 Found 99 hits Enz. Inhib. hit(s) with all data for entry = 50040048
Found 99 hits Enz. Inhib. hit(s) with all data for entry = 50040048 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388467
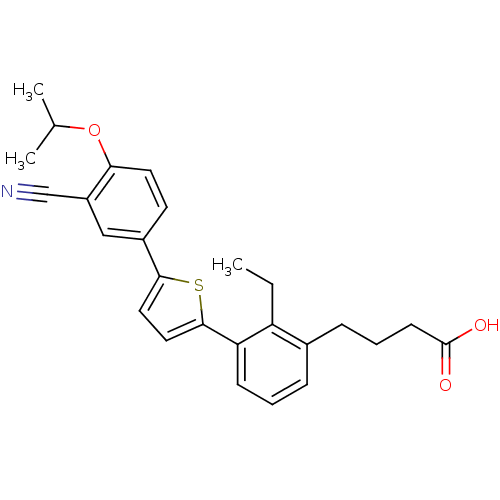
(CHEMBL2059521)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1ccc(s1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C26H27NO3S/c1-4-21-18(8-6-10-26(28)29)7-5-9-22(21)25-14-13-24(31-25)19-11-12-23(30-17(2)3)20(15-19)16-27/h5,7,9,11-15,17H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.58E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388466

(CHEMBL2059520)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1cnc(s1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C25H26N2O3S/c1-4-20-17(8-6-10-24(28)29)7-5-9-21(20)23-15-27-25(31-23)18-11-12-22(30-16(2)3)19(13-18)14-26/h5,7,9,11-13,15-16H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388468
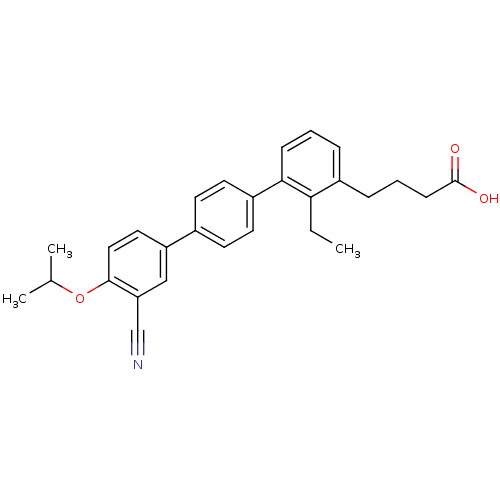
(CHEMBL2059527)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1ccc(cc1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C28H29NO3/c1-4-25-21(8-6-10-28(30)31)7-5-9-26(25)22-13-11-20(12-14-22)23-15-16-27(32-19(2)3)24(17-23)18-29/h5,7,9,11-17,19H,4,6,8,10H2,1-3H3,(H,30,31) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388465
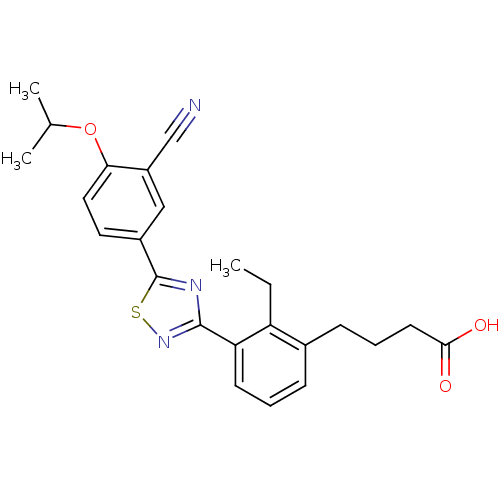
(CHEMBL2059517)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O3S/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)23-26-24(31-27-23)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.01E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50388470
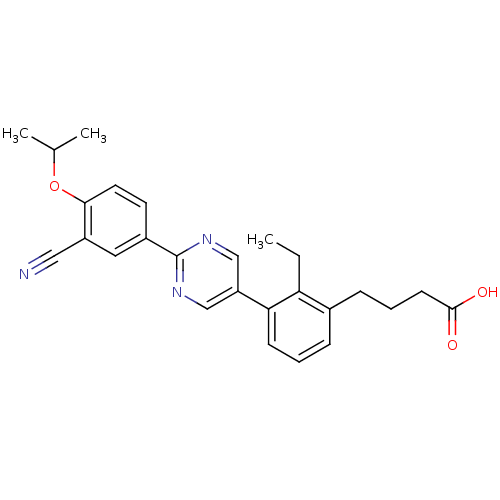
(CHEMBL2057232)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1cnc(nc1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C26H27N3O3/c1-4-22-18(8-6-10-25(30)31)7-5-9-23(22)21-15-28-26(29-16-21)19-11-12-24(32-17(2)3)20(13-19)14-27/h5,7,9,11-13,15-17H,4,6,8,10H2,1-3H3,(H,30,31) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.31E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388473
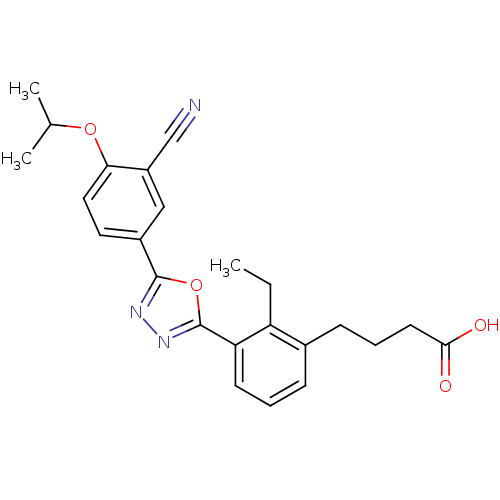
(CHEMBL2059519)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1nnc(o1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O4/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)24-27-26-23(31-24)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50388471

