

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118502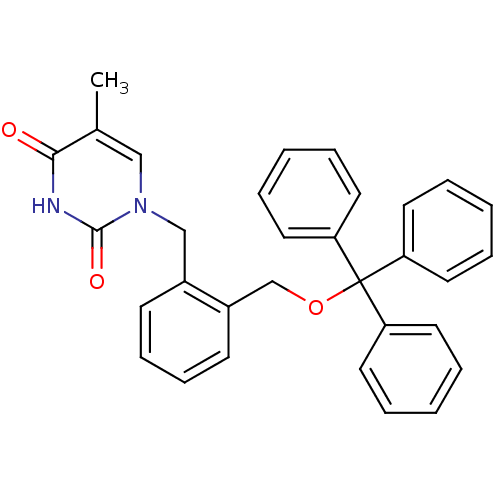 (5-Methyl-1-(2-trityloxymethyl-benzyl)-1H-pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118502 (5-Methyl-1-(2-trityloxymethyl-benzyl)-1H-pyrimidin...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 8 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM1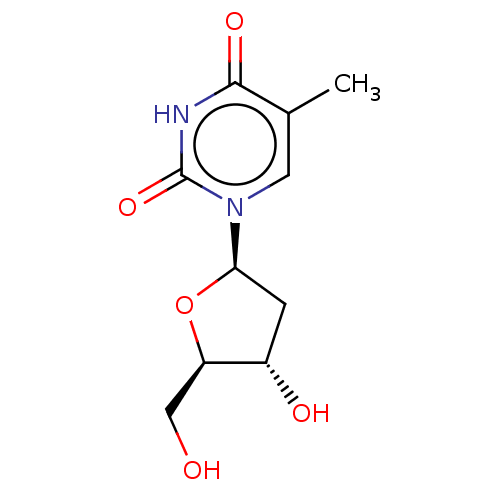 (dT | thymidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity (50 uM) against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM1 (dT | thymidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity (50 uM) against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490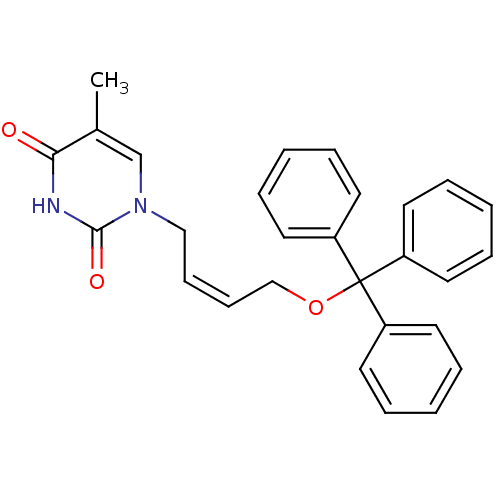 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491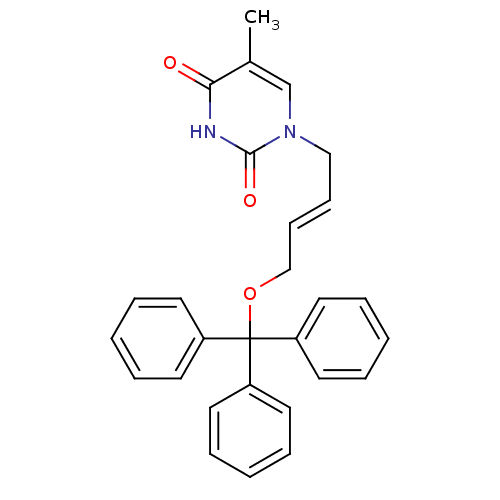 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 230 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with gancicclovir | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 390 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118501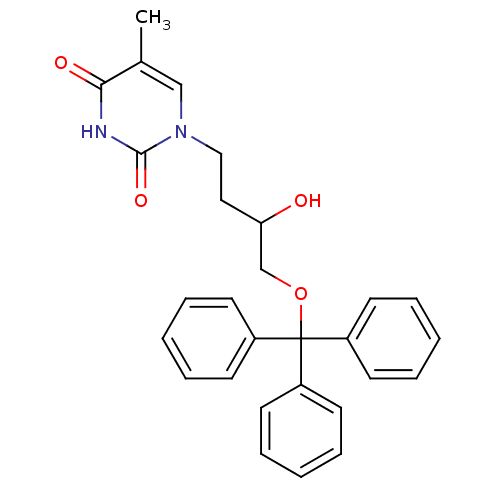 (1-(3-Hydroxy-4-trityloxy-butyl)-5-methyl-1H-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM1 (dT | thymidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118498 (5-Methyl-1-(4-trityloxy-but-2-ynyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory activity against HSV-1 Thymidine kinase in OST-TK-/HSV-1 TK+ cell line in combination with BVAraU | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118496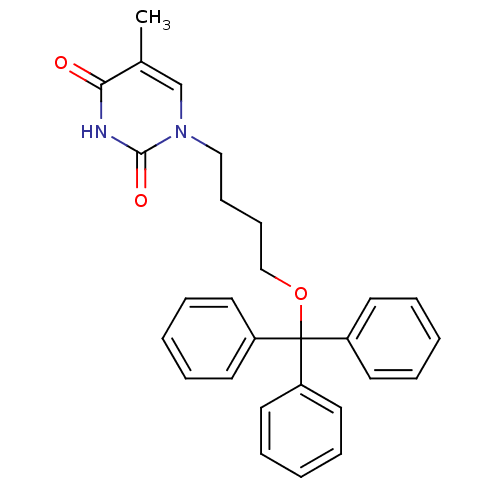 (5-Methyl-1-(4-trityloxy-butyl)-1H-pyrimidine-2,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118501 (1-(3-Hydroxy-4-trityloxy-butyl)-5-methyl-1H-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118503 (5-Iodo-1-(3-trityloxy-propenyl)-1H-pyrimidine-2,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118506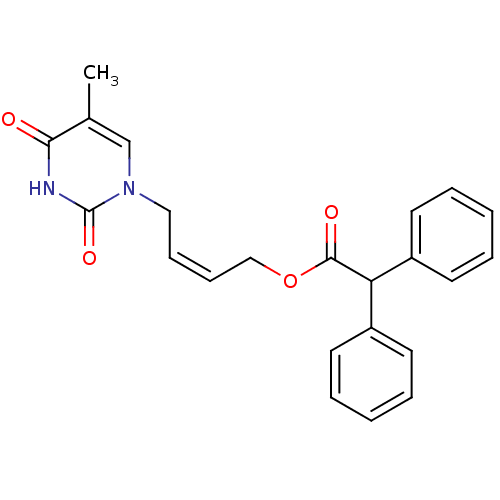 (CHEMBL335866 | Diphenyl-acetic acid 4-(5-methyl-2,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118489 (1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 7.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 8.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118494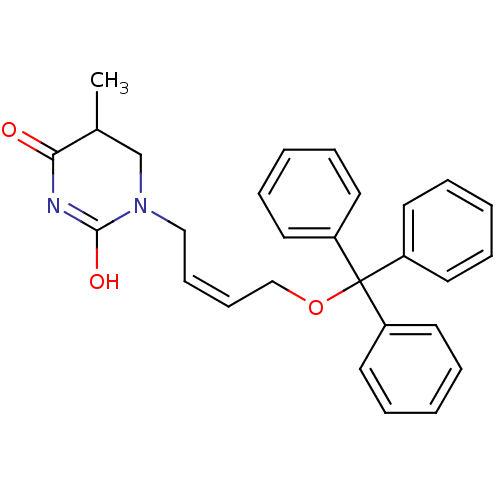 (5-Methyl-1-(4-trityloxy-but-2-enyl)-dihydro-pyrimi...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118496 (5-Methyl-1-(4-trityloxy-butyl)-1H-pyrimidine-2,4-d...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118497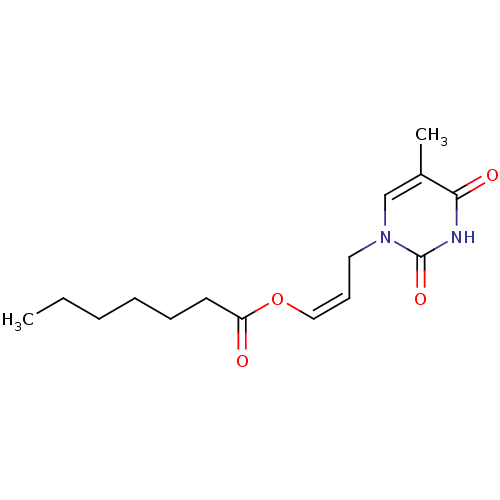 (CHEMBL134449 | Heptanoic acid 3-(5-methyl-2,4-diox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118488 (1-(2,3-Dihydroxy-4-trityloxy-butyl)-5-methyl-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118499 (1-{5-[Bis-(4-methoxy-phenyl)-phenyl-methoxymethyl]...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | PubMed | n/a | n/a | 1.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118488 (1-(2,3-Dihydroxy-4-trityloxy-butyl)-5-methyl-1H-py...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 1.