 Found 22 hits Enz. Inhib. hit(s) with all data for entry = 50037168
Found 22 hits Enz. Inhib. hit(s) with all data for entry = 50037168 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130785
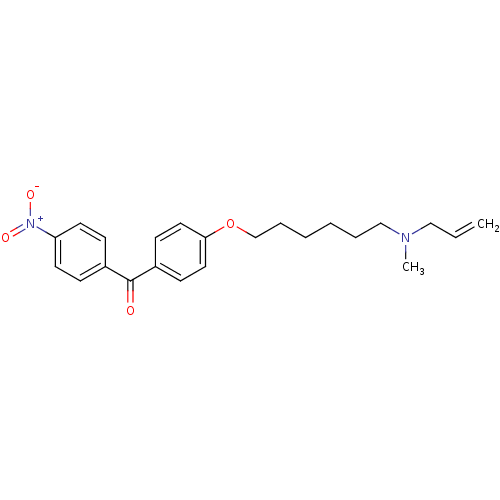
(CHEMBL542453 | CHEMBL609978 | {4-[6-(Allyl-methyl-...)Show SMILES CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(cc1)[N+]([O-])=O)CC=C Show InChI InChI=1S/C23H28N2O4/c1-3-16-24(2)17-6-4-5-7-18-29-22-14-10-20(11-15-22)23(26)19-8-12-21(13-9-19)25(27)28/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128063

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[d]isothiazol-6-...)Show SMILES CN(CCCCCCOc1ccc2c(nsc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C23H27BrN2OS/c1-3-14-26(2)15-6-4-5-7-16-27-20-12-13-21-22(17-20)28-25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128054
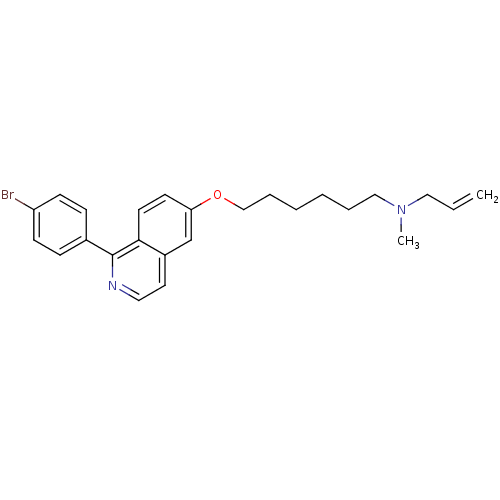
(Allyl-{6-[1-(4-bromo-phenyl)-isoquinolin-6-yloxy]-...)Show SMILES CN(CCCCCCOc1ccc2c(nccc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C25H29BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-15,19H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130788
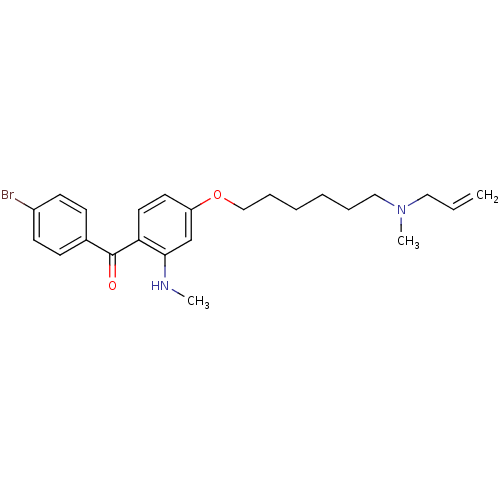
(CHEMBL116705 | CHEMBL611484 | {4-[6-(Allyl-methyl-...)Show SMILES CNc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H31BrN2O2/c1-4-15-27(3)16-7-5-6-8-17-29-21-13-14-22(23(18-21)26-2)24(28)19-9-11-20(25)12-10-19/h4,9-14,18,26H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128055
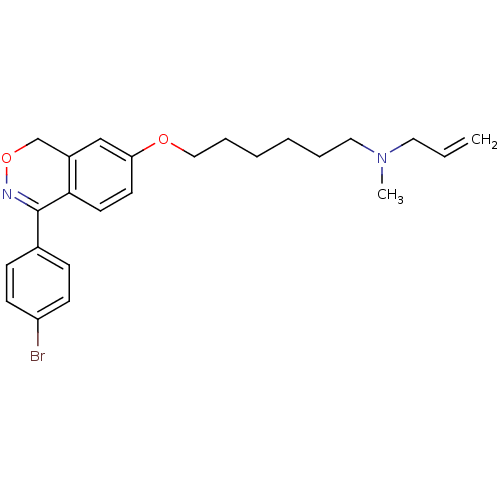
