H2,(H,24,27)/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268441
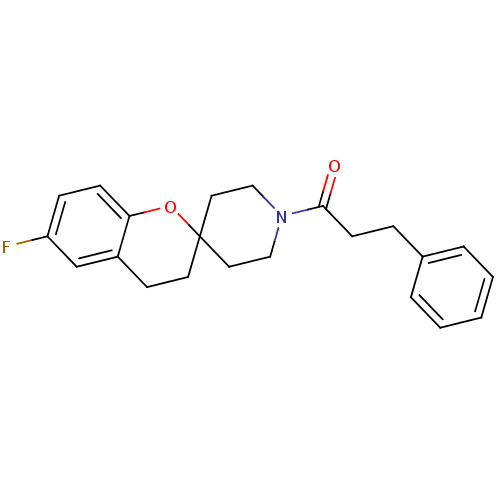
(1-(6-fluorospiro[chroman-2,4'-piperidine]-1'-yl)-3...)Show InChI InChI=1S/C22H24FNO2/c23-19-7-8-20-18(16-19)10-11-22(26-20)12-14-24(15-13-22)21(25)9-6-17-4-2-1-3-5-17/h1-5,7-8,16H,6,9-15H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268380

((+/-)-4-oxo-N-((tans)-2-phenylcyclopropyl)spiro[ch...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ccccc1O2 |r| Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-9-5-4-8-17(20)21)10-12-25(13-11-23)22(27)24-19-14-18(19)16-6-2-1-3-7-16/h1-9,18-19H,10-15H2,(H,24,27)/t18-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268435

((+/-)-6-(1-methyl-1H-pyrazol-5-ylamino)-4-oxo-N-((...)Show SMILES Cn1nccc1Nc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H29N5O3/c1-31-25(9-12-28-31)29-19-7-8-24-21(15-19)23(33)17-27(35-24)10-13-32(14-11-27)26(34)30-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22,29H,10-11,13-14,16-17H2,1H3,(H,30,34)/t20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268322
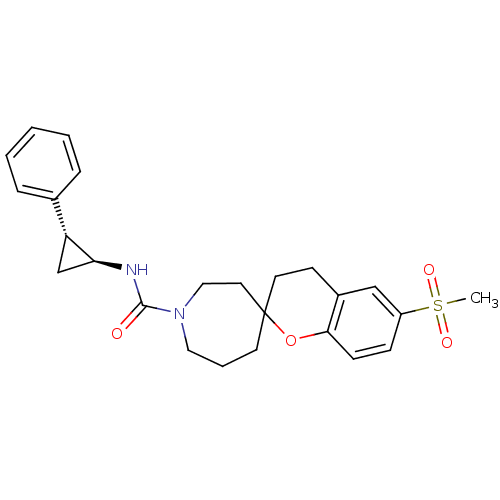
(6'-(methylsulfonyl)-N-((trans)-2-phenylcyclopropyl...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCc2c1)CCCN(CC3)C(=O)N[C@H]1C[C@@H]1c1ccccc1 |r| Show InChI InChI=1S/C25H30N2O4S/c1-32(29,30)20-8-9-23-19(16-20)10-12-25(31-23)11-5-14-27(15-13-25)24(28)26-22-17-21(22)18-6-3-2-4-7-18/h2-4,6-9,16,21-22H,5,10-15,17H2,1H3,(H,26,28)/t21-,22+,25?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268436
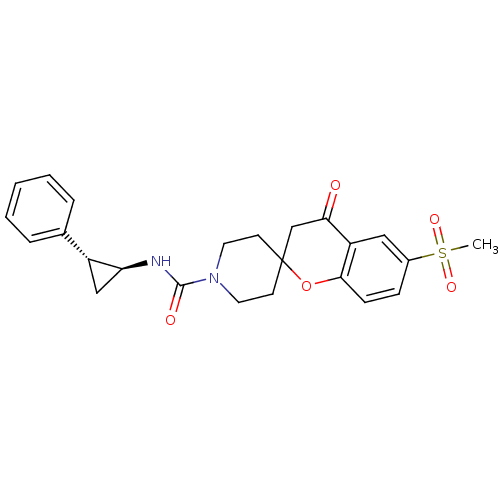
((+/-)-6-(methylsulfonyl)-4-oxo-N-((trans)-2-phenyl...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C24H26N2O5S/c1-32(29,30)17-7-8-22-19(13-17)21(27)15-24(31-22)9-11-26(12-10-24)23(28)25-20-14-18(20)16-5-3-2-4-6-16/h2-8,13,18,20H,9-12,14-15H2,1H3,(H,25,28)/t18-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268440