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118495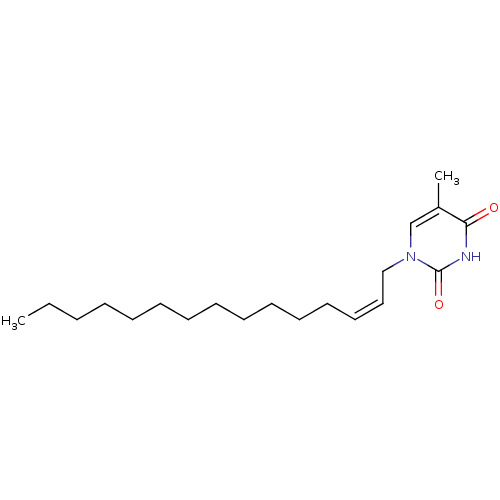 (5-Methyl-1-pentadec-2-enyl-1H-pyrimidine-2,4-dione...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118507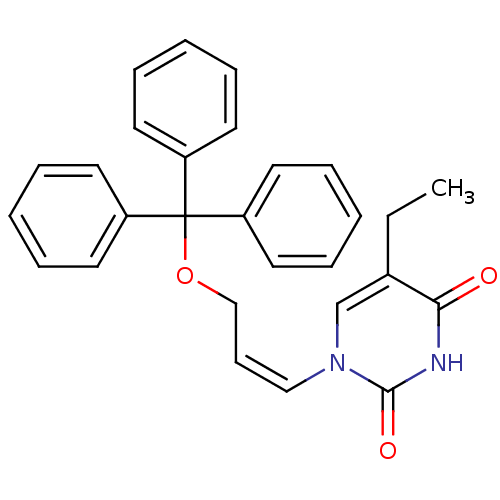 (5-Ethyl-1-(3-trityloxy-propenyl)-1H-pyrimidine-2,4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118504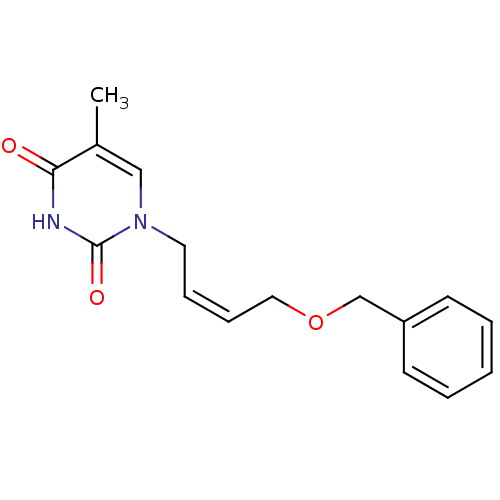 (1-(4-Benzyloxy-but-2-enyl)-5-methyl-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.20E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118493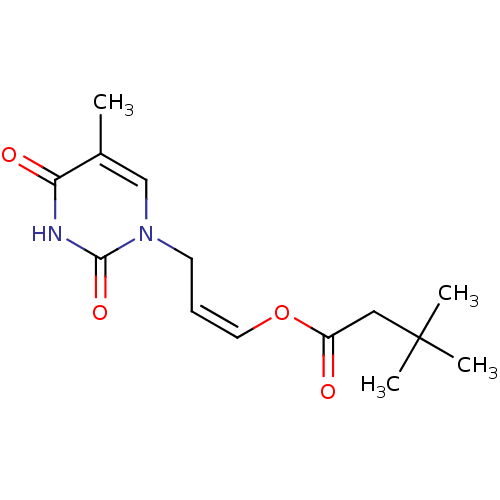 (3,3-Dimethyl-butyric acid 3-(5-methyl-2,4-dioxo-3,...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 2.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118505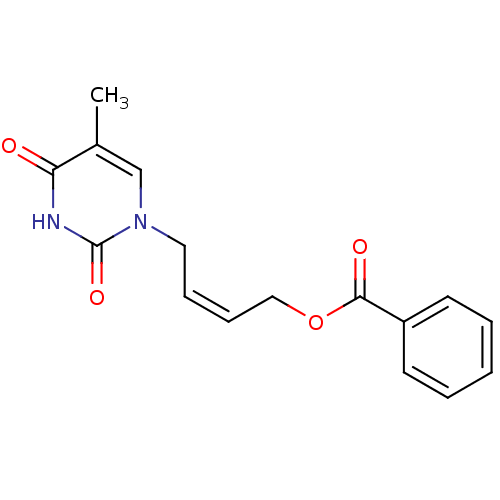 (Benzoic acid 4-(5-methyl-2,4-dioxo-3,4-dihydro-2H-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118489 (1-(4-Hydroxy-5-trityloxymethyl-tetrahydro-furan-2-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 3.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118500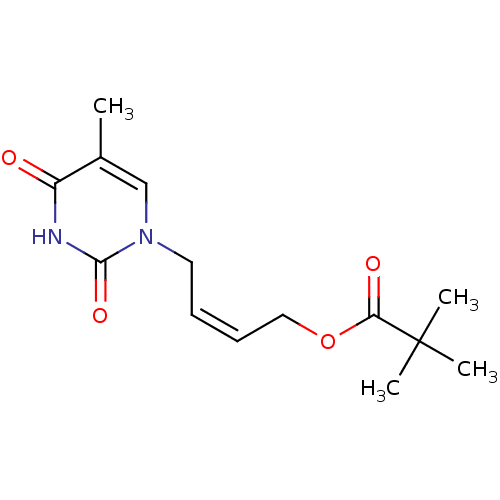 (2,2-Dimethyl-propionic acid 4-(5-methyl-2,4-dioxo-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory effect against phosphorylation of [methyl-3H]dThd by Thymidine Kinase 2 (TK-2) | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118503 (5-Iodo-1-(3-trityloxy-propenyl)-1H-pyrimidine-2,4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Compound was evaluated for inhibitory effect against phosphorylation