

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156581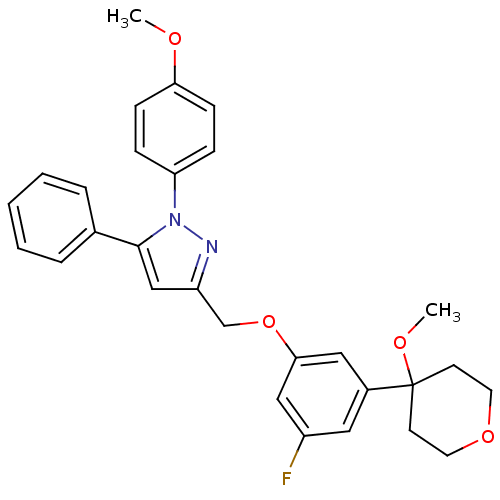 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50000829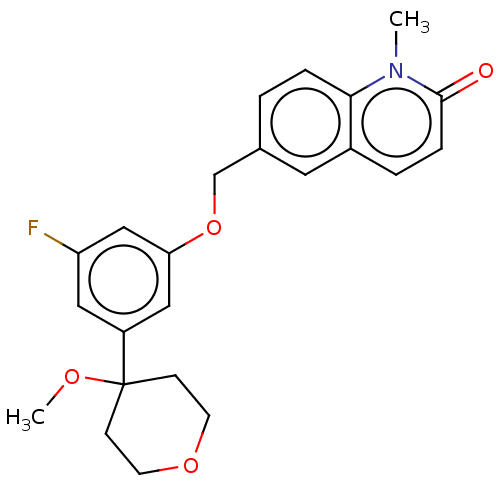 (6-((3-fluoro-5-(4-methoxytetrahydro-2H-pyran-4-yl)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156577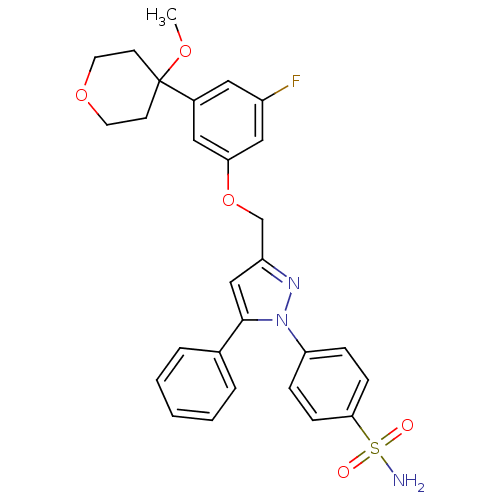 (1-(4-aminosulfonylphenyl)-3-[3-fluoro-5-(4-methoxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 100 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156584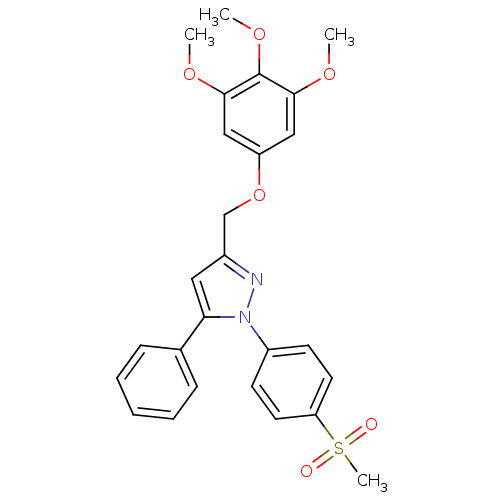 (1-(4-methanesulfonylphenyl)-3-[(3,4,5-trimethoxy)p...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50110484 (3-((3-fluoro-5-(1-methoxycyclohexyl)phenoxy)methyl...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156581 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156594 (3-[(3-fluoro-5-methoxy)phenoxymethyl]-1-(4-methane...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 310 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156580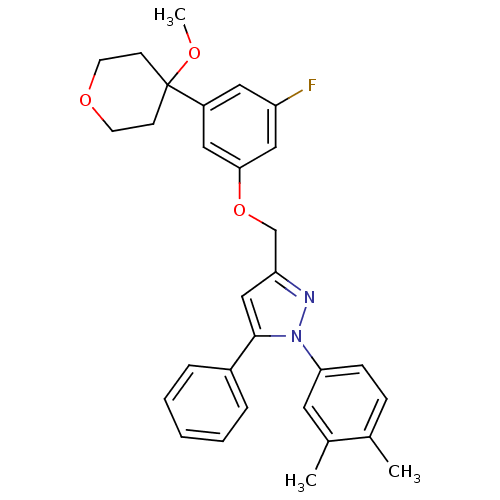 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156590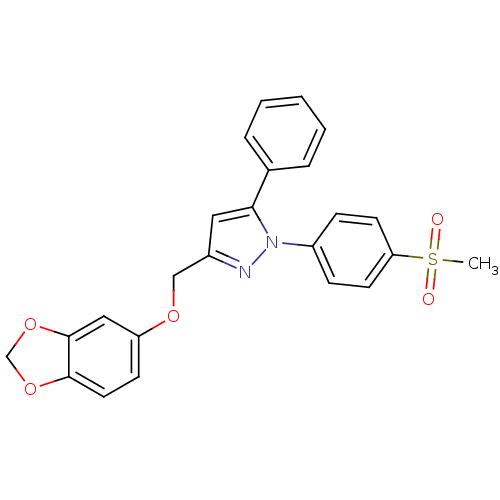 (1-(4-methanesulfonylphenyl)-3-[(3,4-methylenedioxy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 450 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156585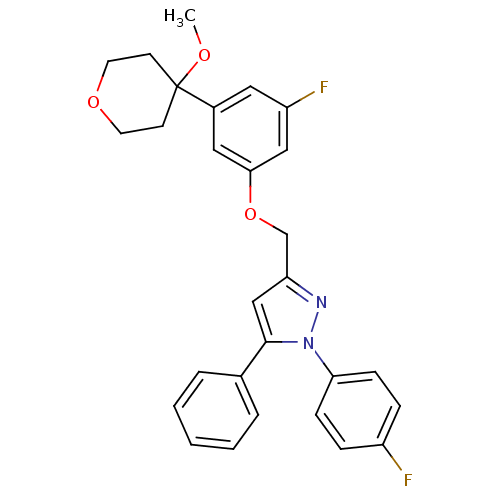 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 480 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156587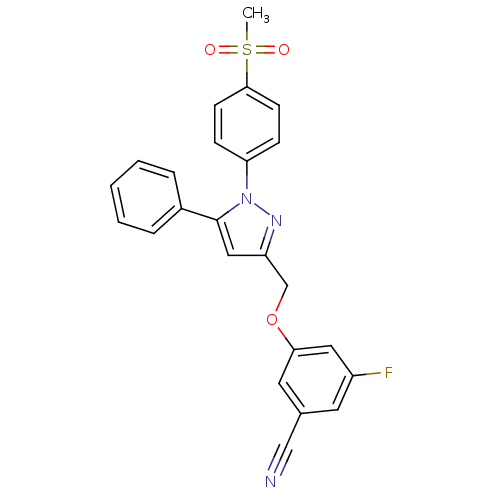 (3-[(5-cyano-3-fluoro)phenoxymethyl]-1-(4-methanesu...