

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50274347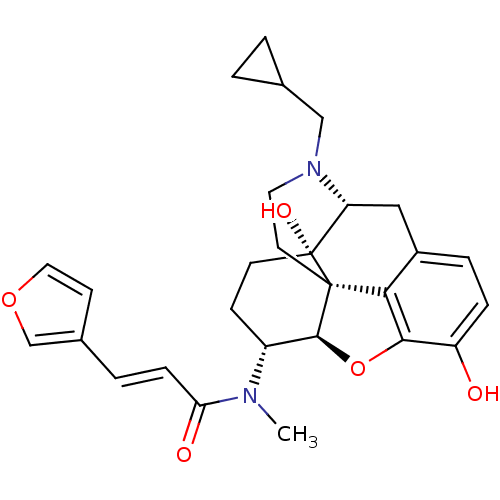 ((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.238 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230035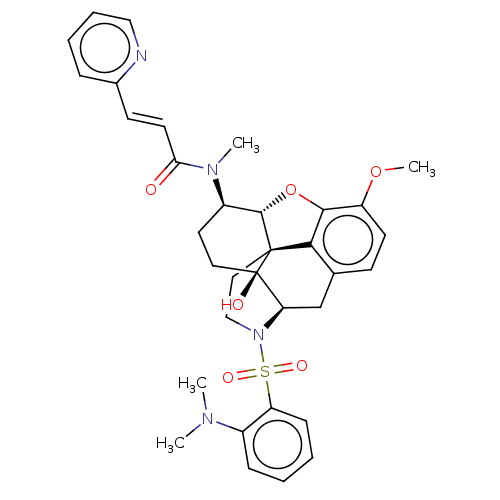 (CHEMBL4091544) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230164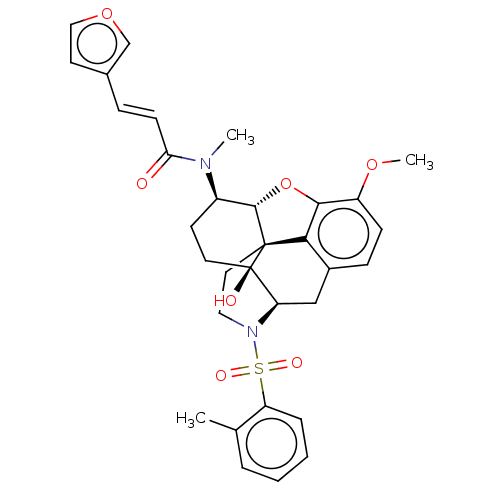 (CHEMBL4091834 | US10377763, Example 2) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230139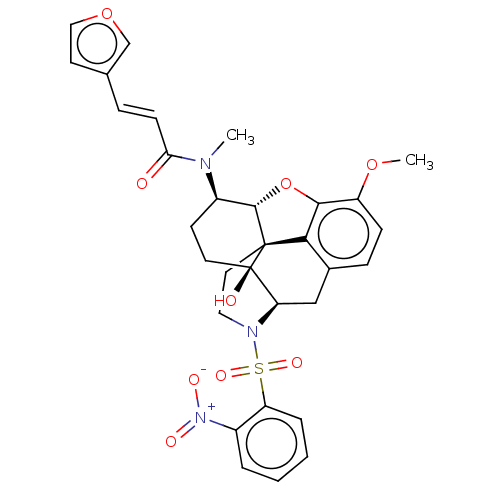 (CHEMBL4105072 | US10377763, Example 8) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230161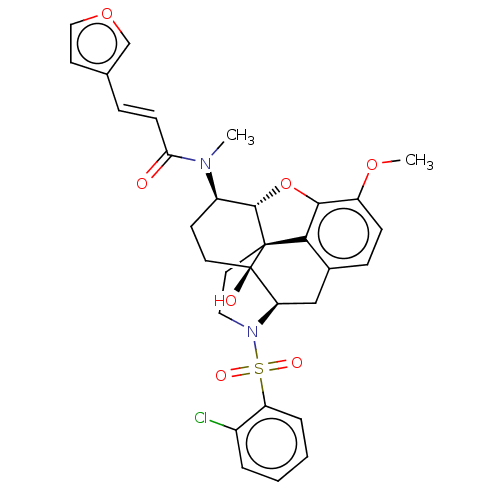 (CHEMBL4089496) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230138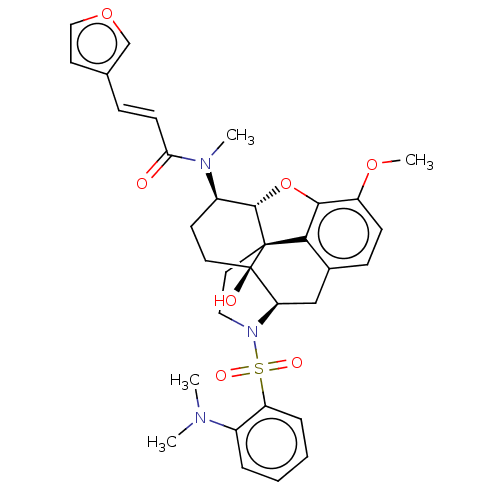 (CHEMBL4094318) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230158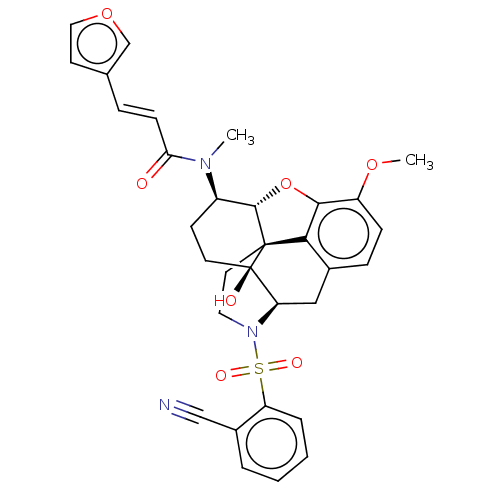 (CHEMBL4073947 | US10377763, Example 11) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230134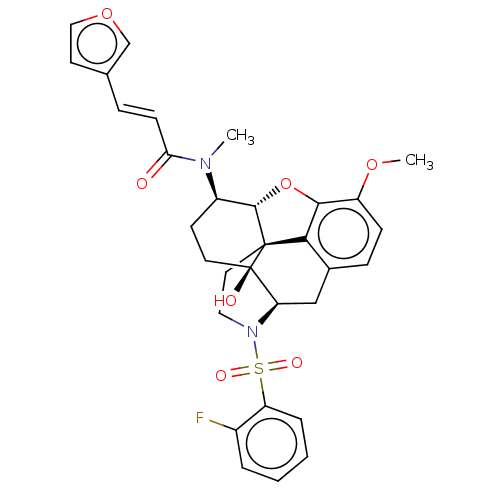 (CHEMBL4087046) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230144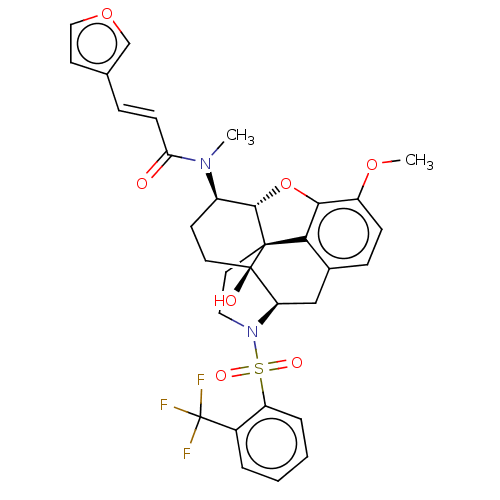 (CHEMBL4081763 | US10377763, Example 5) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230141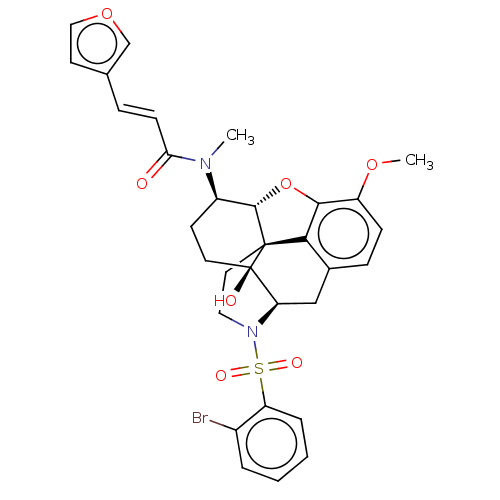 (CHEMBL4083587 | US10377763, Example 20) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230167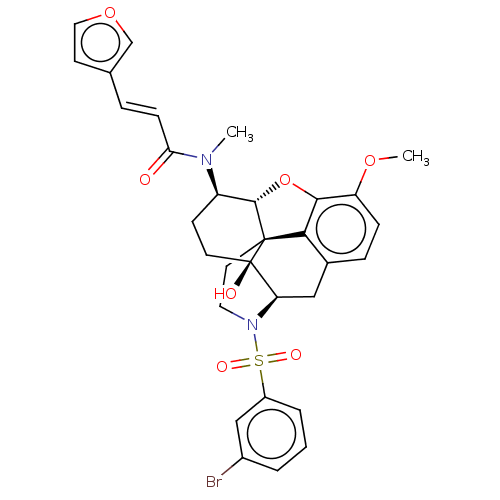 (CHEMBL4071220 | US10377763, Example 21) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230160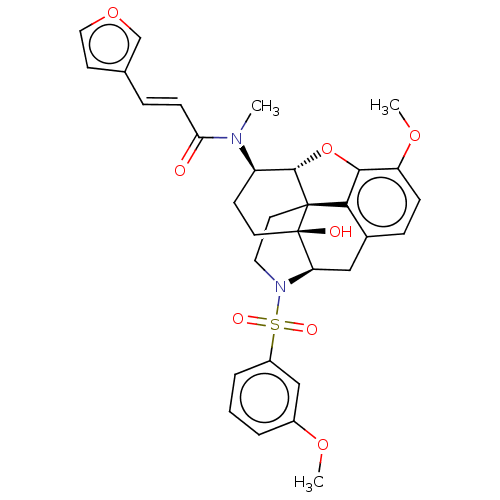 (CHEMBL4065120) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230156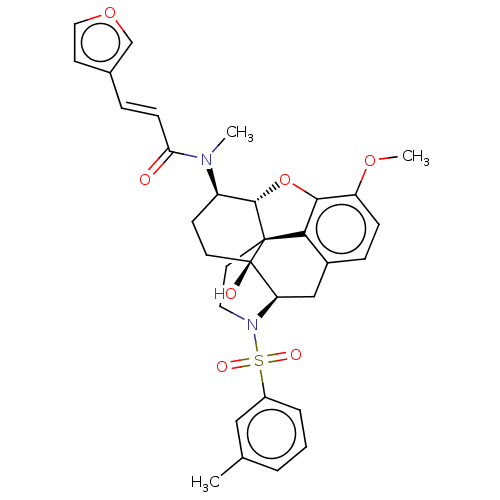 (CHEMBL4097697 | US10377763, Example 3) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230136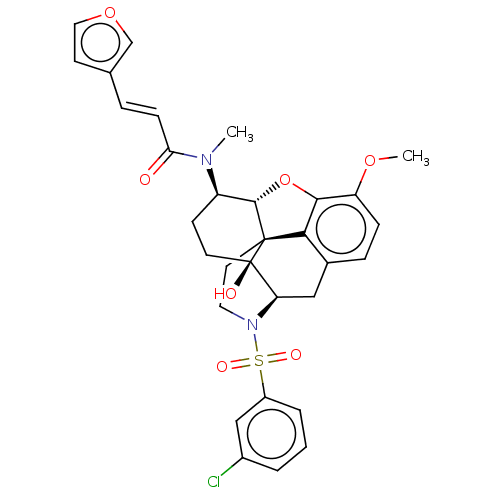 (CHEMBL4068322 | US10377763, Example 18) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230166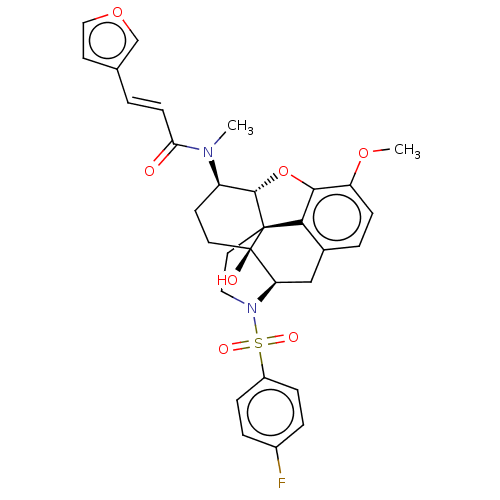 (CHEMBL4079180) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230029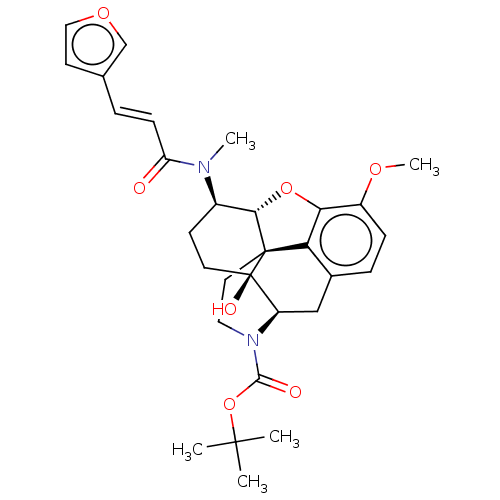 (CHEMBL4071362) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230142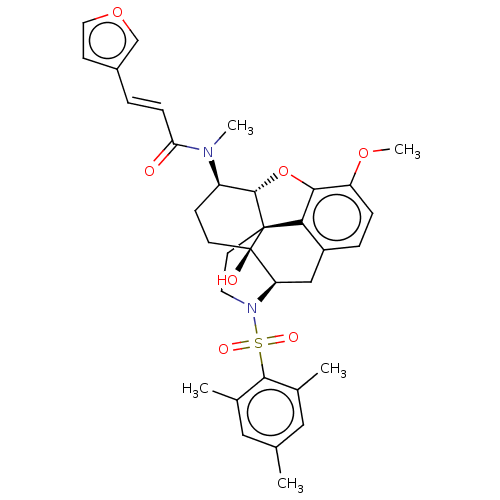 (CHEMBL4099280 | US10377763, Example 23) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230145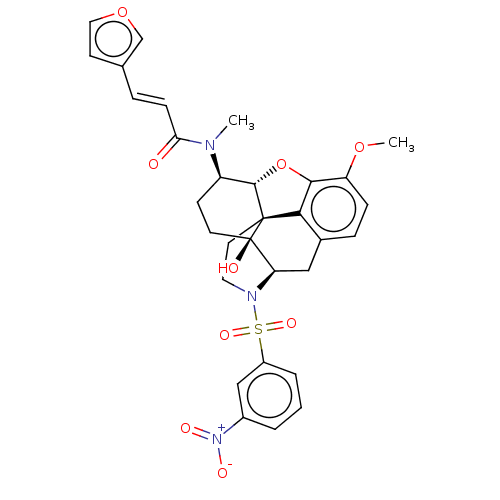 (CHEMBL4080833) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230148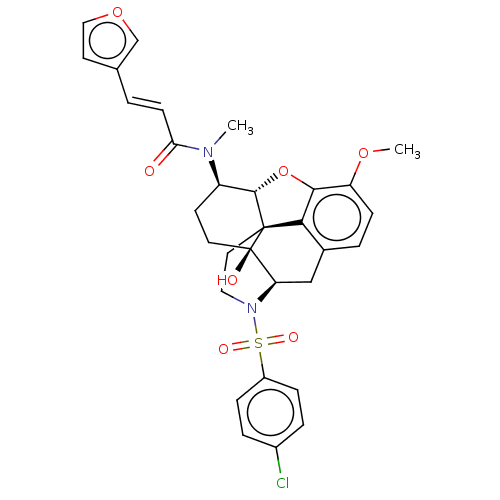 (CHEMBL4101590) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230168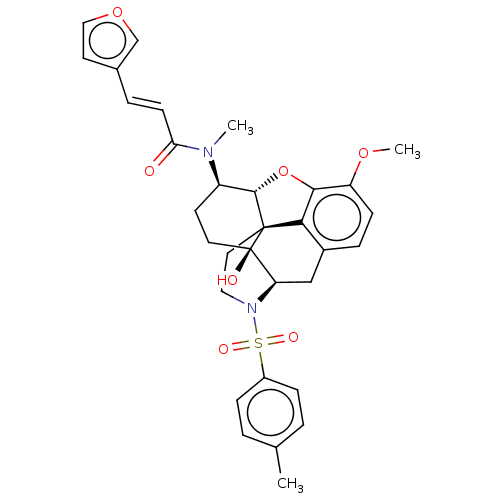 (CHEMBL4079662 | US10377763, Example 4) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230155 (CHEMBL4094746 | US10377763, Example 27) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mu-type opioid receptor (Homo sapiens (Human)) | BDBM50274347 ((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...) | PDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 6 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]DAMGO from human mu opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230163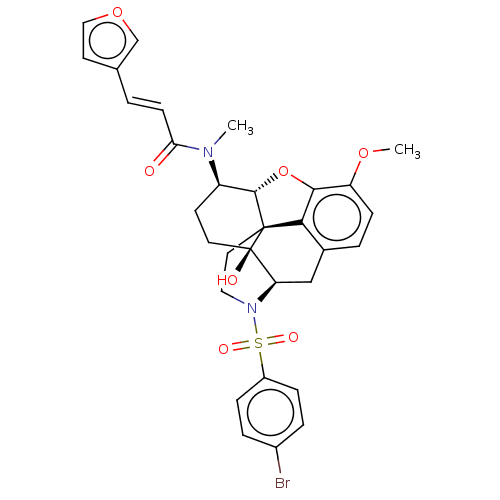 (CHEMBL4092034) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230146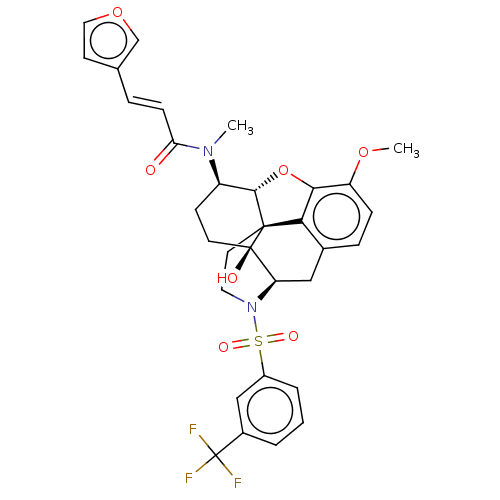 (CHEMBL4099781) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230162 (CHEMBL4084072) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 6.40 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230165 (CHEMBL4062575) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230137 (CHEMBL4073714) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.30 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230154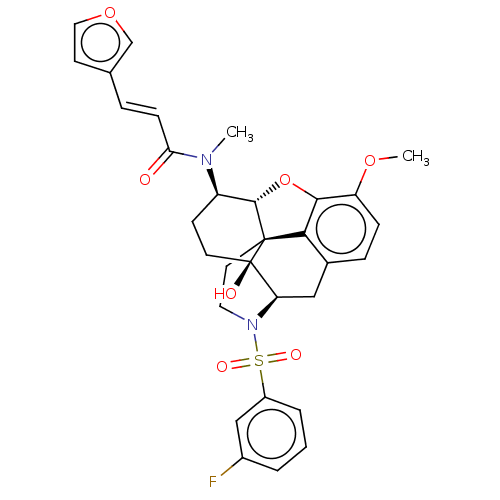 (CHEMBL4097196 | US10377763, Example 15) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.60 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230031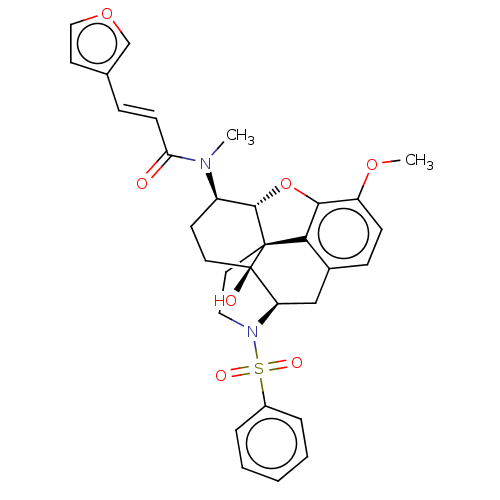 (CHEMBL4080914) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.10 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230143 (CHEMBL4064075) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230133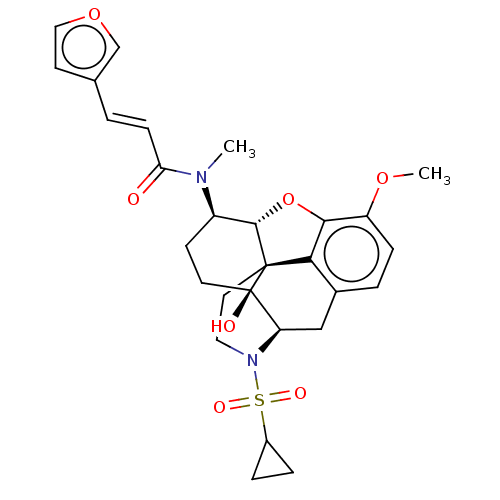 (CHEMBL4067292 | US10377763, Example 28) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230033 (CHEMBL4062717 | US10377763, Example 34) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 12 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230159 (CHEMBL4086564) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230153 (CHEMBL4100720) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230032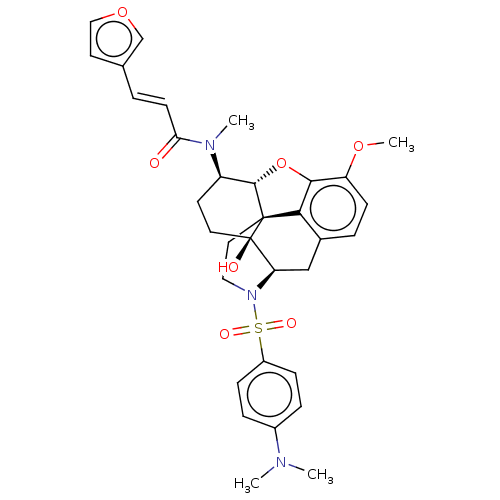 (CHEMBL4102054) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 13 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230147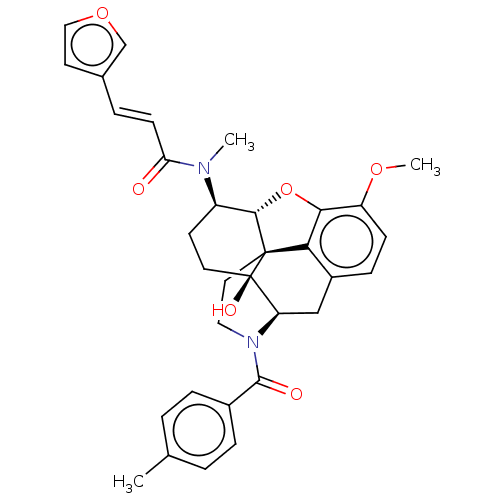 (CHEMBL4094071 | US10377763, Example 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230140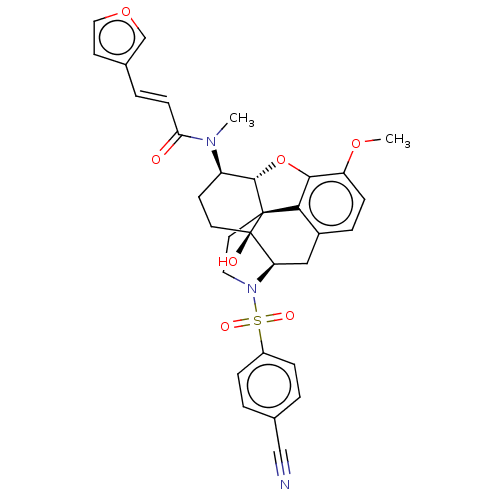 (CHEMBL4084277) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230030 (CHEMBL4066535) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 14 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50423648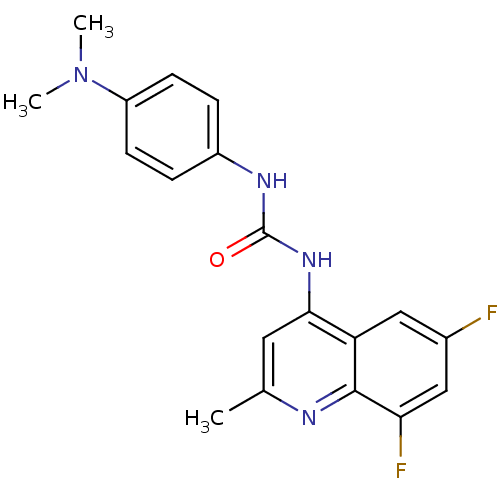 (CHEMBL1334465 | SB-408124) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem | PDB Article PubMed | 19 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230037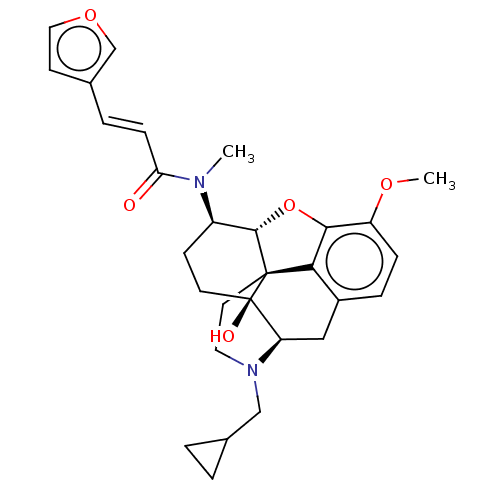 (CHEMBL4103513) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 23 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230036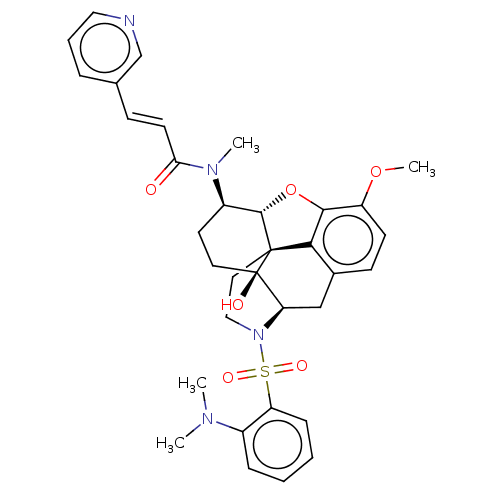 (CHEMBL4063740) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50230029 (CHEMBL4071362) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Kappa-type opioid receptor (Homo sapiens (Human)) | BDBM50230027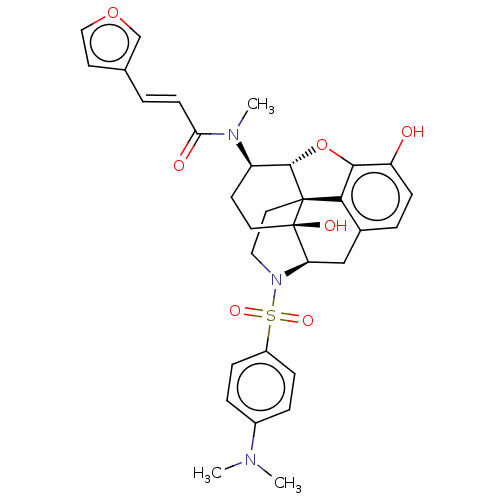 (CHEMBL4101758) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 200 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]U69,593 from human kappa opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50274347 ((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230034 (CHEMBL4072331) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50274175 ((2E)-N-[(5R,6R)-17-(Cyclopropylmethyl)-4,5-epoxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 389 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50230038 (CHEMBL4090683) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 541 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Delta-type opioid receptor (Homo sapiens (Human)) | BDBM50274347 ((2E)-N-[(5R,6R)-17-(cyclopropylmethyl)-4,5-epoxy-3...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | 693 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Displacement of [3H]DPDPE from human delta opioid receptor expressed in CHO cell membranes by micro-beta scintillation counting method | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin receptor type 2 (Homo sapiens (Human)) | BDBM50230147 (CHEMBL4094071 | US10377763, Example 35) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 725 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX2R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Orexin/Hypocretin receptor type 1 (Homo sapiens (Human)) | BDBM50274257 ((2E)-N-[(1S,5R,13R,14R,17S)-4-(cyclopropylmethyl)-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 751 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Tsukuba Curated by ChEMBL | Assay Description Antagonist activity at human OX1R expressed in CHOK1 cells assessed as inhibition of orexin A-induced intracellular calcium level preincubated for 15... | J Med Chem 60: 1018-1040 (2017) Article DOI: 10.1021/acs.jmedchem.6b01418 BindingDB Entry DOI: 10.7270/Q22N54JH | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 74 total ) | Next | Last >> |