

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230326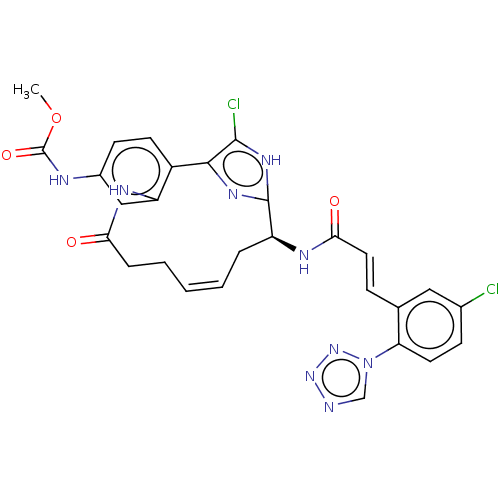 (CHEMBL4060950) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230322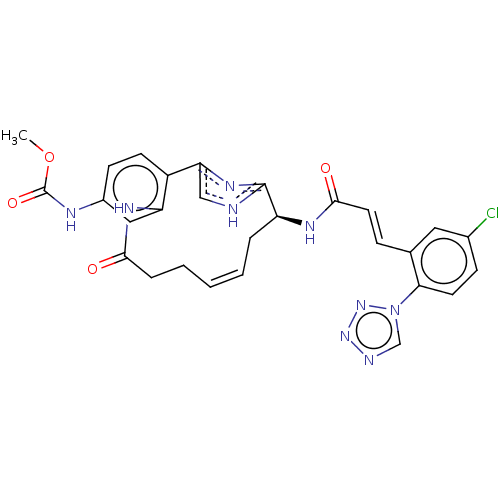 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230325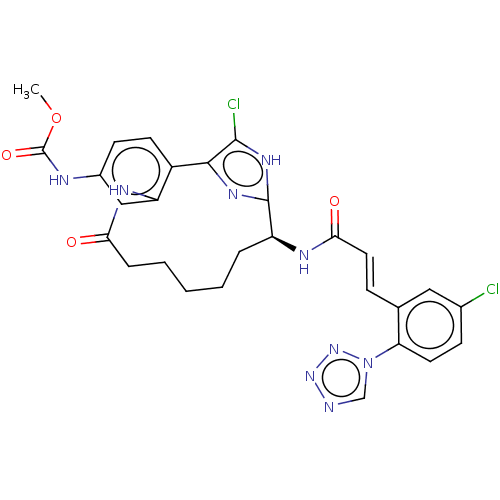 (CHEMBL4062923) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 0.470 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230340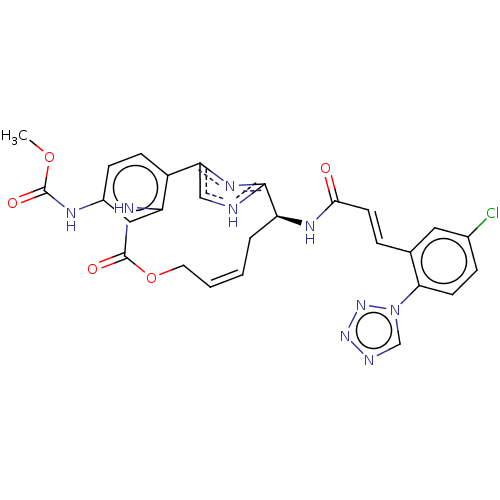 (CHEMBL4087980) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 0.880 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 1 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230324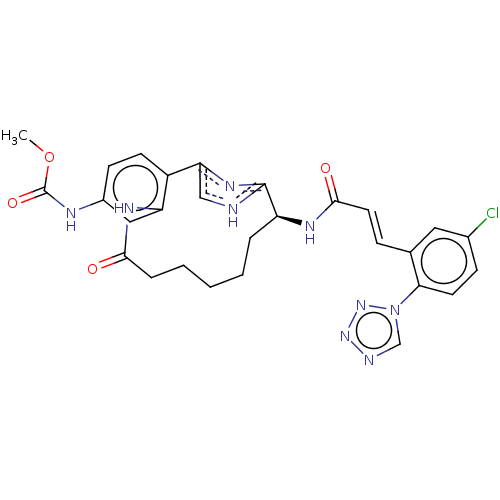 (CHEMBL4090854) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | PDB Article PubMed | 3.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230327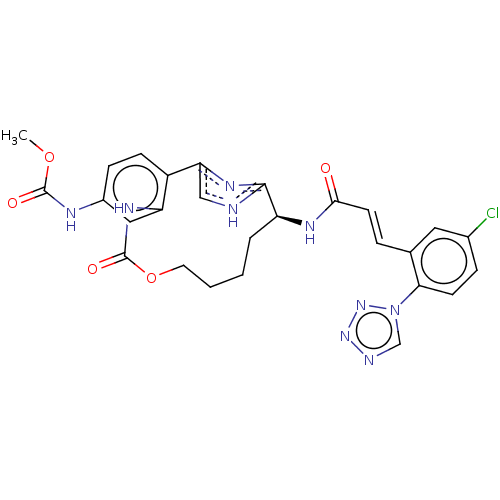 (CHEMBL4082071) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230339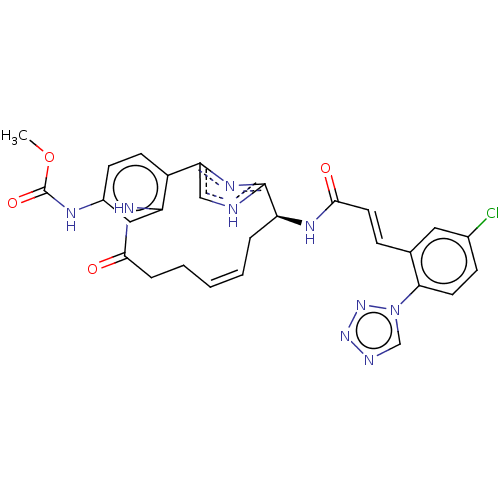 (CHEMBL4098577) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.20 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50063669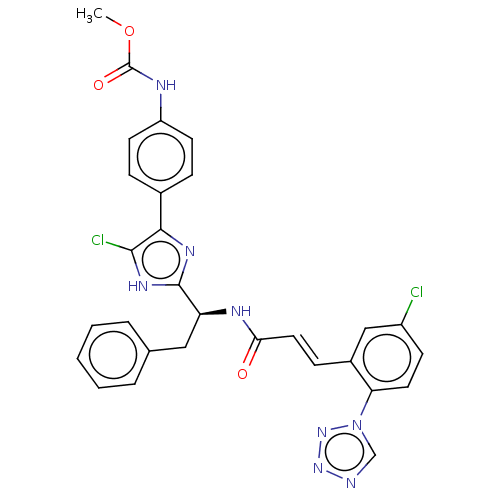 (CHEMBL3398641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 5.80 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 16 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-(D)-Pro-Phe-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230329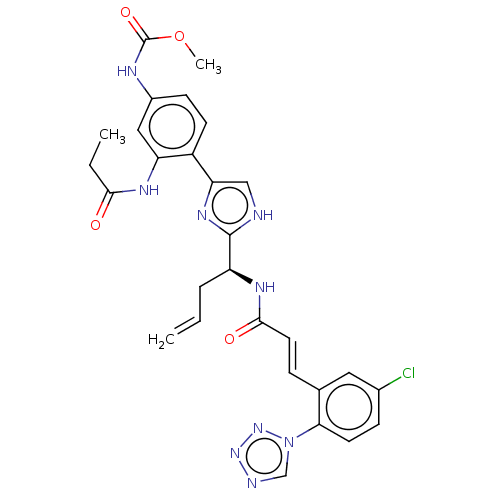 (CHEMBL4079002) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 27 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230332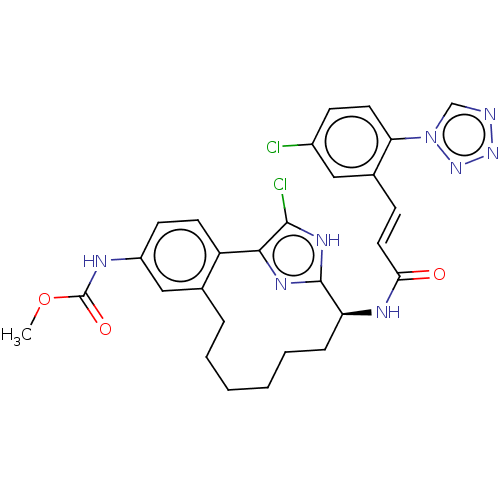 (CHEMBL4063872) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 42 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230335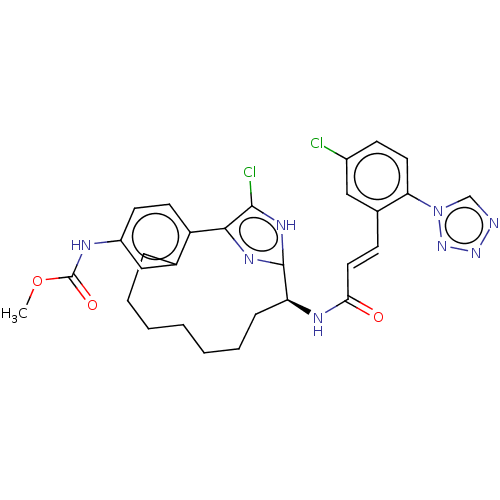 (CHEMBL4072590) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 68 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 70 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-(D)-Pro-Phe-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230323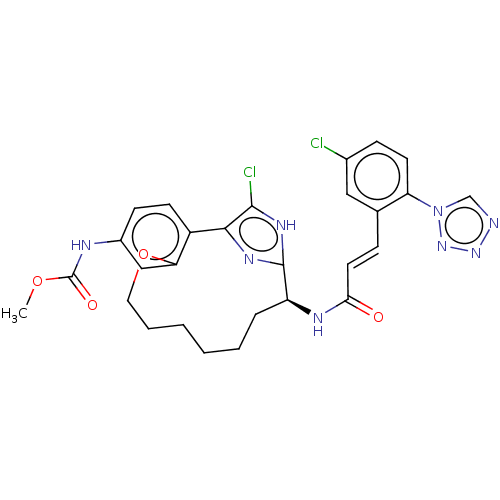 (CHEMBL4101043) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 76 