 Found 17 hits Enz. Inhib. hit(s) with all data for entry = 50015613
Found 17 hits Enz. Inhib. hit(s) with all data for entry = 50015613 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158378
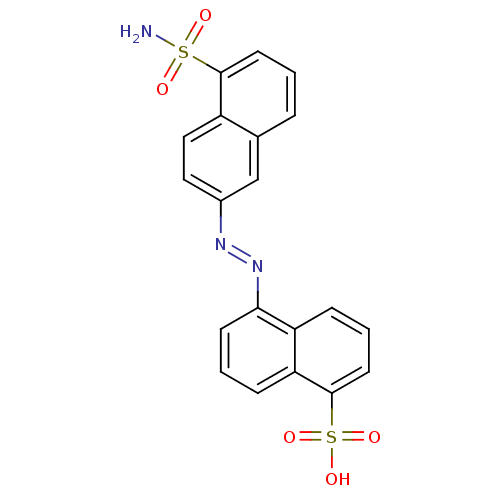
(5-(5-sulfamoyl-naphthalen-2-ylazo)-naphthalene-1-s...)Show SMILES NS(=O)(=O)c1cccc2cc(ccc12)\N=N\c1cccc2c(cccc12)S(O)(=O)=O Show InChI InChI=1S/C20H15N3O5S2/c21-29(24,25)19-8-1-4-13-12-14(10-11-15(13)19)22-23-18-7-2-6-17-16(18)5-3-9-20(17)30(26,27)28/h1-12H,(H2,21,24,25)(H,26,27,28)/b23-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| 154 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158378

(5-(5-sulfamoyl-naphthalen-2-ylazo)-naphthalene-1-s...)Show SMILES NS(=O)(=O)c1cccc2cc(ccc12)\N=N\c1cccc2c(cccc12)S(O)(=O)=O Show InChI InChI=1S/C20H15N3O5S2/c21-29(24,25)19-8-1-4-13-12-14(10-11-15(13)19)22-23-18-7-2-6-17-16(18)5-3-9-20(17)30(26,27)28/h1-12H,(H2,21,24,25)(H,26,27,28)/b23-22+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 600 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22580
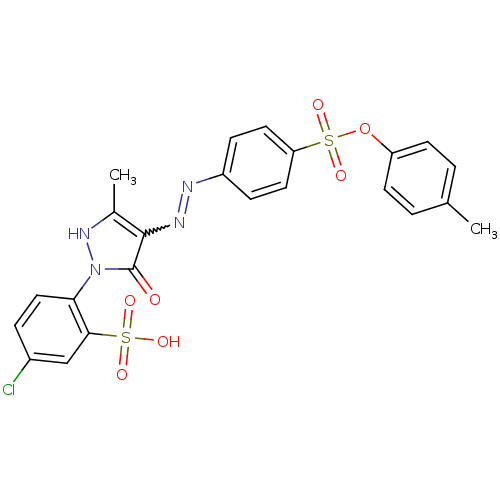
(47729-M | 5-chloro-2-{5-hydroxy-3-methyl-4-[(E)-2-...)Show SMILES Cc1[nH]n(-c2ccc(Cl)cc2S(O)(=O)=O)c(=O)c1N=Nc1ccc(cc1)S(=O)(=O)Oc1ccc(C)cc1 |w:18.19| Show InChI InChI=1S/C23H19ClN4O7S2/c1-14-3-8-18(9-4-14)35-37(33,34)19-10-6-17(7-11-19)25-26-22-15(2)27-28(23(22)29)20-12-5-16(24)13-21(20)36(30,31)32/h3-13,27H,1-2H3,(H,30,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158388
