

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tyrosinase (Homo sapiens (Human)) | BDBM50205807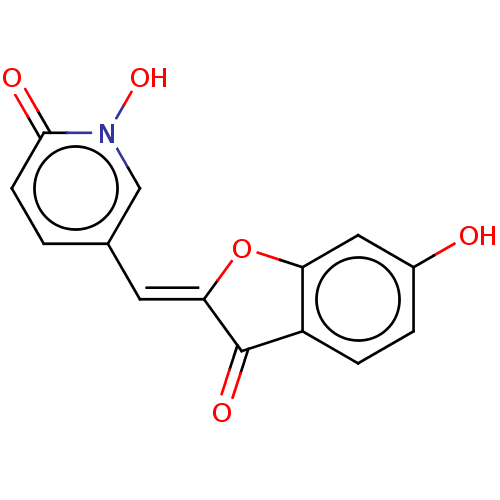 (CHEMBL3978212) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells using L-DOPA as substrate by double-reciprocal plo... | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205807 (CHEMBL3978212) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 350 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells using L-DOPA as substrate by double-reciprocal plo... | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205815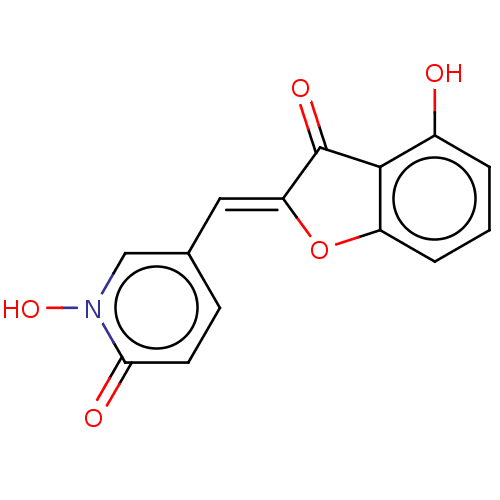 (CHEMBL3907670) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells using L-DOPA as substrate by double-reciprocal plo... | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205815 (CHEMBL3907670) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.02E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells using L-DOPA as substrate by double-reciprocal plo... | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205806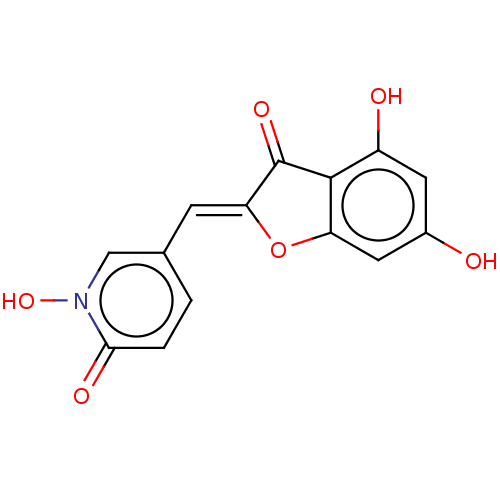 (CHEMBL3969839) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells using L-DOPA as substrate by double-reciprocal plo... | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205806 (CHEMBL3969839) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive inhibition of recombinant human tyrosinase expressed in baculovirus infected Sf9 cells using L-DOPA as substrate by double-reciprocal plo... | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50205814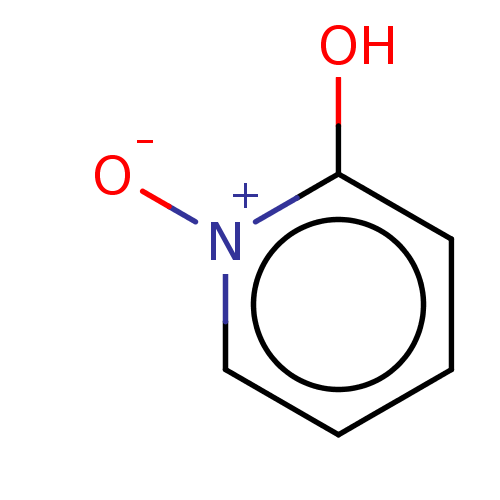 (CHEMBL3898657) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive inhibition of Agaricus bisporus tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50205814 (CHEMBL3898657) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Competitive inhibition of Agaricus bisporus tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205814 (CHEMBL3898657) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50205814 (CHEMBL3898657) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | Article PubMed | 1.28E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50044784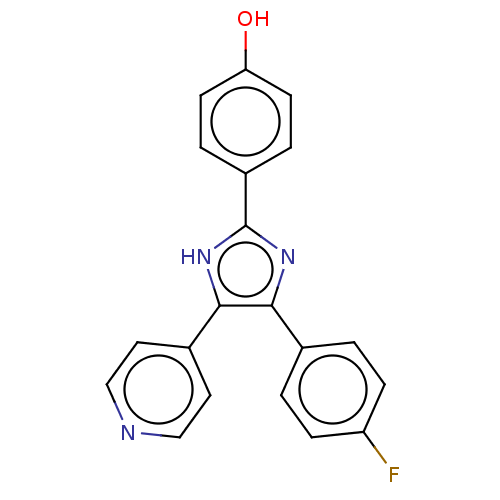 (CHEBI:79090 | SB-202190 | US10865384, Compound SB2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50044784 (CHEBI:79090 | SB-202190 | US10865384, Compound SB2...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50045333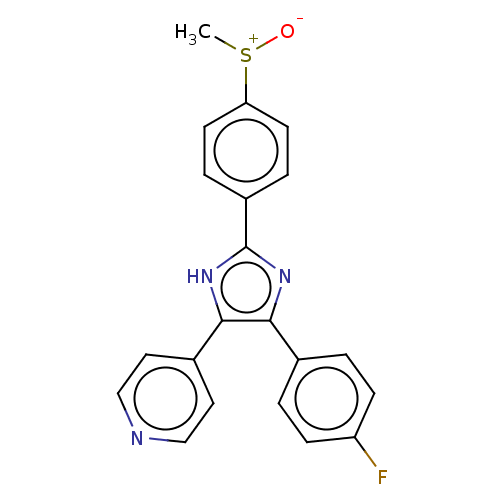 (CHEBI:90705 | SB-203580) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50045333 (CHEBI:90705 | SB-203580) | UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 34 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330795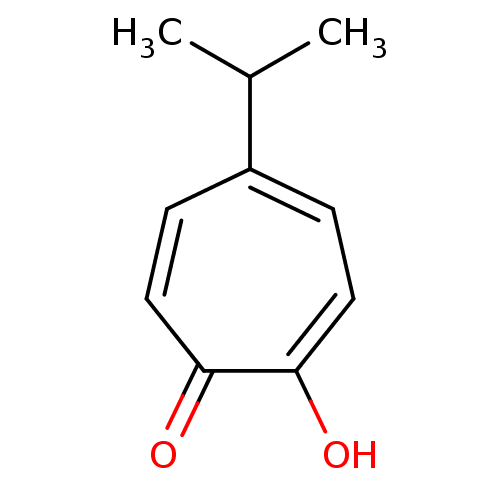 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50203985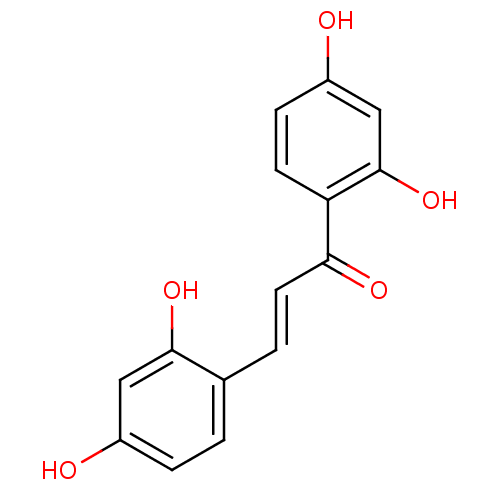 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330795 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50203985 ((E)-1-(2,4-dihydroxyphenyl)-3-(2,4-dihydroxyphenyl...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL KEGG PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50251013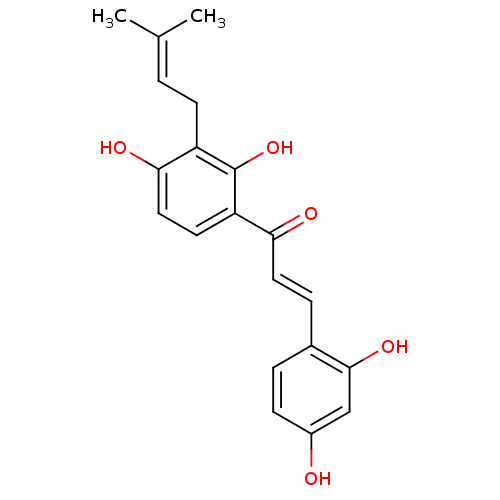 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50251013 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 80 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50230001 (4-(4-(4-fluorophenyl)-2-(4-nitrophenyl)-1H-imidazo...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50230001 (4-(4-(4-fluorophenyl)-2-(4-nitrophenyl)-1H-imidazo...