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM142858 (US10487086, Example 6 | US11136327, Example 6 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 87 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230338 (CHEMBL4083064) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 159 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230333 (CHEMBL4104659) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 184 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM142859 (US10487086, Example 9 | US11136327, Example 9 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 250 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 25 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230330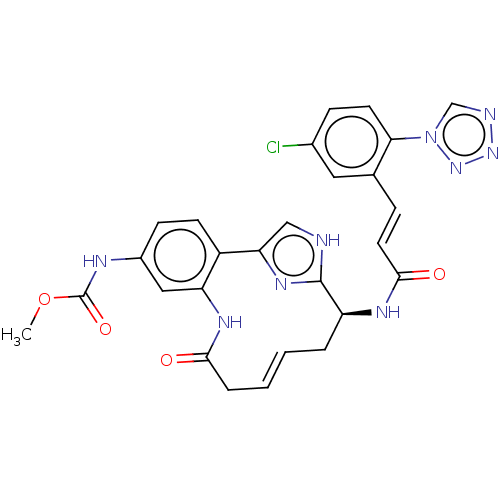 (CHEMBL4092164) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 264 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230331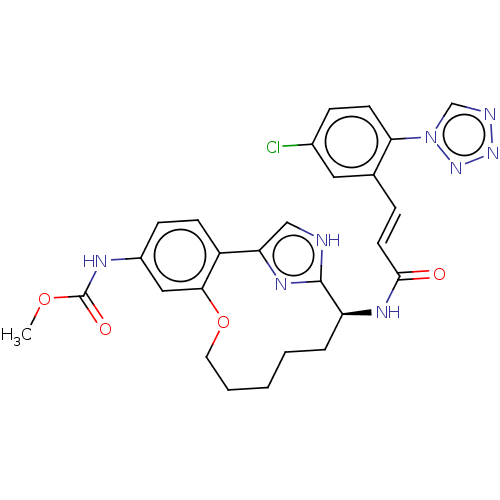 (CHEMBL4081356) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 420 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 25 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230328 (CHEMBL4059685) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 514 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasma kallikrein (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 519 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasma kallikrein using H-(D)-Pro-Phe-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230334 (CHEMBL4086754) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 600 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM142857 (US10487086, Example 4 | US11136327, Example 4 | US...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 636 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 1.67E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 7a using H-(D)-Ile-Pro-Arg-pNA as substrate after 10 to 120 mins at 25 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 12a using H-(D)-CHT-Gly-Arg-pNA as substrate after 10 to 120 mins at 25 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | >3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 12a using H-(D)-CHT-Gly-Arg-pNA as substrate after 10 to 120 mins at 25 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XII (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >3.