(Allyl-{6-[4-(4-bromo-phenyl)-1H-benzo[d][1,2]oxazi...)Show SMILES CN(CCCCCCOc1ccc2C(=NOCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C24H29BrN2O2/c1-3-14-27(2)15-6-4-5-7-16-28-22-12-13-23-20(17-22)18-29-26-24(23)19-8-10-21(25)11-9-19/h3,8-13,17H,1,4-7,14-16,18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.10 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130783

(CHEMBL115020 | CHEMBL611485 | {4-[6-(Allyl-methyl-...)Show SMILES COc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H30BrNO3/c1-4-15-26(2)16-7-5-6-8-17-29-21-13-14-22(23(18-21)28-3)24(27)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 4.60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128050
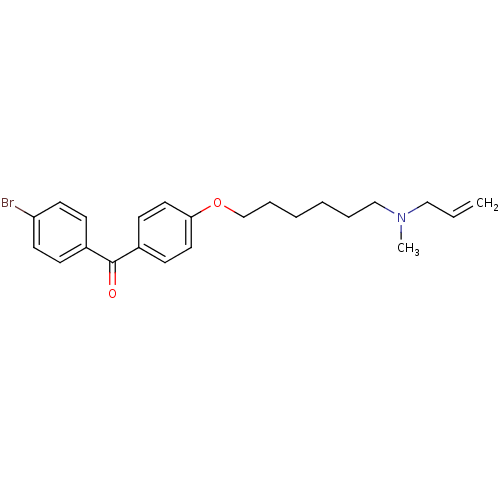
(CHEMBL114259 | CHEMBL416694 | N-allyl-6-(4-(4-brom...)Show InChI InChI=1S/C23H28BrNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 5.40 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130789

(Allyl-{6-[3-(4-bromo-phenyl)-benzo[b]thiophen-6-yl...)Show SMILES CN(CCCCCCOc1ccc2c(csc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C24H28BrNOS/c1-3-14-26(2)15-6-4-5-7-16-27-21-12-13-22-23(18-28-24(22)17-21)19-8-10-20(25)11-9-19/h3,8-13,17-18H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 5.60 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130786
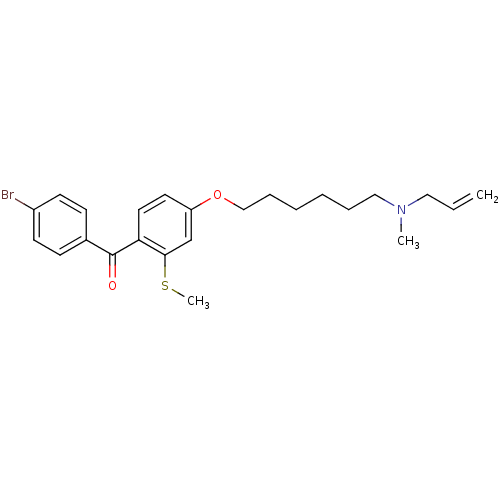
(CHEMBL115923 | CHEMBL611486 | {4-[6-(Allyl-methyl-...)Show SMILES CSc1cc(OCCCCCCN(C)CC=C)ccc1C(=O)c1ccc(Br)cc1 Show InChI InChI=1S/C24H30BrNO2S/c1-4-15-26(2)16-7-5-6-8-17-28-21-13-14-22(23(18-21)29-3)24(27)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130774
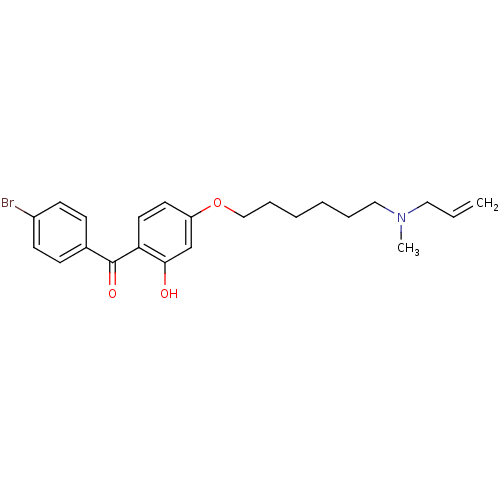
(CHEMBL115375 | CHEMBL611757 | {4-[6-(Allyl-methyl-...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(O)c1)CC=C Show InChI InChI=1S/C23H28BrNO3/c1-3-14-25(2)15-6-4-5-7-16-28-20-12-13-21(22(26)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17,26H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.30 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128065