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 89 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16F10 cells assessed as reduction in melanin synthesis after 2 hrs by spectrophotometric analysis | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330794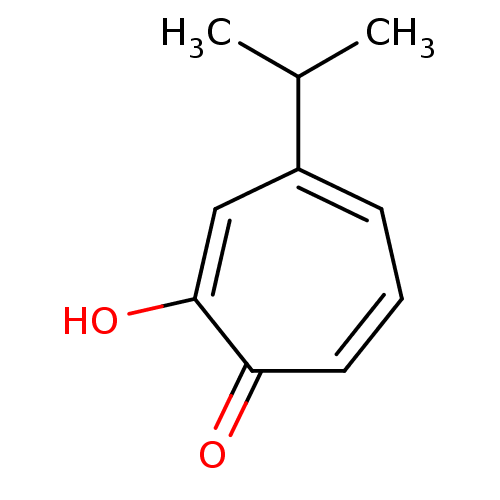 (2-Hydroxy-4-isopropyl-cyclohepta-2,4,6-trienone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50330794 (2-Hydroxy-4-isopropyl-cyclohepta-2,4,6-trienone | ...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50529589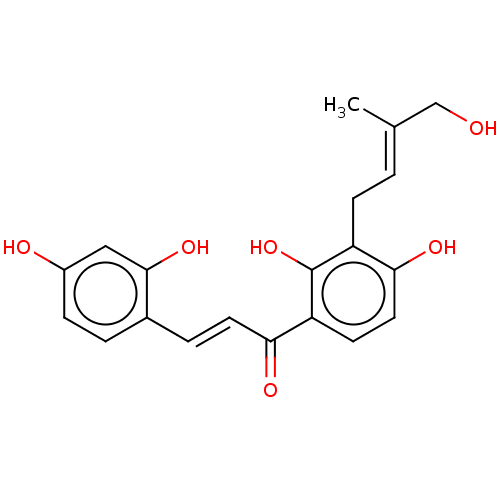 (CHEMBL4239110) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50529589 (CHEMBL4239110) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 170 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50193667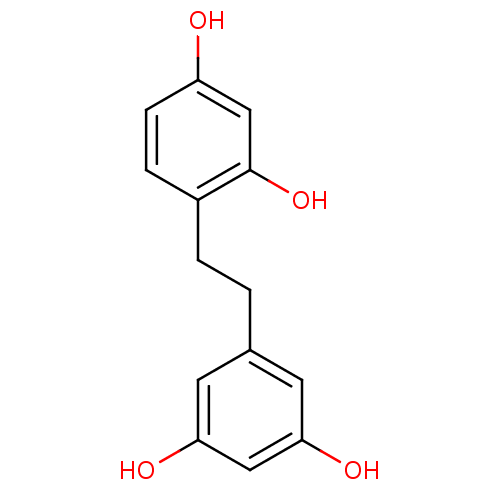 (2,4,3',5'-tetrahydroxybibenzyl | CHEMBL221291) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50193667 (2,4,3',5'-tetrahydroxybibenzyl | CHEMBL221291) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 300 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50529600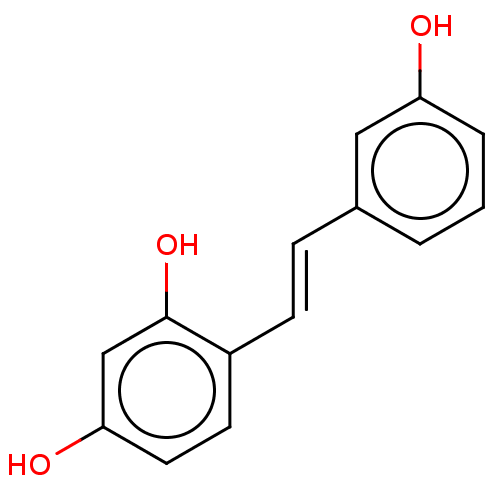 (CHEMBL4445400) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50529600 (CHEMBL4445400) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 800 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50251005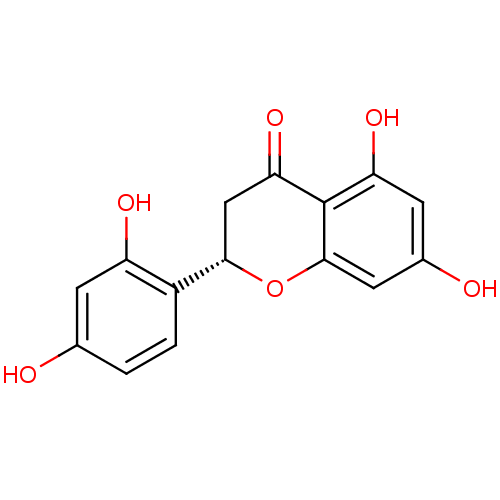 ((2S)-5,7,2',4'-tetrahydroxyflavanone | (S)-2-(2,4-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50251005 ((2S)-5,7,2',4'-tetrahydroxyflavanone | (S)-2-(2,4-...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 980 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50330795 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50330795 (2-hydroxy-5-isopropyl-2,4,6-cycloheptatrien-1-one ...