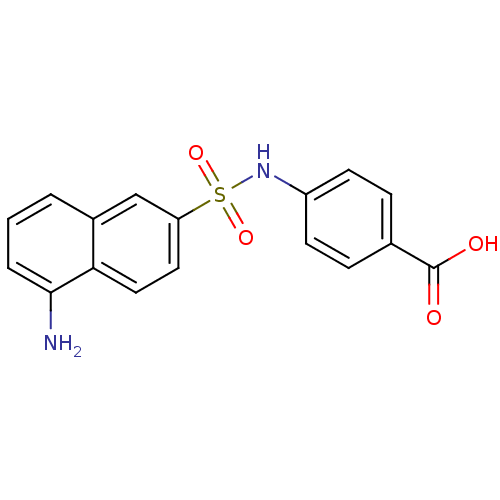
(4-(1-aminonaphthalene-6-sulfonamido)benzoic acid |...)Show SMILES Nc1cccc2cc(ccc12)S(=O)(=O)Nc1ccc(cc1)C(O)=O Show InChI InChI=1S/C17H14N2O4S/c18-16-3-1-2-12-10-14(8-9-15(12)16)24(22,23)19-13-6-4-11(5-7-13)17(20)21/h1-10,19H,18H2,(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158380
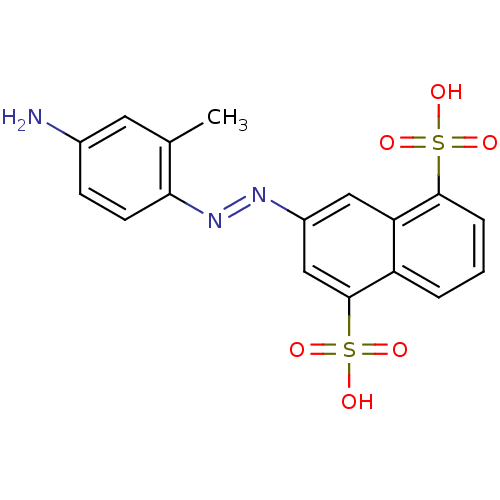
(3-(4-amino-2-methyl-phenylazo)-naphthalene-1,5-dis...)Show SMILES Cc1cc(N)ccc1\N=N\c1cc(c2cccc(c2c1)S(O)(=O)=O)S(O)(=O)=O Show InChI InChI=1S/C17H15N3O6S2/c1-10-7-11(18)5-6-15(10)20-19-12-8-14-13(17(9-12)28(24,25)26)3-2-4-16(14)27(21,22)23/h2-9H,18H2,1H3,(H,21,22,23)(H,24,25,26)/b20-19+ | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 7.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158381
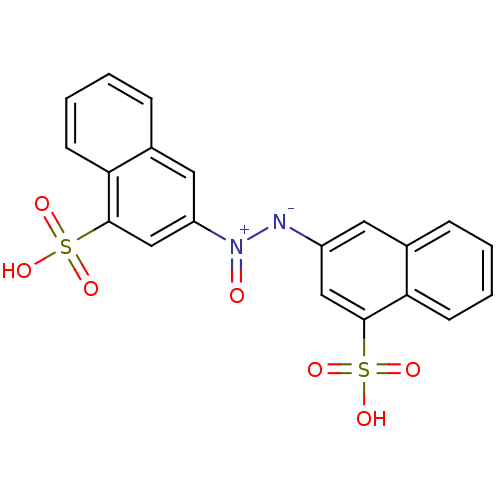
((Z)-1,2-bis(4-sulfonaphthalen-2-yl)diazene oxide |...)Show SMILES OS(=O)(=O)c1cc([N-][N+](=O)c2cc(c3ccccc3c2)S(O)(=O)=O)cc2ccccc12 Show InChI InChI=1S/C20H14N2O7S2/c23-22(16-10-14-6-2-4-8-18(14)20(12-16)31(27,28)29)21-15-9-13-5-1-3-7-17(13)19(11-15)30(24,25)26/h1-12H,(H,24,25,26)(H,27,28,29)/b22-21- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158387
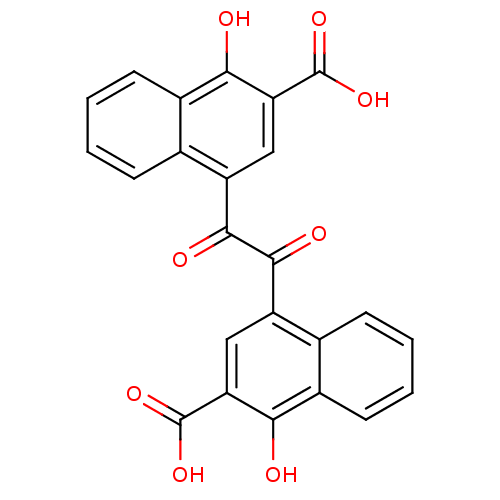