(CHEMBL2057282)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C27H32ClN3O3S/c1-4-21-18(10-13-31-14-11-19(12-15-31)27(32)33)6-5-7-22(21)25-29-26(35-30-25)20-8-9-24(23(28)16-20)34-17(2)3/h5-9,16-17,19H,4,10-15H2,1-3H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50388464
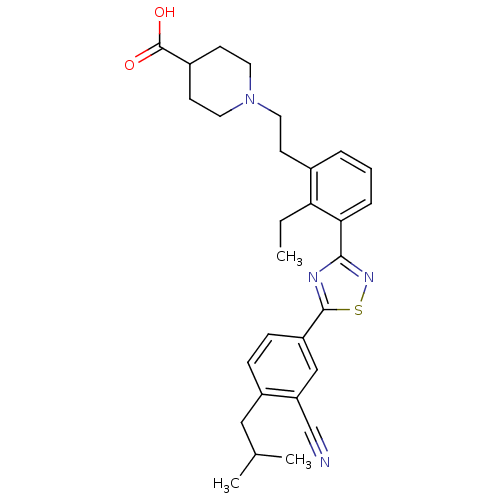
(CHEMBL2057285)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C29H34N4O2S/c1-4-25-20(10-13-33-14-11-21(12-15-33)29(34)35)6-5-7-26(25)27-31-28(36-32-27)23-9-8-22(16-19(2)3)24(17-23)18-30/h5-9,17,19,21H,4,10-16H2,1-3H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50388464

(CHEMBL2057285)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C29H34N4O2S/c1-4-25-20(10-13-33-14-11-21(12-15-33)29(34)35)6-5-7-26(25)27-31-28(36-32-27)23-9-8-22(16-19(2)3)24(17-23)18-30/h5-9,17,19,21H,4,10-16H2,1-3H3,(H,34,35) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50388464

(CHEMBL2057285)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C29H34N4O2S/c1-4-25-20(10-13-33-14-11-21(12-15-33)29(34)35)6-5-7-26(25)27-31-28(36-32-27)23-9-8-22(16-19(2)3)24(17-23)18-30/h5-9,17,19,21H,4,10-16H2,1-3H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50388472

(CHEMBL2059515)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1noc(n1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O4/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)23-26-24(31-27-23)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50388472