(2-(2,4-dichlorophenyl)-1-(6-(methylsulfonyl)spiro[...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)Cc3ccc(Cl)cc3Cl)CCc2c1 Show InChI InChI=1S/C22H23Cl2NO4S/c1-30(27,28)18-4-5-20-16(12-18)6-7-22(29-20)8-10-25(11-9-22)21(26)13-15-2-3-17(23)14-19(15)24/h2-5,12,14H,6-11,13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268438

(CHEMBL524992 | spiro[chroman-2,4'-piperidine]-1'-y...)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)N1CCC2(CC1)CCc1ccccc1O2 Show InChI InChI=1S/C21H20F3NO2/c22-21(23,24)17-7-5-16(6-8-17)19(26)25-13-11-20(12-14-25)10-9-15-3-1-2-4-18(15)27-20/h1-8H,9-14H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268437

((+/-)-N-((trans)-2-phenylcyclopropyl)spiro[chroman...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CCc1ccccc1O2 |r| Show InChI InChI=1S/C23H26N2O2/c26-22(24-20-16-19(20)17-6-2-1-3-7-17)25-14-12-23(13-15-25)11-10-18-8-4-5-9-21(18)27-23/h1-9,19-20H,10-16H2,(H,24,26)/t19-,20+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268434

((+/-)-6-(1-methyl-1H-pyrazol-5-yl)-4-oxo-N-((tans)...)Show SMILES Cn1nccc1-c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H28N4O3/c1-30-23(9-12-28-30)19-7-8-25-21(15-19)24(32)17-27(34-25)10-13-31(14-11-27)26(33)29-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22H,10-11,13-14,16-17H2,1H3,(H,29,33)/t20-,22+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268382
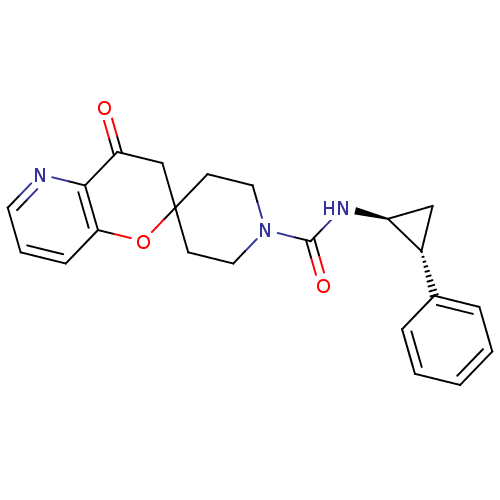
(4'-oxo-N-((trans)-2-phenylcyclopropyl)-3',4'-dihyd...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ncccc1O2 |r| Show InChI InChI=1S/C22H23N3O3/c26-18-14-22(28-19-7-4-10-23-20(18)19)8-11-25(12-9-22)21(27)24-17-13-16(17)15-5-2-1-3-6-15/h1-7,10,16-17H,8-9,11-14H2,(H,24,27)/t16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268442
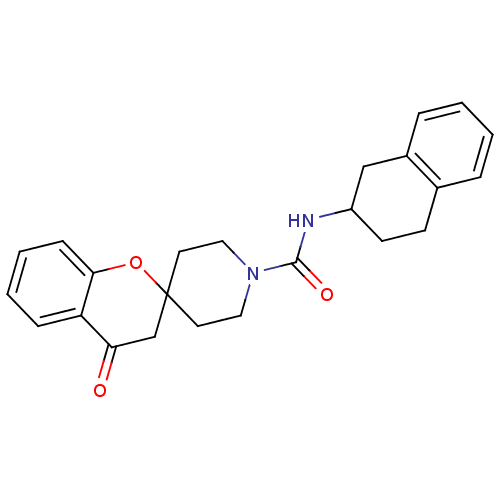
((+/-)-4-oxo-N-(1,2,3,4-tetrahydronaphthalen-2-yl)s...)Show SMILES O=C(NC1CCc2ccccc2C1)N1CCC2(CC1)CC(=O)c1ccccc1O2 Show InChI InChI=1S/C24H26N2O3/c27-21-16-24(29-22-8-4-3-7-20(21)22)11-13-26(14-12-24)23(28)25-19-10-9-17-5-1-2-6-18(17)15-19/h1-8,19H,9-16H2,(H,25,28) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268384