(4-[(3-carboxy-4-hydroxy-1-naphthyl)(oxo)acetyl]-1-...)Show SMILES OC(=O)c1cc(C(=O)C(=O)c2cc(C(O)=O)c(O)c3ccccc23)c2ccccc2c1O Show InChI InChI=1S/C24H14O8/c25-19-13-7-3-1-5-11(13)15(9-17(19)23(29)30)21(27)22(28)16-10-18(24(31)32)20(26)14-8-4-2-6-12(14)16/h1-10,25-26H,(H,29,30)(H,31,32) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158382
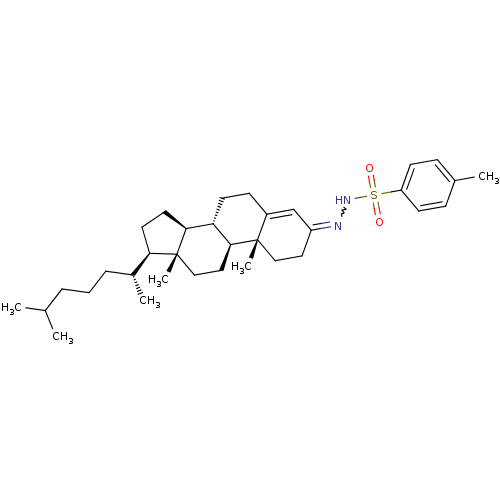
(CHEMBL376528 | N'-((8S,9S,10R,13R,14S,17R)-10,13-d...)Show SMILES CC(C)CCC[C@@H](C)[C@H]1CC[C@H]2[C@@H]3CCC4=CC(CC[C@]4(C)[C@H]3CC[C@]12C)=NNS(=O)(=O)c1ccc(C)cc1 |r,w:27.31,t:15| Show InChI InChI=1S/C34H52N2O2S/c1-23(2)8-7-9-25(4)30-16-17-31-29-15-12-26-22-27(18-20-33(26,5)32(29)19-21-34(30,31)6)35-36-39(37,38)28-13-10-24(3)11-14-28/h10-11,13-14,22-23,25,29-32,36H,7-9,12,15-21H2,1-6H3/t25-,29+,30-,31+,32+,33+,34-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22582
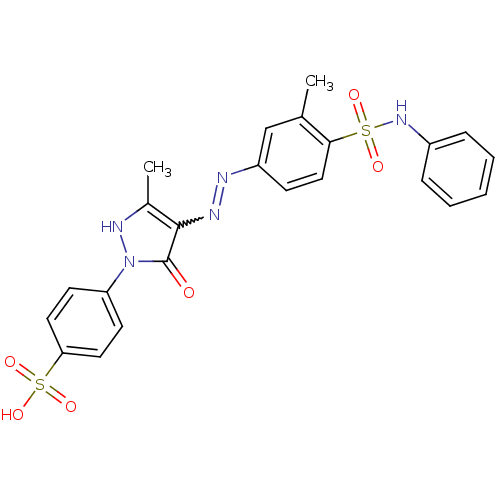
(324572-F | 4-{3-methyl-4-[(E)-2-[3-methyl-4-(pheny...)Show SMILES Cc1[nH]n(-c2ccc(cc2)S(O)(=O)=O)c(=O)c1N=Nc1ccc(c(C)c1)S(=O)(=O)Nc1ccccc1 |w:17.18| Show InChI InChI=1S/C23H21N5O6S2/c1-15-14-18(8-13-21(15)35(30,31)27-17-6-4-3-5-7-17)24-25-22-16(2)26-28(23(22)29)19-9-11-20(12-10-19)36(32,33)34/h3-14,26-27H,1-2H3,(H,32,33,34) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158386
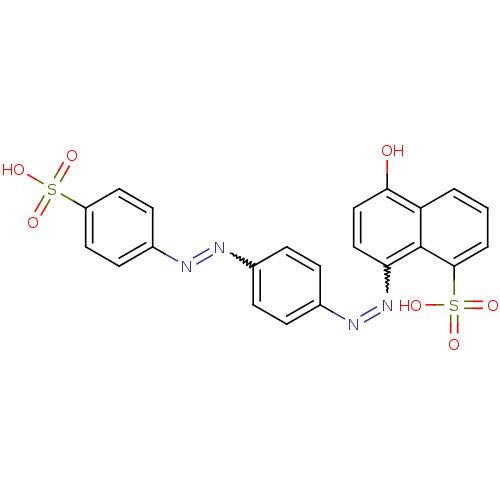
