

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50270591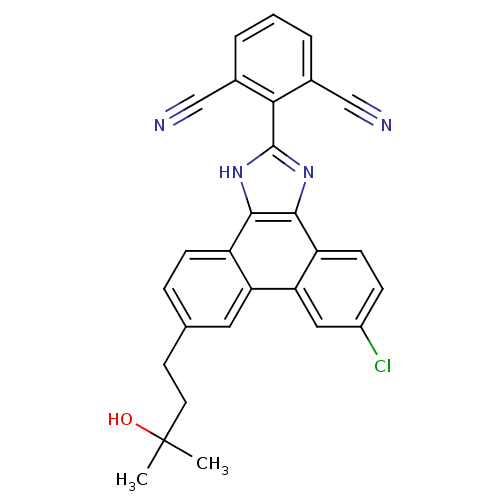 (2-(6-chloro-9-(3-hydroxy-3-methylbutyl)-1H-phenant...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168766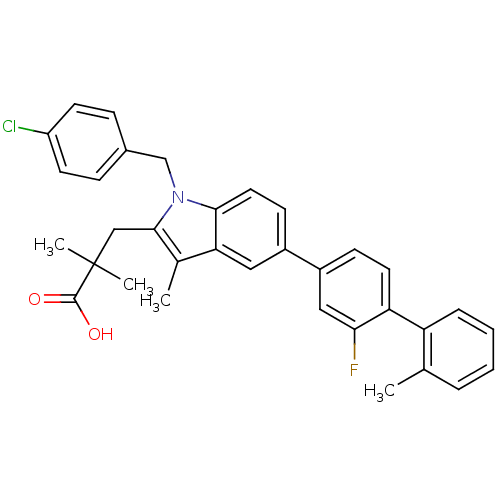 (3-(1-(4-chlorobenzyl)-5-(2-fluoro-2'-methylbipheny...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168776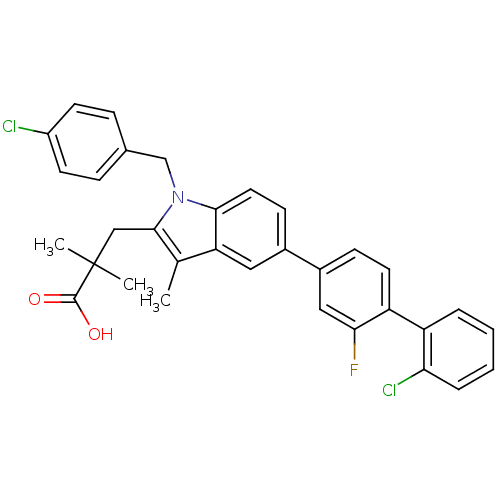 (3-(5-(2'-chloro-2-fluorobiphenyl-4-yl)-1-(4-chloro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168761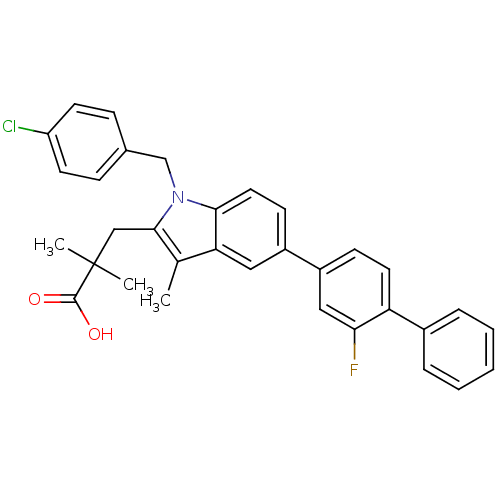 (3-(1-(4-chlorobenzyl)-5-(2-fluorobiphenyl-4-yl)-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168768 (3-(5-(biphenyl-4-yl)-1-(4-chlorobenzyl)-3-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343537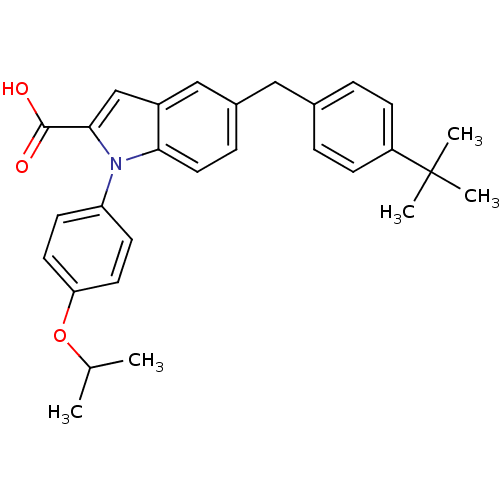 (5-(4-tert-butylbenzyl)-1-(4-isopropoxyphenyl)-1H-i...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343535 (3-(1-(4-chlorobenzyl)-3-(benzylthio)-5-(2-(4-chlor...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 60 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343539 (5-(4-tert-butylbenzyl)-3-(4-isopropoxyphenyl)-1-(4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 110 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168771 (3-[1-(4-Chloro-benzyl)-3-(3,3-dimethyl-butyryl)-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM22334 (BW A4C | BW4C | BWA4C | BWA4C, 10 | CHEMBL314360 |...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 360 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50270594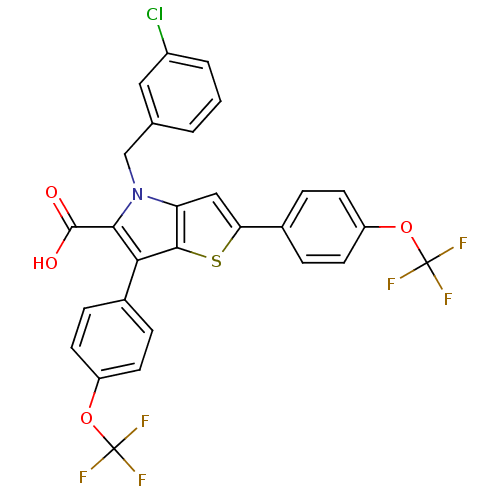 (4-(3-chlorobenzyl)-2,6-bis(4-(trifluoromethoxy)phe...