(CHEMBL522211 | N-((trans)-2-phenylcyclopropyl)-3',...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CCc1ncccc1O2 |r| Show InChI InChI=1S/C22H25N3O2/c26-21(24-19-15-17(19)16-5-2-1-3-6-16)25-13-10-22(11-14-25)9-8-18-20(27-22)7-4-12-23-18/h1-7,12,17,19H,8-11,13-15H2,(H,24,26)/t17-,19+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268326
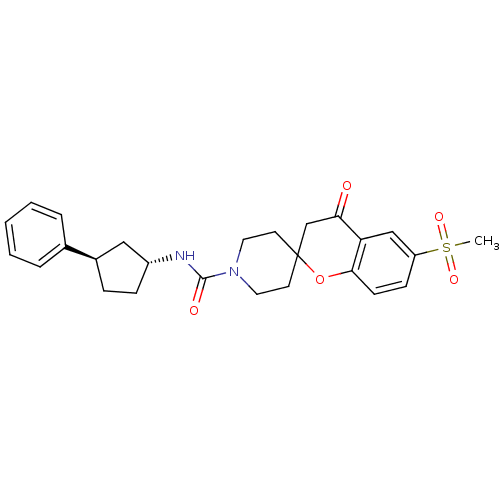
(6-(methylsulfonyl)-4-oxo-N-((trans)-3-phenylcyclop...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@@H]3CC[C@H](C3)c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C26H30N2O5S/c1-34(31,32)21-9-10-24-22(16-21)23(29)17-26(33-24)11-13-28(14-12-26)25(30)27-20-8-7-19(15-20)18-5-3-2-4-6-18/h2-6,9-10,16,19-20H,7-8,11-15,17H2,1H3,(H,27,30)/t19-,20-/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268381

(4-oxo-N-((trans)-2-phenylcyclopropyl)-6-(pyridin-4...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1cc(ccc1O2)-c1ccncc1 |r| Show InChI InChI=1S/C28H27N3O3/c32-25-18-28(34-26-7-6-21(16-23(25)26)19-8-12-29-13-9-19)10-14-31(15-11-28)27(33)30-24-17-22(24)20-4-2-1-3-5-20/h1-9,12-13,16,22,24H,10-11,14-15,17-18H2,(H,30,33)/t22-,24+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268441