(CHEMBL2059515)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1noc(n1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O4/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)23-26-24(31-27-23)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50388472

(CHEMBL2059515)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1noc(n1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O4/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)23-26-24(31-27-23)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50388472

(CHEMBL2059515)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1noc(n1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O4/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)23-26-24(31-27-23)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388469
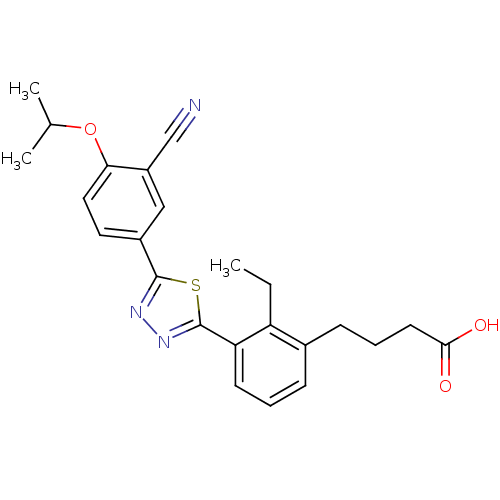
(CHEMBL2059516)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1nnc(s1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O3S/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)24-27-26-23(31-24)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388472

(CHEMBL2059515)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1noc(n1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O4/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)23-26-24(31-27-23)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388464

(CHEMBL2057285)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C29H34N4O2S/c1-4-25-20(10-13-33-14-11-21(12-15-33)29(34)35)6-5-7-26(25)27-31-28(36-32-27)23-9-8-22(16-19(2)3)24(17-23)18-30/h5-9,17,19,21H,4,10-16H2,1-3H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50388469

(CHEMBL2059516)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1nnc(s1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O3S/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)24-27-26-23(31-24)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50388471

(CHEMBL2057282)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C27H32ClN3O3S/c1-4-21-18(10-13-31-14-11-19(12-15-31)27(32)33)6-5-7-22(21)25-29-26(35-30-25)20-8-9-24(23(28)16-20)34-17(2)3/h5-9,16-17,19H,4,10-15H2,1-3H3,(H,32,33) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C19
(Homo sapiens (Human)) | BDBM50388471

(CHEMBL2057282)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C27H32ClN3O3S/c1-4-21-18(10-13-31-14-11-19(12-15-31)27(32)33)6-5-7-22(21)25-29-26(35-30-25)20-8-9-24(23(28)16-20)34-17(2)3/h5-9,16-17,19H,4,10-15H2,1-3H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C19 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50388471

(CHEMBL2057282)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C27H32ClN3O3S/c1-4-21-18(10-13-31-14-11-19(12-15-31)27(32)33)6-5-7-22(21)25-29-26(35-30-25)20-8-9-24(23(28)16-20)34-17(2)3/h5-9,16-17,19H,4,10-15H2,1-3H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50388469

(CHEMBL2059516)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1nnc(s1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O3S/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)24-27-26-23(31-24)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50388469

(CHEMBL2059516)Show SMILES CCc1c(CCCC(O)=O)cccc1-c1nnc(s1)-c1ccc(OC(C)C)c(c1)C#N Show InChI InChI=1S/C24H25N3O3S/c1-4-19-16(8-6-10-22(28)29)7-5-9-20(19)24-27-26-23(31-24)17-11-12-21(30-15(2)3)18(13-17)14-25/h5,7,9,11-13,15H,4,6,8,10H2,1-3H3,(H,28,29) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50388464

(CHEMBL2057285)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C29H34N4O2S/c1-4-25-20(10-13-33-14-11-21(12-15-33)29(34)35)6-5-7-26(25)27-31-28(36-32-27)23-9-8-22(16-19(2)3)24(17-23)18-30/h5-9,17,19,21H,4,10-16H2,1-3H3,(H,34,35) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50388471

(CHEMBL2057282)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C27H32ClN3O3S/c1-4-21-18(10-13-31-14-11-19(12-15-31)27(32)33)6-5-7-22(21)25-29-26(35-30-25)20-8-9-24(23(28)16-20)34-17(2)3/h5-9,16-17,19H,4,10-15H2,1-3H3,(H,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420108