(CHEMBL304858 | CHEMBL324162 | N-allyl-6-(4-(4-brom...)Show SMILES CN(CCCCCCOc1ccc(C(=O)c2ccc(Br)cc2)c(F)c1)CC=C Show InChI InChI=1S/C23H27BrFNO2/c1-3-14-26(2)15-6-4-5-7-16-28-20-12-13-21(22(25)17-20)23(27)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
MMDB
PDB
Article
PubMed
| n/a | n/a | 6.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130775
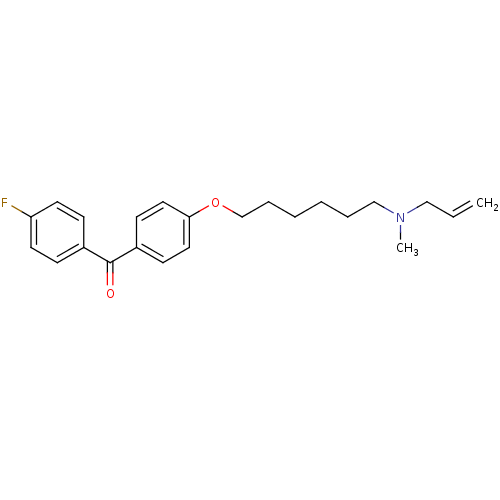
(CHEMBL554209 | CHEMBL611758 | {4-[6-(Allyl-methyl-...)Show InChI InChI=1S/C23H28FNO2/c1-3-16-25(2)17-6-4-5-7-18-27-22-14-10-20(11-15-22)23(26)19-8-12-21(24)13-9-19/h3,8-15H,1,4-7,16-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130772
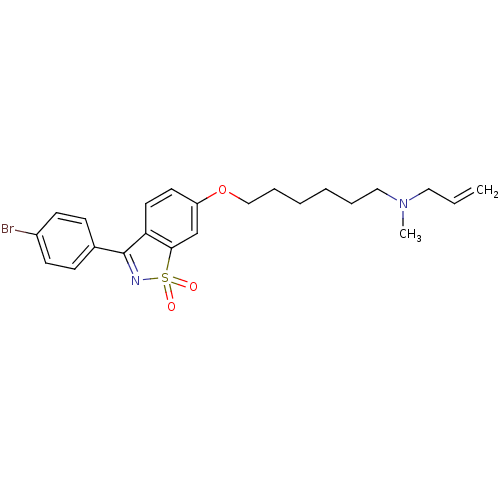
(Allyl-{6-[3-(4-bromo-phenyl)-1,1-dioxo-1H-1lambda*...)Show SMILES CN(CCCCCCOc1ccc2C(=NS(=O)(=O)c2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C23H27BrN2O3S/c1-3-14-26(2)15-6-4-5-7-16-29-20-12-13-21-22(17-20)30(27,28)25-23(21)18-8-10-19(24)11-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.80 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130784

(Allyl-{6-[1-(4-bromo-phenyl)-3,4-dihydro-isoquinol...)Show SMILES CN(CCCCCCOc1ccc2C(=NCCc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C25H31BrN2O/c1-3-16-28(2)17-6-4-5-7-18-29-23-12-13-24-21(19-23)14-15-27-25(24)20-8-10-22(26)11-9-20/h3,8-13,19H,1,4-7,14-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 7.90 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130787
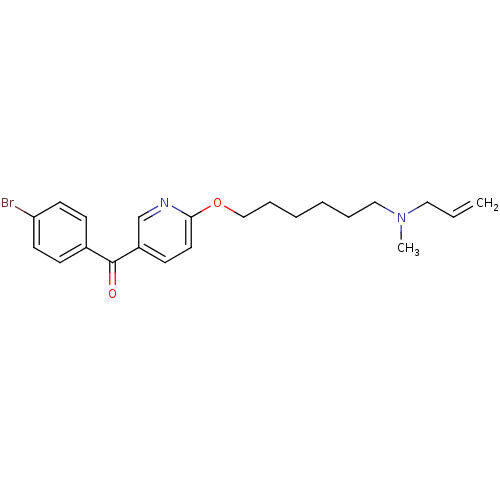