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.15E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50205807 (CHEMBL3978212) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Polyphenol oxidase 2 (Agaricus bisporus (Common mushroom)) | BDBM50205807 (CHEMBL3978212) | UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of mushroom tyrosinase | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50108046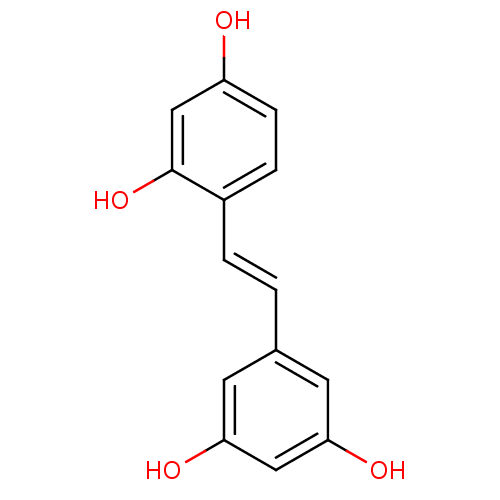 ((oxyresveratrol)4-[(E)-2-(3,5-dihydroxyphenyl)viny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50108046 ((oxyresveratrol)4-[(E)-2-(3,5-dihydroxyphenyl)viny...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 1.70E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50174577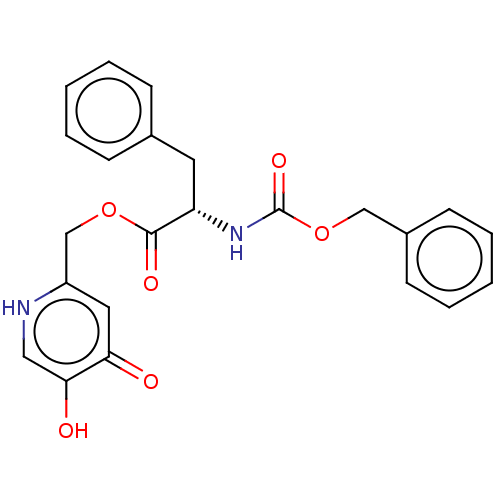 (CHEMBL3810334) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) assessed as reduction in monophenolase activity | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50174577 (CHEMBL3810334) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.95E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) assessed as reduction in monophenolase activity | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50174577 (CHEMBL3810334) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) assessed as reduction in diphenolase activity | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50174577 (CHEMBL3810334) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.79E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) assessed as reduction in diphenolase activity | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM4374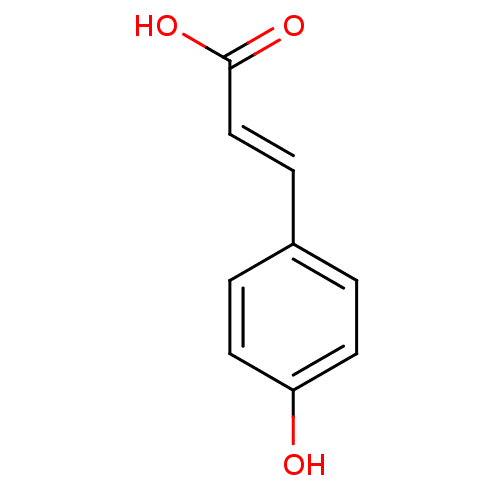 ((2E)-3-(4-hydroxyphenyl)prop-2-enoic acid | (2E)-3...