(CHEMBL2059681)Show SMILES CCc1c(CN(C)CC(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C24H26F3N3O3S/c1-5-17-16(12-30(4)13-21(31)32)7-6-8-18(17)22-28-23(34-29-22)15-9-10-20(33-14(2)3)19(11-15)24(25,26)27/h6-11,14H,5,12-13H2,1-4H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 10 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420108

(CHEMBL2059681)Show SMILES CCc1c(CN(C)CC(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C24H26F3N3O3S/c1-5-17-16(12-30(4)13-21(31)32)7-6-8-18(17)22-28-23(34-29-22)15-9-10-20(33-14(2)3)19(11-15)24(25,26)27/h6-11,14H,5,12-13H2,1-4H3,(H,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420109

(CHEMBL2059682)Show SMILES CCc1c(CN(C)C(C)(C)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C26H30F3N3O3S/c1-7-18-17(14-32(6)25(4,5)24(33)34)9-8-10-19(18)22-30-23(36-31-22)16-11-12-21(35-15(2)3)20(13-16)26(27,28)29/h8-13,15H,7,14H2,1-6H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420109

(CHEMBL2059682)Show SMILES CCc1c(CN(C)C(C)(C)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C26H30F3N3O3S/c1-7-18-17(14-32(6)25(4,5)24(33)34)9-8-10-19(18)22-30-23(36-31-22)16-11-12-21(35-15(2)3)20(13-16)26(27,28)29/h8-13,15H,7,14H2,1-6H3,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420110

(CHEMBL2059683)Show SMILES CCc1c(CCNCC(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C24H26F3N3O3S/c1-4-17-15(10-11-28-13-21(31)32)6-5-7-18(17)22-29-23(34-30-22)16-8-9-20(33-14(2)3)19(12-16)24(25,26)27/h5-9,12,14,28H,4,10-11,13H2,1-3H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0158 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420111
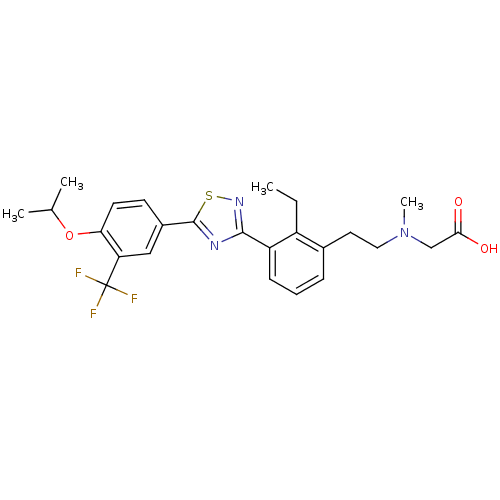
(CHEMBL2059684)Show SMILES CCc1c(CCN(C)CC(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C25H28F3N3O3S/c1-5-18-16(11-12-31(4)14-22(32)33)7-6-8-19(18)23-29-24(35-30-23)17-9-10-21(34-15(2)3)20(13-17)25(26,27)28/h6-10,13,15H,5,11-12,14H2,1-4H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.0200 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420111

(CHEMBL2059684)Show SMILES CCc1c(CCN(C)CC(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C25H28F3N3O3S/c1-5-18-16(11-12-31(4)14-22(32)33)7-6-8-19(18)23-29-24(35-30-23)17-9-10-21(34-15(2)3)20(13-17)25(26,27)28/h6-10,13,15H,5,11-12,14H2,1-4H3,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420112

(CHEMBL2059685)Show SMILES CCc1c(CN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C27H30F3N3O3S/c1-4-20-19(15-33-12-10-17(11-13-33)26(34)35)6-5-7-21(20)24-31-25(37-32-24)18-8-9-23(36-16(2)3)22(14-18)27(28,29)30/h5-9,14,16-17H,4,10-13,15H2,1-3H3,(H,34,35) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 15.8 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420112