(1-(6-fluorospiro[chroman-2,4'-piperidine]-1'-yl)-3...)Show InChI InChI=1S/C22H24FNO2/c23-19-7-8-20-18(16-19)10-11-22(26-20)12-14-24(15-13-22)21(25)9-6-17-4-2-1-3-5-17/h1-5,7-8,16H,6,9-15H2 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268383
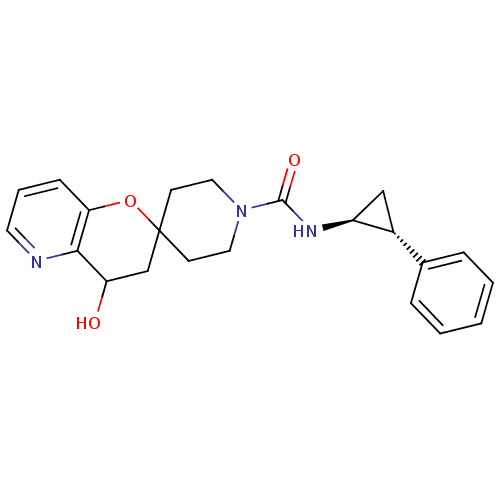
(4'-hydroxy-N-((trans)-2-phenylcyclopropyl)-3',4'-d...)Show SMILES OC1CC2(CCN(CC2)C(=O)N[C@H]2C[C@@H]2c2ccccc2)Oc2cccnc12 |r| Show InChI InChI=1S/C22H25N3O3/c26-18-14-22(28-19-7-4-10-23-20(18)19)8-11-25(12-9-22)21(27)24-17-13-16(17)15-5-2-1-3-6-15/h1-7,10,16-18,26H,8-9,11-14H2,(H,24,27)/t16-,17+,18?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268325
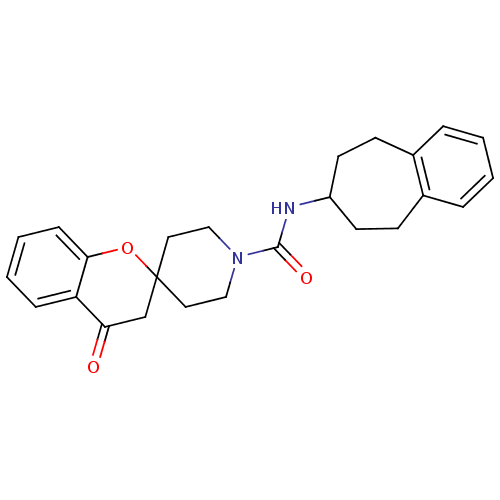
((+/-)-4-oxo-N-(6,7,8,9-tetrahydro-5H-benzo[7]annul...)Show SMILES O=C(NC1CCc2ccccc2CC1)N1CCC2(CC1)CC(=O)c1ccccc1O2 Show InChI InChI=1S/C25H28N2O3/c28-22-17-25(30-23-8-4-3-7-21(22)23)13-15-27(16-14-25)24(29)26-20-11-9-18-5-1-2-6-19(18)10-12-20/h1-8,20H,9-17H2,(H,26,29) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268383

(4'-hydroxy-N-((trans)-2-phenylcyclopropyl)-3',4'-d...)Show SMILES OC1CC2(CCN(CC2)C(=O)N[C@H]2C[C@@H]2c2ccccc2)Oc2cccnc12 |r| Show InChI InChI=1S/C22H25N3O3/c26-18-14-22(28-19-7-4-10-23-20(18)19)8-11-25(12-9-22)21(27)24-17-13-16(17)15-5-2-1-3-6-15/h1-7,10,16-18,26H,8-9,11-14H2,(H,24,27)/t16-,17+,18?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase in HEK293 cells assessed as DHET production |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268382

(4'-oxo-N-((trans)-2-phenylcyclopropyl)-3',4'-dihyd...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ncccc1O2 |r| Show InChI InChI=1S/C22H23N3O3/c26-18-14-22(28-19-7-4-10-23-20(18)19)8-11-25(12-9-22)21(27)24-17-13-16(17)15-5-2-1-3-6-15/h1-7,10,16-17H,8-9,11-14H2,(H,24,27)/t16-,17+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268380

((+/-)-4-oxo-N-((tans)-2-phenylcyclopropyl)spiro[ch...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ccccc1O2 |r| Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-9-5-4-8-17(20)21)10-12-25(13-11-23)22(27)24-19-14-18(19)16-6-2-1-3-7-16/h1-9,18-19H,10-15H2,(H,24,27)/t18-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268380

((+/-)-4-oxo-N-((tans)-2-phenylcyclopropyl)spiro[ch...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ccccc1O2 |r| Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-9-5-4-8-17(20)21)10-12-25(13-11-23)22(27)24-19-14-18(19)16-6-2-1-3-7-16/h1-9,18-19H,10-15H2,(H,24,27)/t18-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268441