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156582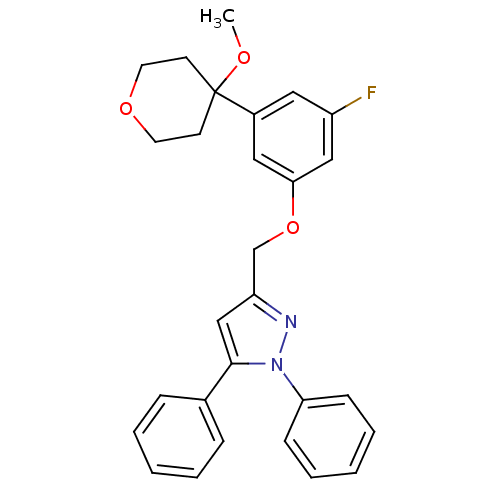 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 570 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156582 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 610 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156579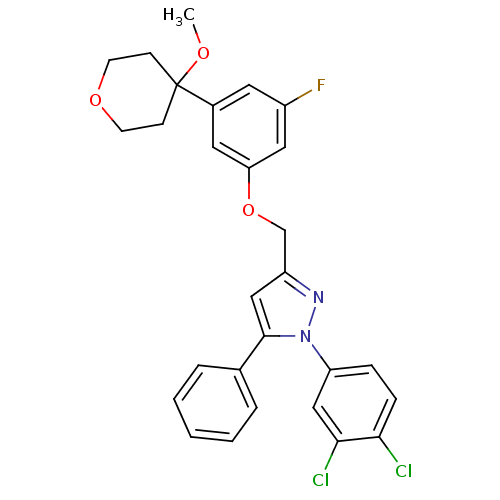 (1-(3,4-dichlorophenyl)-3-[3-fluoro-5-(4-methoxytet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156583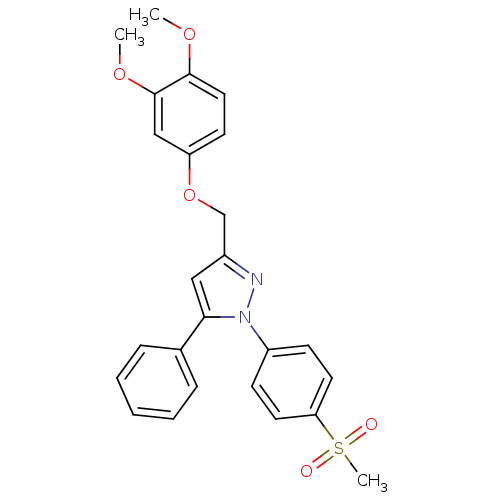 (1-(4-methanesulfonylphenyl)-3-[(3,4-dimethoxy)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 700 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156586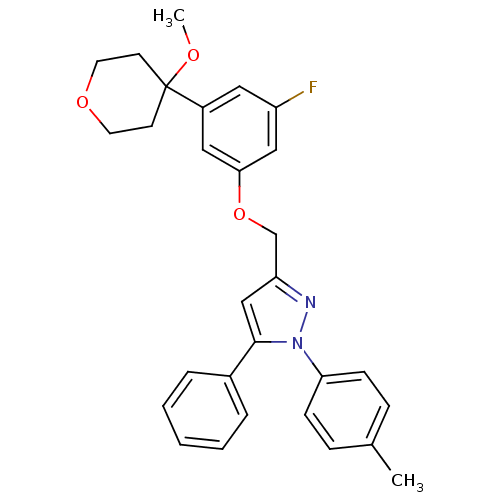 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 720 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156591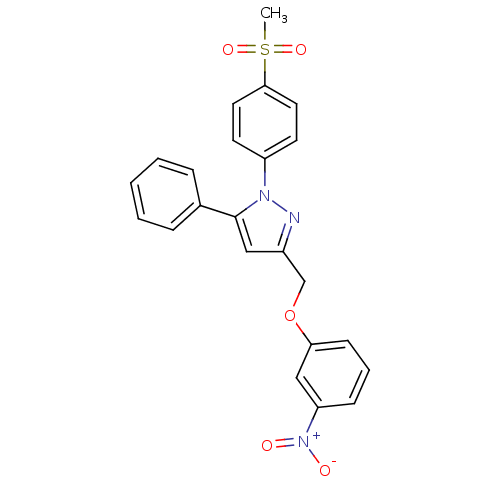 (1-(4-methanesulfonylphenyl)-3-[(3-nitro)phenoxymet...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156577 (1-(4-aminosulfonylphenyl)-3-[3-fluoro-5-(4-methoxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156580 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 760 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156581 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 770 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156579 (1-(3,4-dichlorophenyl)-3-[3-fluoro-5-(4-methoxytet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156588 (1-(4-trifluoromethoxyphenyl)-3-[3-fluoro-5-(4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 820 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156589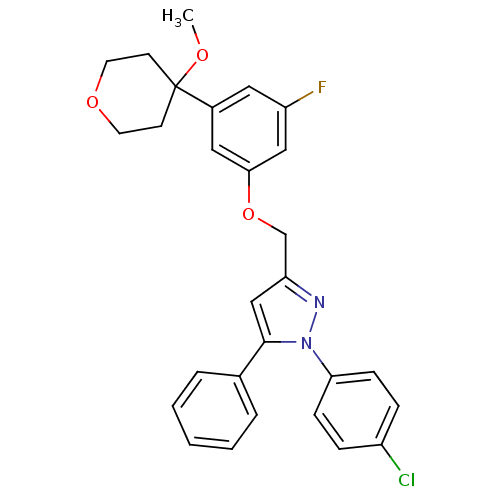 (1-(4-chlorophenyl)-3-[3-fluoro-5-(4-methoxytetrahy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156588 (1-(4-trifluoromethoxyphenyl)-3-[3-fluoro-5-(4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156592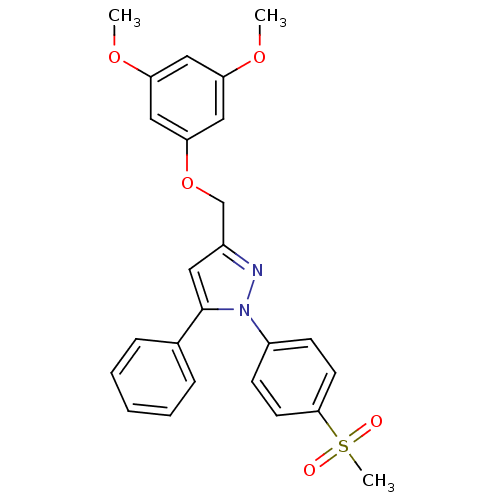 (1-(4-methanesulfonylphenyl)-3-[(3,5-dimethoxy)phen...