(5-hydroxy-8-[4-(4-sulfo-phenylazo)-phenylazo]-naph...)Show SMILES Oc1ccc(N=Nc2ccc(cc2)N=Nc2ccc(cc2)S(O)(=O)=O)c2c(cccc12)S(O)(=O)=O |w:13.13,5.4| Show InChI InChI=1S/C22H16N4O7S2/c27-20-13-12-19(22-18(20)2-1-3-21(22)35(31,32)33)26-25-15-6-4-14(5-7-15)23-24-16-8-10-17(11-9-16)34(28,29)30/h1-13,27H,(H,28,29,30)(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158383
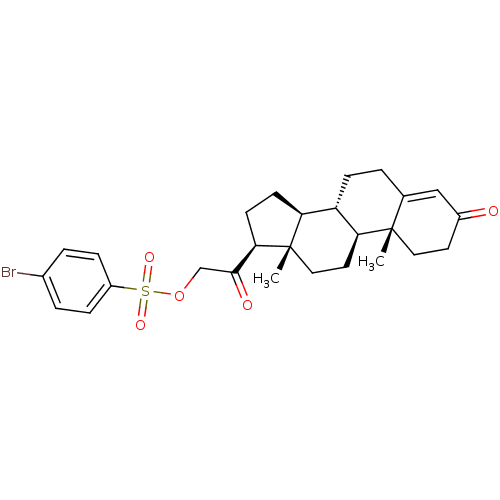
(2-((8S,9S,10R,13S,14S,17S)-10,13-dimethyl-3-oxo-2,...)Show SMILES C[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@]34C)[C@@H]1CC[C@@H]2C(=O)COS(=O)(=O)c1ccc(Br)cc1 |r,t:8| Show InChI InChI=1S/C27H33BrO5S/c1-26-13-11-19(29)15-17(26)3-8-21-22-9-10-24(27(22,2)14-12-23(21)26)25(30)16-33-34(31,32)20-6-4-18(28)5-7-20/h4-7,15,21-24H,3,8-14,16H2,1-2H3/t21-,22-,23-,24+,26-,27-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.39E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158385
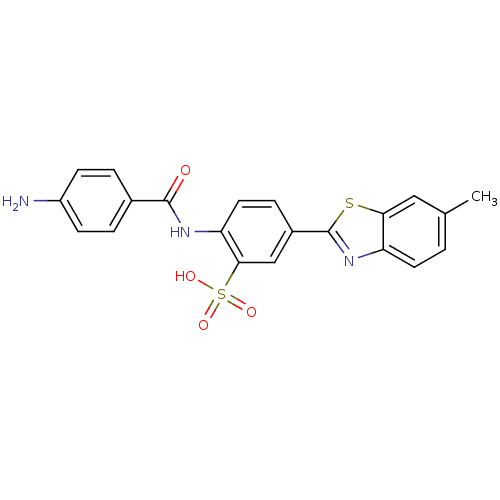
(2-(4-aminobenzamido)-5-(6-methylbenzo[d]thiazol-2-...)Show SMILES Cc1ccc2nc(sc2c1)-c1ccc(NC(=O)c2ccc(N)cc2)c(c1)S(O)(=O)=O Show InChI InChI=1S/C21H17N3O4S2/c1-12-2-8-16-18(10-12)29-21(24-16)14-5-9-17(19(11-14)30(26,27)28)23-20(25)13-3-6-15(22)7-4-13/h2-11H,22H2,1H3,(H,23,25)(H,26,27,28) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.69E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM22583
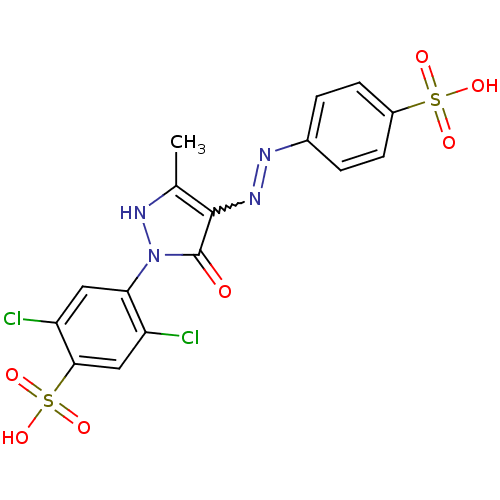