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343531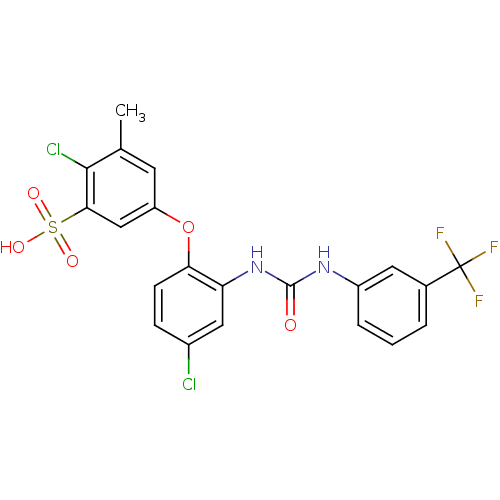 (2-chloro-5-(4-chloro-2-(3-(3-(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 400 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343533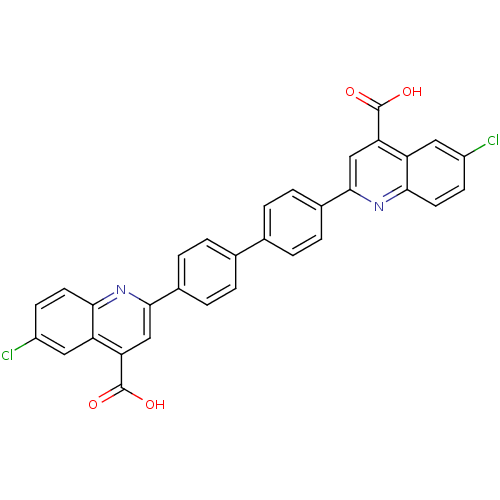 (2,2'-(biphenyl-4,4'-diyl)bis(6-chloroquinoline-4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343526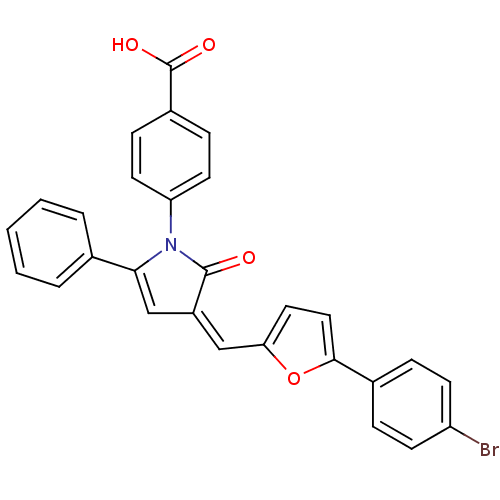 ((Z)-4-(3-((5-(4-Bromophenyl)furan-2-yl)methylene)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343530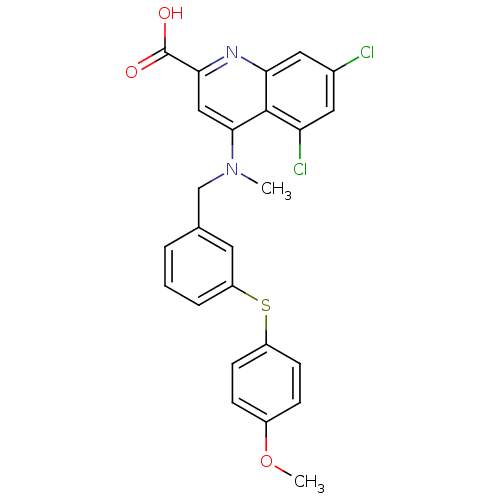 (5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 850 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168781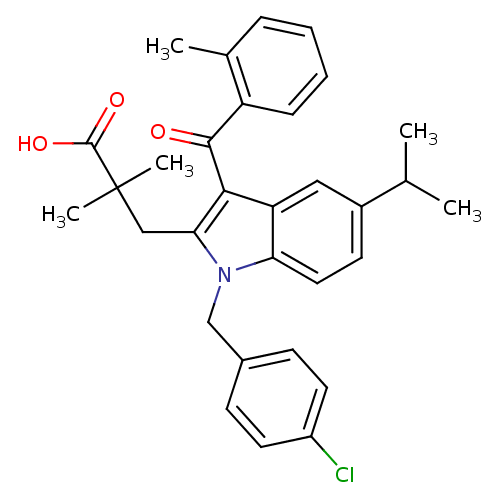 (3-[1-(4-Chloro-benzyl)-5-isopropyl-3-(2-methyl-ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343540 ((R)-2-(4-(biphenyl-4-ylmethylamino)-6-chloropyrimi...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50273441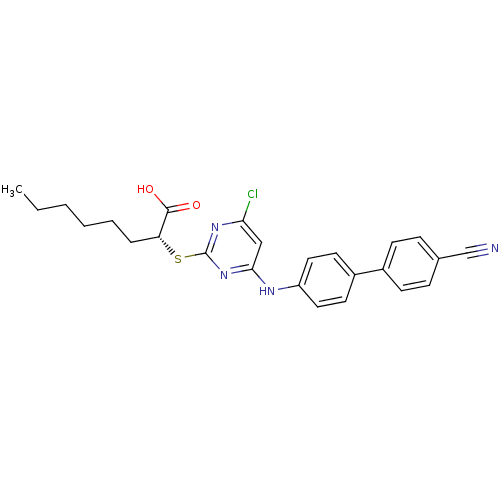 ((R)-2-(4-Chloro-6-(4'-cyanobiphenyl-4-ylamino)pyri...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343533 (2,2'-(biphenyl-4,4'-diyl)bis(6-chloroquinoline-4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343533 (2,2'-(biphenyl-4,4'-diyl)bis(6-chloroquinoline-4-c...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343525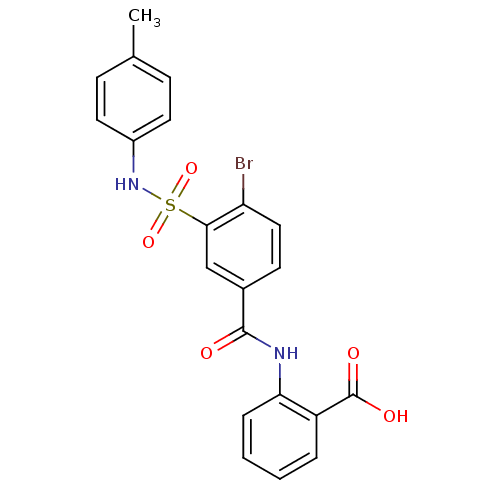 (2-(4-bromo-3-(N-p-tolylsulfamoyl)benzamido)benzoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343528 (3-(9-(3-bromo-4-(4-chlorobenzyloxy)phenyl)-3,3,6,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343536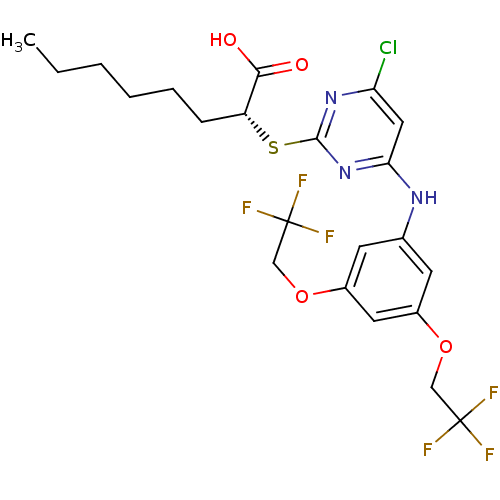 ((R)-2-(4-(3,5-bis(2,2,2-trifluoroethoxy)phenylamin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343531 (2-chloro-5-(4-chloro-2-(3-(3-(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343526 ((Z)-4-(3-((5-(4-Bromophenyl)furan-2-yl)methylene)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50513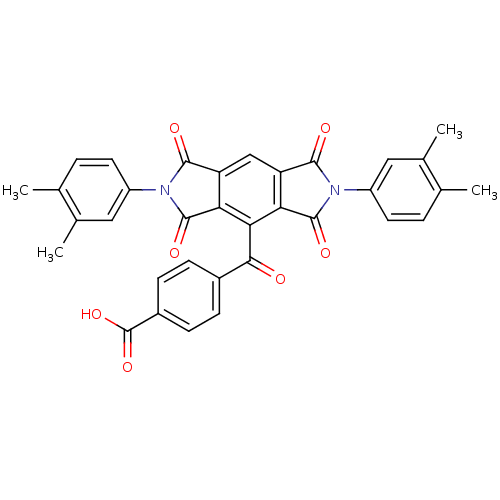 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343531 (2-chloro-5-(4-chloro-2-(3-(3-(trifluoromethyl)phen...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343530 (5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168764 (3-[3-tert-Butylsulfanyl-5-isopropyl-1-(3-phenyl-pr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343532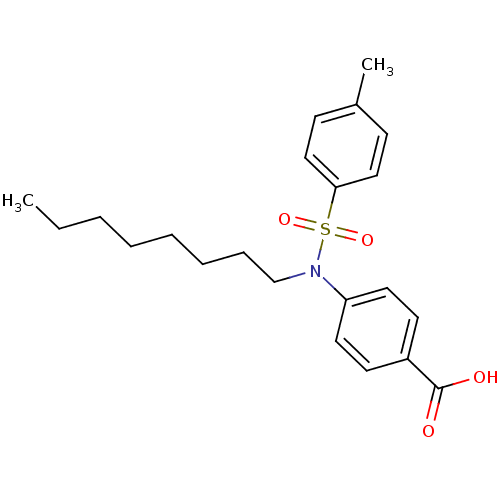 (4-(4-methyl-N-octylphenylsulfonamido)benzoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343538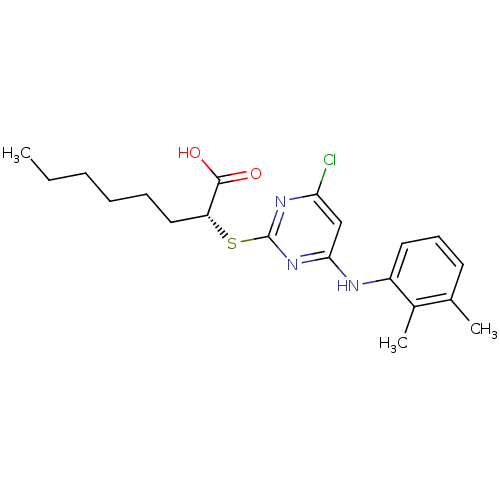 ((R)-2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343532 (4-(4-methyl-N-octylphenylsulfonamido)benzoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343525 (2-(4-bromo-3-(N-p-tolylsulfamoyl)benzamido)benzoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343534 ((R)-2-(4-chloro-6-(2,3-dimethylphenylamino)pyrimid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343530 (5,7-dichloro-4-((3-(4-methoxyphenylthio)benzyl)(me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343526 ((Z)-4-(3-((5-(4-Bromophenyl)furan-2-yl)methylene)-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343532 (4-(4-methyl-N-octylphenylsulfonamido)benzoic acid ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50168778 (3-(1-Allyl-3-tert-butylsulfanyl-5-isopropyl-1H-ind...