(CHEMBL2059685)Show SMILES CCc1c(CN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C27H30F3N3O3S/c1-4-20-19(15-33-12-10-17(11-13-33)26(34)35)6-5-7-21(20)24-31-25(37-32-24)18-8-9-23(36-16(2)3)22(14-18)27(28,29)30/h5-9,14,16-17H,4,10-13,15H2,1-3H3,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420113

(CHEMBL2059686)Show SMILES CCc1c(CN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C25H26F3N3O3S/c1-4-18-16(11-31-12-17(13-31)24(32)33)6-5-7-19(18)22-29-23(35-30-22)15-8-9-21(34-14(2)3)20(10-15)25(26,27)28/h5-10,14,17H,4,11-13H2,1-3H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420113

(CHEMBL2059686)Show SMILES CCc1c(CN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C25H26F3N3O3S/c1-4-18-16(11-31-12-17(13-31)24(32)33)6-5-7-19(18)22-29-23(35-30-22)15-8-9-21(34-14(2)3)20(10-15)25(26,27)28/h5-10,14,17H,4,11-13H2,1-3H3,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420114
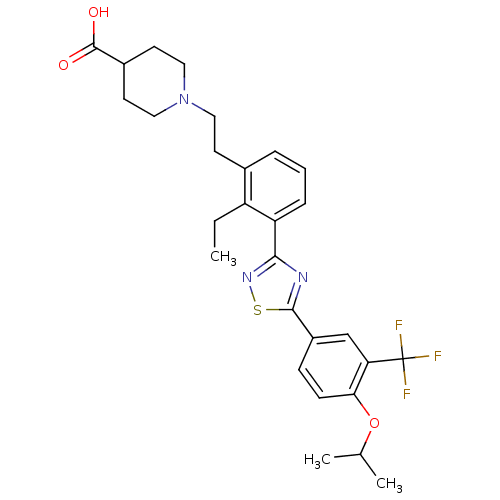
(CHEMBL2059687)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C28H32F3N3O3S/c1-4-21-18(10-13-34-14-11-19(12-15-34)27(35)36)6-5-7-22(21)25-32-26(38-33-25)20-8-9-24(37-17(2)3)23(16-20)28(29,30)31/h5-9,16-17,19H,4,10-15H2,1-3H3,(H,35,36) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420114

(CHEMBL2059687)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C28H32F3N3O3S/c1-4-21-18(10-13-34-14-11-19(12-15-34)27(35)36)6-5-7-22(21)25-32-26(38-33-25)20-8-9-24(37-17(2)3)23(16-20)28(29,30)31/h5-9,16-17,19H,4,10-15H2,1-3H3,(H,35,36) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420115
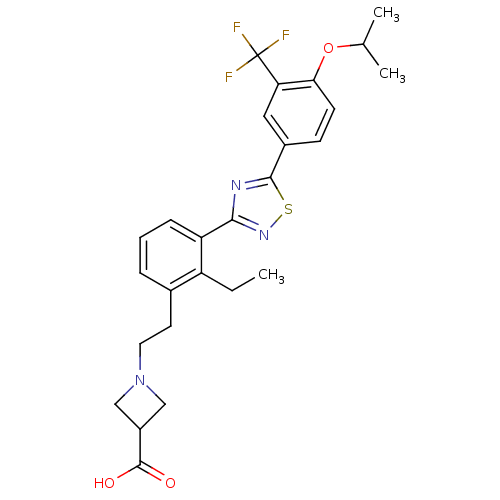
(CHEMBL2059688)Show SMILES CCc1c(CCN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C26H28F3N3O3S/c1-4-19-16(10-11-32-13-18(14-32)25(33)34)6-5-7-20(19)23-30-24(36-31-23)17-8-9-22(35-15(2)3)21(12-17)26(27,28)29/h5-9,12,15,18H,4,10-11,13-14H2,1-3H3,(H,33,34) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420115