(2,5-dichloro-4-{3-methyl-5-oxo-4-[(E)-2-(4-sulfoph...)Show SMILES Cc1[nH]n(-c2cc(Cl)c(cc2Cl)S(O)(=O)=O)c(=O)c1N=Nc1ccc(cc1)S(O)(=O)=O |w:19.20| Show InChI InChI=1S/C16H12Cl2N4O7S2/c1-8-15(20-19-9-2-4-10(5-3-9)30(24,25)26)16(23)22(21-8)13-6-12(18)14(7-11(13)17)31(27,28)29/h2-7,21H,1H3,(H,24,25,26)(H,27,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.01E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158390
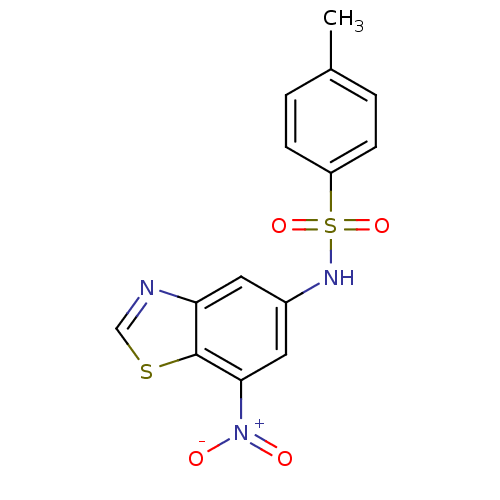
(4-methyl-N-(7-nitrobenzo[d]thiazol-5-yl)benzenesul...)Show SMILES Cc1ccc(cc1)S(=O)(=O)Nc1cc([N+]([O-])=O)c2scnc2c1 Show InChI InChI=1S/C14H11N3O4S2/c1-9-2-4-11(5-3-9)23(20,21)16-10-6-12-14(22-8-15-12)13(7-10)17(18)19/h2-8,16H,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.61E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158379
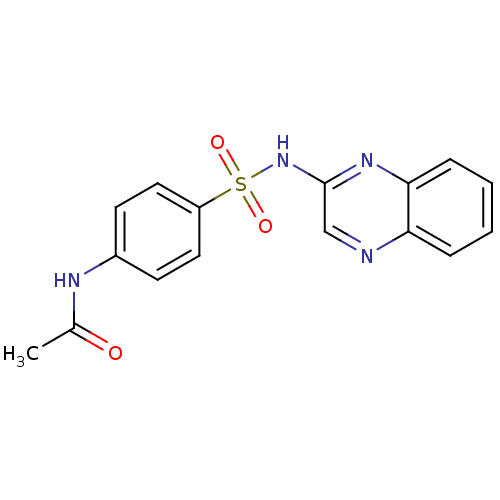
(CHEMBL225266 | N-[4-(quinoxalin-2-ylsulfamoyl)-phe...)Show InChI InChI=1S/C16H14N4O3S/c1-11(21)18-12-6-8-13(9-7-12)24(22,23)20-16-10-17-14-4-2-3-5-15(14)19-16/h2-10H,1H3,(H,18,21)(H,19,20) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | 5.47E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50158384
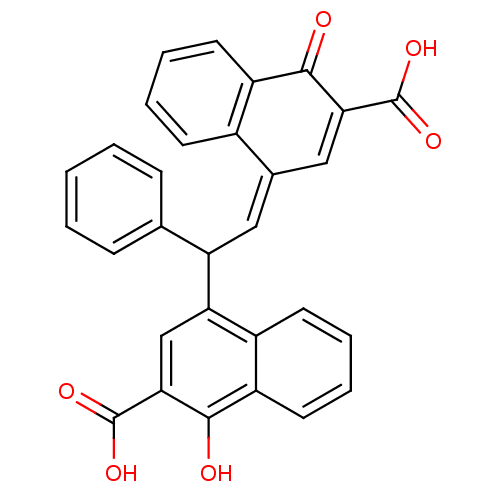