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 870 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156593 (3-[(3-ethoxy)phenoxymethyl]-1-(4-methanesulfonylph...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156578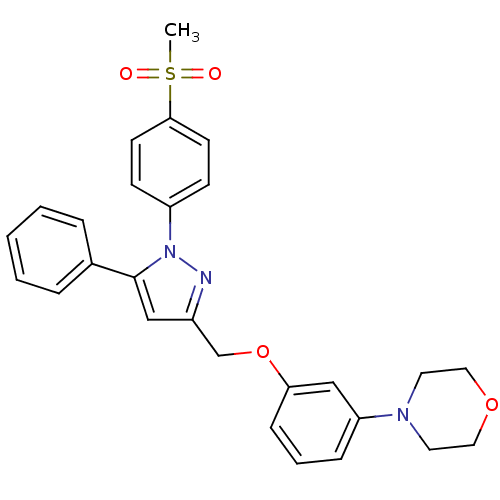 (1-(4-methanesulfonylphenyl)-3-[3-(4-morpholino)phe...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 920 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156586 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156589 (1-(4-chlorophenyl)-3-[3-fluoro-5-(4-methoxytetrahy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 930 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 2 (Homo sapiens (Human)) | BDBM50156585 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 960 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX2 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156585 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156586 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.56E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156589 (1-(4-chlorophenyl)-3-[3-fluoro-5-(4-methoxytetrahy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156582 (3-[3-fluoro-5-(4-methoxytetrahydropyran-4-yl)pheno...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.14E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156590 (1-(4-methanesulfonylphenyl)-3-[(3,4-methylenedioxy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156591 (1-(4-methanesulfonylphenyl)-3-[(3-nitro)phenoxymet...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156584 (1-(4-methanesulfonylphenyl)-3-[(3,4,5-trimethoxy)p...