(CHEMBL2059688)Show SMILES CCc1c(CCN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(c1)C(F)(F)F Show InChI InChI=1S/C26H28F3N3O3S/c1-4-19-16(10-11-32-13-18(14-32)25(33)34)6-5-7-20(19)23-30-24(36-31-23)17-8-9-22(35-15(2)3)21(12-17)26(27,28)29/h5-9,12,15,18H,4,10-11,13-14H2,1-3H3,(H,33,34) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.16E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420116
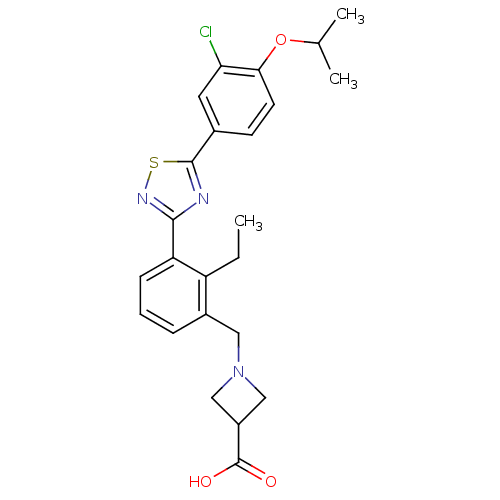
(CHEMBL2059689)Show SMILES CCc1c(CN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C24H26ClN3O3S/c1-4-18-16(11-28-12-17(13-28)24(29)30)6-5-7-19(18)22-26-23(32-27-22)15-8-9-21(20(25)10-15)31-14(2)3/h5-10,14,17H,4,11-13H2,1-3H3,(H,29,30) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 3.98 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420116

(CHEMBL2059689)Show SMILES CCc1c(CN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C24H26ClN3O3S/c1-4-18-16(11-28-12-17(13-28)24(29)30)6-5-7-19(18)22-26-23(32-27-22)15-8-9-21(20(25)10-15)31-14(2)3/h5-10,14,17H,4,11-13H2,1-3H3,(H,29,30) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50388471

(CHEMBL2057282)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C27H32ClN3O3S/c1-4-21-18(10-13-31-14-11-19(12-15-31)27(32)33)6-5-7-22(21)25-29-26(35-30-25)20-8-9-24(23(28)16-20)34-17(2)3/h5-9,16-17,19H,4,10-15H2,1-3H3,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420117
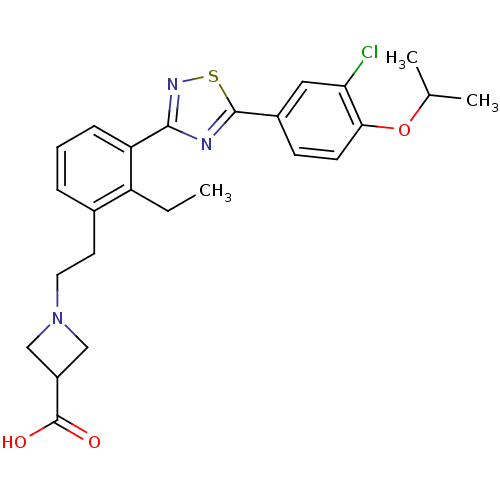
(CHEMBL2057283)Show SMILES CCc1c(CCN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C25H28ClN3O3S/c1-4-19-16(10-11-29-13-18(14-29)25(30)31)6-5-7-20(19)23-27-24(33-28-23)17-8-9-22(21(26)12-17)32-15(2)3/h5-9,12,15,18H,4,10-11,13-14H2,1-3H3,(H,30,31) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.251 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420117