(4-(2-(3-carboxy-4-oxonaphthalen-1(4H)-ylidene)-1-p...)Show SMILES OC(=O)C1=C\C(=C\C(c2ccccc2)c2cc(C(O)=O)c(O)c3ccccc23)c2ccccc2C1=O |t:3| Show InChI InChI=1S/C30H20O6/c31-27-21-12-6-4-10-19(21)18(15-25(27)29(33)34)14-23(17-8-2-1-3-9-17)24-16-26(30(35)36)28(32)22-13-7-5-11-20(22)24/h1-16,23,32H,(H,33,34)(H,35,36)/b18-14- | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.04E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |
Bifunctional purine biosynthesis protein ATIC
(Homo sapiens (Human)) | BDBM50292288
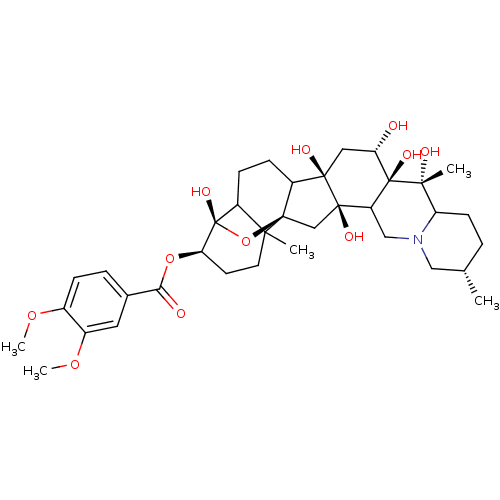
(CHEMBL451227 | NSC-7524 | Veratridine)Show SMILES COc1ccc(cc1OC)C(=O)O[C@@H]1CCC2(C)C3CCC4[C@]5(O)C[C@H](O)[C@@]6(O)C(CN7C[C@@H](C)CCC7[C@@]6(C)O)[C@]5(O)C[C@]24O[C@]13O |r,TLB:12:13:18:44.45,13:46:21.19.20:16,22:21:16:46.45,43:44:18:15.14.13,47:46:21.19.20:16,17:16:21.19.20:46.45,THB:21:44:18:15.14.13| Show InChI InChI=1S/C36H51NO11/c1-19-6-11-26-31(3,40)35(43)25(17-37(26)16-19)33(42)18-34-24(32(33,41)15-27(35)38)10-9-23-30(34,2)13-12-28(36(23,44)48-34)47-29(39)20-7-8-21(45-4)22(14-20)46-5/h7-8,14,19,23-28,38,40-44H,6,9-13,15-18H2,1-5H3/t19-,23?,24?,25?,26?,27-,28+,30?,31+,32+,33+,34+,35-,36-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.31E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
The Scripps Research Institute
Curated by ChEMBL
| Assay Description
Inhibition of human AICAR Tfase |
J Med Chem 47: 6681-90 (2004)
Article DOI: 10.1021/jm049504o
BindingDB Entry DOI: 10.7270/Q2PZ589Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data