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 12a using H-(D)-CHT-Gly-Arg-pNA as substrate after 10 to 120 mins at 25 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | 3.19E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 7a using H-(D)-Ile-Pro-Arg-pNA as substrate after 10 to 120 mins at 25 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor XI (Homo sapiens (Human)) | BDBM50230336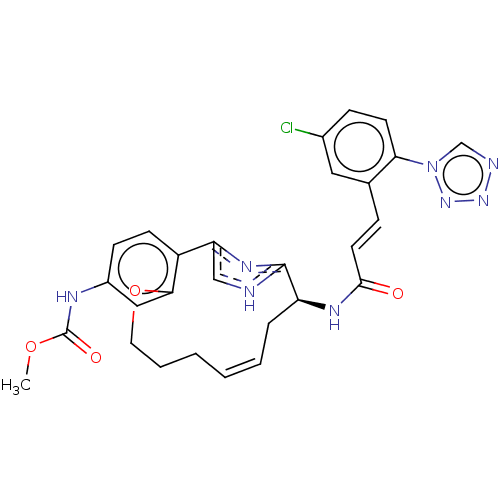 (CHEMBL4093309) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | 3.92E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 11a using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | 5.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human urokinase using pyro-Glu-Gly-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human tissue plasminogen activator using methylsulfonyl-D-cyclohexylalanyl-GlyArg-pNA as substrate after 10 to 120 mins at 37 degC by s... | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | >6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human tissue plasminogen activator using methylsulfonyl-D-cyclohexylalanyl-GlyArg-pNA as substrate after 10 to 120 mins at 37 degC by s... | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tissue-type plasminogen activator (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >6.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human tissue plasminogen activator using methylsulfonyl-D-cyclohexylalanyl-GlyArg-pNA as substrate after 10 to 120 mins at 37 degC by s... | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human thrombin using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | >1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human thrombin using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Prothrombin (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human thrombin using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotom... | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | >1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotom... | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor X (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.33E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 10a using N-benzoyl-Ile-Glu-(OH,OMe)-Gly-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotom... | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Coagulation factor VII (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.34E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human coagulation factor 7a using H-(D)-Ile-Pro-Arg-pNA as substrate after 10 to 120 mins at 25 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >1.42E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-(D)-Val-Leu-LyspNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | >1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human urokinase using pyro-Glu-Gly-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Urokinase-type plasminogen activator (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.51E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human urokinase using pyro-Glu-Gly-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-(D)-Val-Leu-LyspNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Plasminogen (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | >1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human plasmin using H-(D)-Val-Leu-LyspNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50230322 (CHEMBL4071545) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | >2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM50063669 (CHEMBL3398641) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | >2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Vitamin K-dependent protein C (Homo sapiens (Human)) | BDBM47108 (US10487086, Example 10 | US11136327, Example 10 | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem Similars | Article PubMed | >2.15E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Bristol-Myers Squibb Company Curated by ChEMBL | Assay Description Inhibition of human activated protein C using pyroGlu-Pro-Arg-pNA as substrate after 10 to 120 mins at 37 degC by spectrophotometric method | J Med Chem 60: 1060-1075 (2017) Article DOI: 10.1021/acs.jmedchem.6b01460 BindingDB Entry DOI: 10.7270/Q25D8V31 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 53 total ) | Next | Last >> |