of [methyl-3H]dThd by HSV-1 Thymidine kinase | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118491 (5-Methyl-1-(4-trityloxy-but-2-enyl)-1H-pyrimidine-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 4.80E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (A167Y) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50118490 (1-[(Z)-4-(triphenylmethoxy)-2-butenyl]thymine | 5-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | >5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (A167Y) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50422404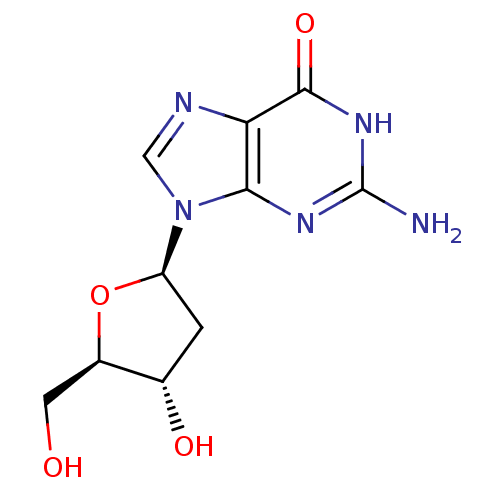 (2''-Deoxyguanosine | DEOXYGUANOSINE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 1.15E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (A167Y) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM50422404 (2''-Deoxyguanosine | DEOXYGUANOSINE) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Similars | PubMed | n/a | n/a | 3.64E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (WT) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine kinase, cytosolic (Homo sapiens (Human)) | BDBM1 (dT | thymidine) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | >5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Instituto de Qu£mica M£dica (CSIC) Curated by ChEMBL | Assay Description Inhibitory concentration against HSV-1 TK (A167Y) catalyzed [3H]-GCV phosphorylation | J Med Chem 45: 4254-63 (2002) BindingDB Entry DOI: 10.7270/Q2GQ6ZGB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||