(CHEMBL114266 | CHEMBL612025 | {6-[6-(Allyl-methyl-...)Show InChI InChI=1S/C22H27BrN2O2/c1-3-14-25(2)15-6-4-5-7-16-27-21-13-10-19(17-24-21)22(26)18-8-11-20(23)12-9-18/h3,8-13,17H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 8.70 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130776

(Allyl-{6-[4-(4-bromo-phenyl)-2H-chromen-7-yloxy]-h...)Show SMILES CN(CCCCCCOc1ccc2C(=CCOc2c1)c1ccc(Br)cc1)CC=C |c:13| Show InChI InChI=1S/C25H30BrNO2/c1-3-15-27(2)16-6-4-5-7-17-28-22-12-13-24-23(14-18-29-25(24)19-22)20-8-10-21(26)11-9-20/h3,8-14,19H,1,4-7,15-18H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 11.4 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130781
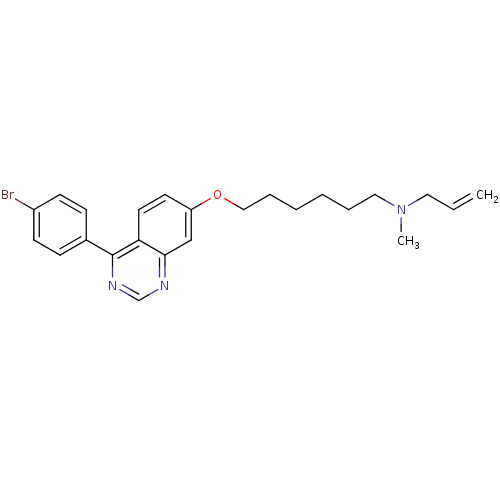
(Allyl-{6-[4-(4-bromo-phenyl)-quinazolin-7-yloxy]-h...)Show SMILES CN(CCCCCCOc1ccc2c(ncnc2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C24H28BrN3O/c1-3-14-28(2)15-6-4-5-7-16-29-21-12-13-22-23(17-21)26-18-27-24(22)19-8-10-20(25)11-9-19/h3,8-13,17-18H,1,4-7,14-16H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12.3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130777
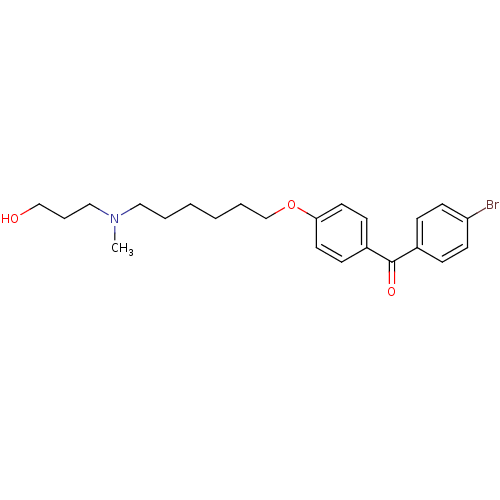
((4-Bromo-phenyl)-(4-{6-[(3-hydroxy-propyl)-methyl-...)Show InChI InChI=1S/C23H30BrNO3/c1-25(16-6-17-26)15-4-2-3-5-18-28-22-13-9-20(10-14-22)23(27)19-7-11-21(24)12-8-19/h7-14,26H,2-6,15-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 15.7 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50128058
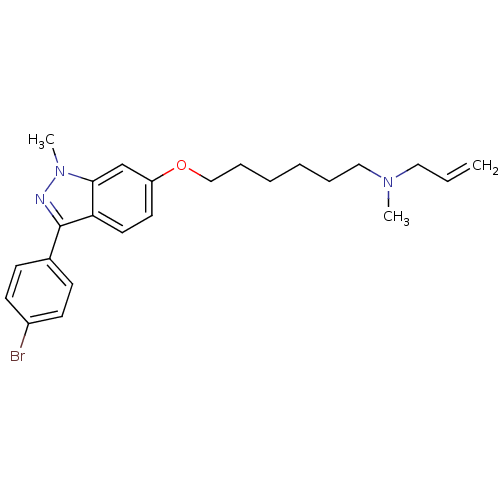
(ALLYL-{6-[3-(4-BROMO-PHENYL)-1-METHYL-1H-INDAZOL-6...)Show SMILES CN(CCCCCCOc1ccc2c(nn(C)c2c1)-c1ccc(Br)cc1)CC=C Show InChI InChI=1S/C24H30BrN3O/c1-4-15-27(2)16-7-5-6-8-17-29-21-13-14-22-23(18-21)28(3)26-24(22)19-9-11-20(25)12-10-19/h4,9-14,18H,1,5-8,15-17H2,2-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