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 6.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50343529 (2-(6-(4-tert-butylbenzamido)benzo[d]thiazol-2-ylth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 7.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prostaglandin E synthase (Homo sapiens (Human)) | BDBM50513 (4-(2,6-bis(3,4-dimethylphenyl)-1,3,5,7-tetraoxo-1,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human microsomal PGES1 in cell-free system assessed as inhibition of conversion of PGH2 to PGE2 by HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343529 (2-(6-(4-tert-butylbenzamido)benzo[d]thiazol-2-ylth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343528 (3-(9-(3-bromo-4-(4-chlorobenzyloxy)phenyl)-3,3,6,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343528 (3-(9-(3-bromo-4-(4-chlorobenzyloxy)phenyl)-3,3,6,6...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343529 (2-(6-(4-tert-butylbenzamido)benzo[d]thiazol-2-ylth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of 5-LO in human polymorphonuclear leukocytes assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyunsaturated fatty acid 5-lipoxygenase (Homo sapiens (Human)) | BDBM50343525 (2-(4-bromo-3-(N-p-tolylsulfamoyl)benzamido)benzoic...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Innsbruck Curated by ChEMBL | Assay Description Inhibition of human recombinant 5-LO in cell-free system assessed as inhibition of LTB4 production after 10 mins by RP-HPLC assay | J Med Chem 54: 3163-74 (2011) Article DOI: 10.1021/jm101309g BindingDB Entry DOI: 10.7270/Q2MS3T3J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||