(CHEMBL2057283)Show SMILES CCc1c(CCN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(OC(C)C)c(Cl)c1 Show InChI InChI=1S/C25H28ClN3O3S/c1-4-19-16(10-11-29-13-18(14-29)25(30)31)6-5-7-20(19)23-27-24(33-28-23)17-8-9-22(21(26)12-17)32-15(2)3/h5-9,12,15,18H,4,10-11,13-14H2,1-3H3,(H,30,31) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420118
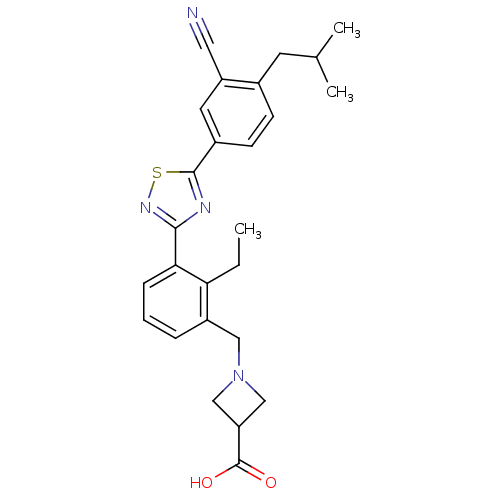
(CHEMBL2057284)Show SMILES CCc1c(CN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C26H28N4O2S/c1-4-22-19(13-30-14-21(15-30)26(31)32)6-5-7-23(22)24-28-25(33-29-24)18-9-8-17(10-16(2)3)20(11-18)12-27/h5-9,11,16,21H,4,10,13-15H2,1-3H3,(H,31,32) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 7.94 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420118

(CHEMBL2057284)Show SMILES CCc1c(CN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C26H28N4O2S/c1-4-22-19(13-30-14-21(15-30)26(31)32)6-5-7-23(22)24-28-25(33-29-24)18-9-8-17(10-16(2)3)20(11-18)12-27/h5-9,11,16,21H,4,10,13-15H2,1-3H3,(H,31,32) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50388464

(CHEMBL2057285)Show SMILES CCc1c(CCN2CCC(CC2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C29H34N4O2S/c1-4-25-20(10-13-33-14-11-21(12-15-33)29(34)35)6-5-7-26(25)27-31-28(36-32-27)23-9-8-22(16-19(2)3)24(17-23)18-30/h5-9,17,19,21H,4,10-16H2,1-3H3,(H,34,35) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | >3.16E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 1
(Homo sapiens (Human)) | BDBM50420119
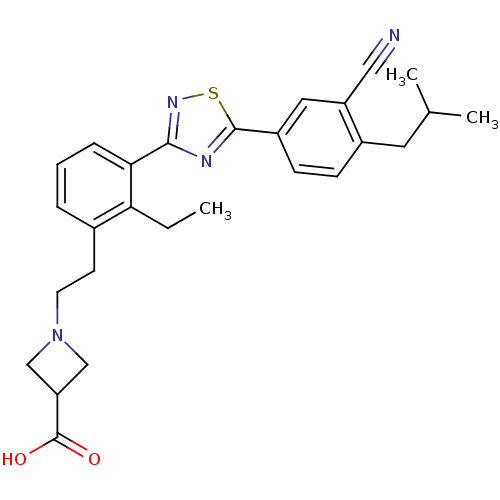
(CHEMBL2057286)Show SMILES CCc1c(CCN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C27H30N4O2S/c1-4-23-18(10-11-31-15-22(16-31)27(32)33)6-5-7-24(23)25-29-26(34-30-25)20-9-8-19(12-17(2)3)21(13-20)14-28/h5-9,13,17,22H,4,10-12,15-16H2,1-3H3,(H,32,33) | PDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 0.794 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P1 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |
Sphingosine 1-phosphate receptor 3
(Homo sapiens (Human)) | BDBM50420119

(CHEMBL2057286)Show SMILES CCc1c(CCN2CC(C2)C(O)=O)cccc1-c1nsc(n1)-c1ccc(CC(C)C)c(c1)C#N Show InChI InChI=1S/C27H30N4O2S/c1-4-23-18(10-11-31-15-22(16-31)27(32)33)6-5-7-24(23)25-29-26(34-30-25)20-9-8-19(12-17(2)3)21(13-20)14-28/h5-9,13,17,22H,4,10-12,15-16H2,1-3H3,(H,32,33) | PDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | n/a | 2.51E+3 | n/a | n/a | n/a | n/a |
GlaxoSmithKline
Curated by ChEMBL
| Assay Description
Agonist activity against S1P3 receptor by cell based FRET assay |
J Med Chem 55: 4286-96 (2012)
Article DOI: 10.1021/jm2016107
BindingDB Entry DOI: 10.7270/Q2F190SQ |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data