DrugBank
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 19.6 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130779
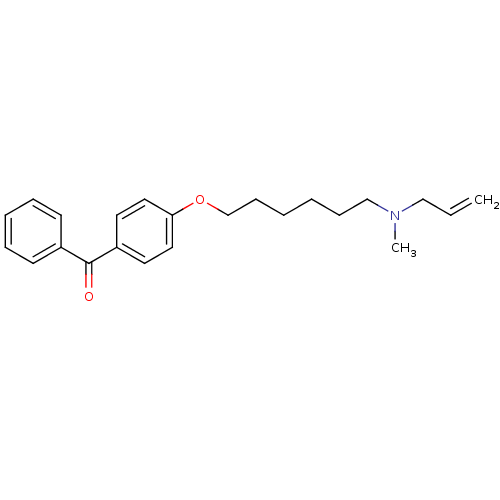
(CHEMBL553281 | CHEMBL611755 | {4-[6-(Allyl-methyl-...)Show InChI InChI=1S/C23H29NO2/c1-3-17-24(2)18-9-4-5-10-19-26-22-15-13-21(14-16-22)23(25)20-11-7-6-8-12-20/h3,6-8,11-16H,1,4-5,9-10,17-19H2,2H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22.5 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130771
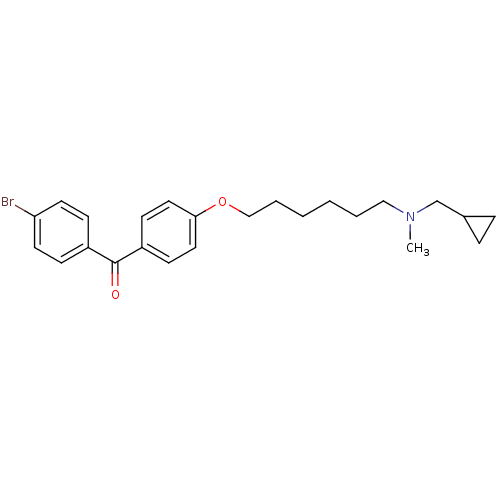
((4-Bromo-phenyl)-{4-[6-(cyclopropylmethyl-methyl-a...)Show SMILES CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(Br)cc1)CC1CC1 Show InChI InChI=1S/C24H30BrNO2/c1-26(18-19-6-7-19)16-4-2-3-5-17-28-23-14-10-21(11-15-23)24(27)20-8-12-22(25)13-9-20/h8-15,19H,2-7,16-18H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 31.8 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |
Lanosterol synthase
(Homo sapiens (Human)) | BDBM50130778
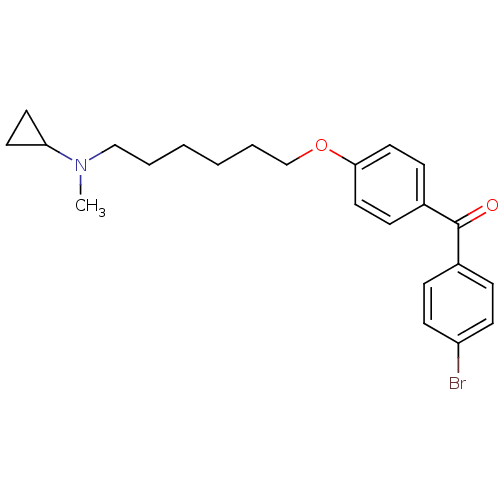
((4-Bromo-phenyl)-{4-[6-(cyclopropyl-methyl-amino)-...)Show SMILES CN(CCCCCCOc1ccc(cc1)C(=O)c1ccc(Br)cc1)C1CC1 Show InChI InChI=1S/C23H28BrNO2/c1-25(21-12-13-21)16-4-2-3-5-17-27-22-14-8-19(9-15-22)23(26)18-6-10-20(24)11-7-18/h6-11,14-15,21H,2-5,12-13,16-17H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35.3 | n/a | n/a | n/a | n/a | n/a | n/a |
F. Hoffmann-La Roche Ltd.
Curated by ChEMBL
| Assay Description
In vitro inhibition of human 2,3-oxidosqualene cyclase. |
J Med Chem 46: 3354-70 (2003)
Article DOI: 10.1021/jm021120f
BindingDB Entry DOI: 10.7270/Q29G5NKM |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data