(1-(6-fluorospiro[chroman-2,4'-piperidine]-1'-yl)-3...)Show InChI InChI=1S/C22H24FNO2/c23-19-7-8-20-18(16-19)10-11-22(26-20)12-14-24(15-13-22)21(25)9-6-17-4-2-1-3-5-17/h1-5,7-8,16H,6,9-15H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268438

(CHEMBL524992 | spiro[chroman-2,4'-piperidine]-1'-y...)Show SMILES FC(F)(F)c1ccc(cc1)C(=O)N1CCC2(CC1)CCc1ccccc1O2 Show InChI InChI=1S/C21H20F3NO2/c22-21(23,24)17-7-5-16(6-8-17)19(26)25-13-11-20(12-14-25)10-9-15-3-1-2-4-18(15)27-20/h1-8H,9-14H2 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268437

((+/-)-N-((trans)-2-phenylcyclopropyl)spiro[chroman...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CCc1ccccc1O2 |r| Show InChI InChI=1S/C23H26N2O2/c26-22(24-20-16-19(20)17-6-2-1-3-7-17)25-14-12-23(13-15-25)11-10-18-8-4-5-9-21(18)27-23/h1-9,19-20H,10-16H2,(H,24,26)/t19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268322

(6'-(methylsulfonyl)-N-((trans)-2-phenylcyclopropyl...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCc2c1)CCCN(CC3)C(=O)N[C@H]1C[C@@H]1c1ccccc1 |r| Show InChI InChI=1S/C25H30N2O4S/c1-32(29,30)20-8-9-23-19(16-20)10-12-25(31-23)11-5-14-27(15-13-25)24(28)26-22-17-21(22)18-6-3-2-4-7-18/h2-4,6-9,16,21-22H,5,10-15,17H2,1H3,(H,26,28)/t21-,22+,25?/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268436

((+/-)-6-(methylsulfonyl)-4-oxo-N-((trans)-2-phenyl...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C24H26N2O5S/c1-32(29,30)17-7-8-22-19(13-17)21(27)15-24(31-22)9-11-26(12-10-24)23(28)25-20-14-18(20)16-5-3-2-4-6-16/h2-8,13,18,20H,9-12,14-15H2,1H3,(H,25,28)/t18-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268435

((+/-)-6-(1-methyl-1H-pyrazol-5-ylamino)-4-oxo-N-((...)Show SMILES Cn1nccc1Nc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H29N5O3/c1-31-25(9-12-28-31)29-19-7-8-24-21(15-19)23(33)17-27(35-24)10-13-32(14-11-27)26(34)30-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22,29H,10-11,13-14,16-17H2,1H3,(H,30,34)/t20-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268320

((+/-)-6-fluoro-N-((trans)-2-phenylcyclopropyl)spir...)Show SMILES Fc1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C23H25FN2O2/c24-18-6-7-21-17(14-18)8-9-23(28-21)10-12-26(13-11-23)22(27)25-20-15-19(20)16-4-2-1-3-5-16/h1-7,14,19-20H,8-13,15H2,(H,25,27)/t19-,20+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268439

((6-(methylsulfonyl)spiro[chroman-2,4'-piperidine]-...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)c3ccc(cc3)C(F)(F)F)CCc2c1 Show InChI InChI=1S/C22H22F3NO4S/c1-31(28,29)18-6-7-19-16(14-18)8-9-21(30-19)10-12-26(13-11-21)20(27)15-2-4-17(5-3-15)22(23,24)25/h2-7,14H,8-13H2,1H3 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Homo sapiens (Human)) | BDBM50268324
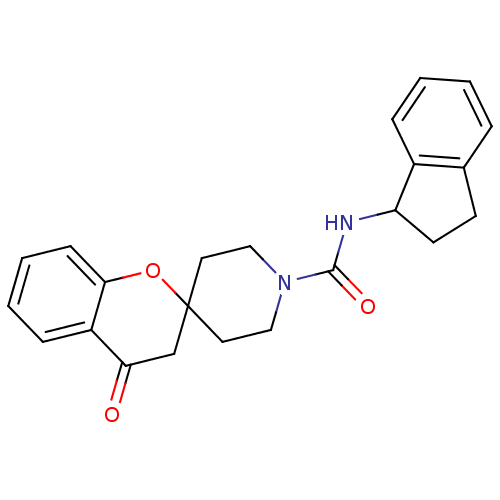
((+/-)-N-(2,3-dihydro-1H-inden-1-yl)-4-oxospiro[chr...)Show SMILES O=C(NC1CCc2ccccc12)N1CCC2(CC1)CC(=O)c1ccccc1O2 Show InChI InChI=1S/C23H24N2O3/c26-20-15-23(28-21-8-4-3-7-18(20)21)11-13-25(14-12-23)22(27)24-19-10-9-16-5-1-2-6-17(16)19/h1-8,19H,9-15H2,(H,24,27) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of human soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268382