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156583 (1-(4-methanesulfonylphenyl)-3-[(3,4-dimethoxy)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156578 (1-(4-methanesulfonylphenyl)-3-[3-(4-morpholino)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156587 (3-[(5-cyano-3-fluoro)phenoxymethyl]-1-(4-methanesu...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156594 (3-[(3-fluoro-5-methoxy)phenoxymethyl]-1-(4-methane...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156593 (3-[(3-ethoxy)phenoxymethyl]-1-(4-methanesulfonylph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50156592 (1-(4-methanesulfonylphenyl)-3-[(3,5-dimethoxy)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of 5LOX in human whole blood assessed as inhibition of calcium ionophore A 23187-stimulated 5HETE production by HPLC analysis | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.35E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156590 (1-(4-methanesulfonylphenyl)-3-[(3,4-methylenedioxy...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.58E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156584 (1-(4-methanesulfonylphenyl)-3-[(3,4,5-trimethoxy)p...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.76E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin G/H synthase 1 (Homo sapiens (Human)) | BDBM50156592 (1-(4-methanesulfonylphenyl)-3-[(3,5-dimethoxy)phen...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Université de Lille 2 Curated by ChEMBL | Assay Description Inhibition of COX1 expressed in CHO cells assessed as inhibition of arachidonic acid-stimulated PGE2 production by enzyme immunoassay | J Med Chem 47: 6195-206 (2004) Article DOI: 10.1021/jm0407761 BindingDB Entry DOI: 10.7270/Q2DR2V01 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 60 total ) | Next | Last >> |