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human tyrosinase after 120 mins | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM4374 ((2E)-3-(4-hydroxyphenyl)prop-2-enoic acid | (2E)-3...) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 3.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of human tyrosinase after 120 mins | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50251013 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin synthesis after 1 hr | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50251013 (2,4,2',4'-tetrahydroxy-3'-prenylchalcone | CHEMBL4...) | UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin synthesis after 1 hr | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50529589 (CHEMBL4239110) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin synthesis after 1 hr | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Mus musculus (Mouse)) | BDBM50529589 (CHEMBL4239110) | UniProtKB/SwissProt GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase in mouse B16 cells assessed as reduction in melanin synthesis after 1 hr | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50529598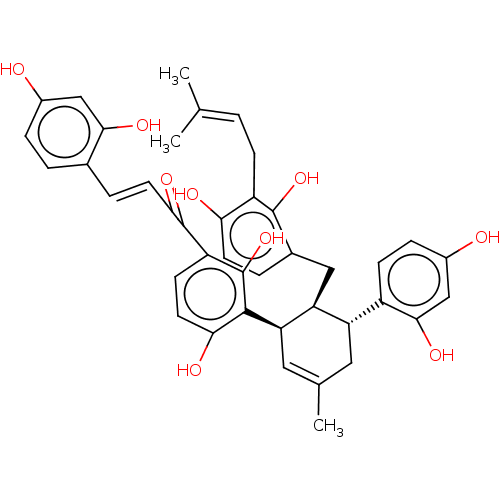 (CHEMBL4436373) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Tyrosinase (Homo sapiens (Human)) | BDBM50529598 (CHEMBL4436373) | KEGG UniProtKB/SwissProt DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | n/a | n/a | 4.62E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Bonn Curated by ChEMBL | Assay Description Inhibition of tyrosinase (unknown origin) | J Med Chem 61: 7395-7418 (2018) Article DOI: 10.1021/acs.jmedchem.7b00967 BindingDB Entry DOI: 10.7270/Q2CN77C2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 132 total ) | Next | Last >> |