(4'-oxo-N-((trans)-2-phenylcyclopropyl)-3',4'-dihyd...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CC(=O)c1ncccc1O2 |r| Show InChI InChI=1S/C22H23N3O3/c26-18-14-22(28-19-7-4-10-23-20(18)19)8-11-25(12-9-22)21(27)24-17-13-16(17)15-5-2-1-3-6-15/h1-7,10,16-17H,8-9,11-14H2,(H,24,27)/t16-,17+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268434

((+/-)-6-(1-methyl-1H-pyrazol-5-yl)-4-oxo-N-((tans)...)Show SMILES Cn1nccc1-c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C27H28N4O3/c1-30-23(9-12-28-30)19-7-8-25-21(15-19)24(32)17-27(34-25)10-13-31(14-11-27)26(33)29-22-16-20(22)18-5-3-2-4-6-18/h2-9,12,15,20,22H,10-11,13-14,16-17H2,1H3,(H,29,33)/t20-,22+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268384

(CHEMBL522211 | N-((trans)-2-phenylcyclopropyl)-3',...)Show SMILES O=C(N[C@H]1C[C@@H]1c1ccccc1)N1CCC2(CC1)CCc1ncccc1O2 |r| Show InChI InChI=1S/C22H25N3O2/c26-21(24-19-15-17(19)16-5-2-1-3-6-16)25-13-10-22(11-14-25)9-8-18-20(27-22)7-4-12-23-18/h1-7,12,17,19H,8-11,13-15H2,(H,24,26)/t17-,19+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268326

(6-(methylsulfonyl)-4-oxo-N-((trans)-3-phenylcyclop...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@@H]3CC[C@H](C3)c3ccccc3)CC(=O)c2c1 |r| Show InChI InChI=1S/C26H30N2O5S/c1-34(31,32)21-9-10-24-22(16-21)23(29)17-26(33-24)11-13-28(14-12-26)25(30)27-20-8-7-19(15-20)18-5-3-2-4-6-18/h2-6,9-10,16,19-20H,7-8,11-15,17H2,1H3,(H,27,30)/t19-,20-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |
Bifunctional epoxide hydrolase 2
(Rattus norvegicus) | BDBM50268321

(6-(methylsulfonyl)-N-((trans)-2-phenylcyclopropyl)...)Show SMILES CS(=O)(=O)c1ccc2OC3(CCN(CC3)C(=O)N[C@H]3C[C@@H]3c3ccccc3)CCc2c1 |r| Show InChI InChI=1S/C24H28N2O4S/c1-31(28,29)19-7-8-22-18(15-19)9-10-24(30-22)11-13-26(14-12-24)23(27)25-21-16-20(21)17-5-3-2-4-6-17/h2-8,15,20-21H,9-14,16H2,1H3,(H,25,27)/t20-,21+/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories
Curated by ChEMBL
| Assay Description
Inhibition of rat soluble epoxide hydrolase |
Bioorg Med Chem Lett 19: 3398-404 (2009)
Article DOI: 10.1016/j.bmcl.2009.05.036
BindingDB Entry DOI: 10.7270/Q20C4VP7 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data