 Found 145 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 6411
Found 145 hits Enz. Inhib. hit(s) with all data for assayid = 1 entry = 6411 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127387
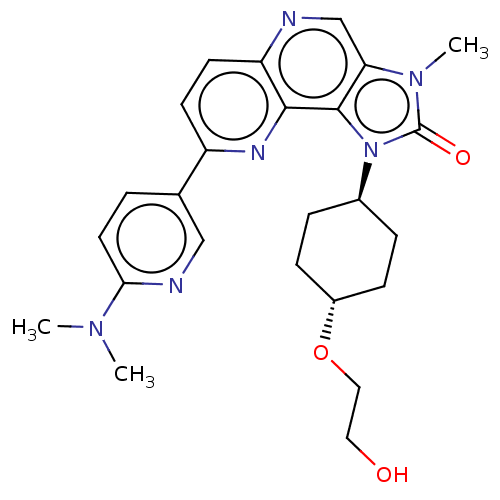
(US8791131, 149)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@@H](CC4)OCCO)c3c2n1 |r,wU:21.21,wD:24.28,(-7.32,-.2,;-5.98,.57,;-5.98,2.11,;-4.65,-.2,;-4.65,-1.74,;-3.32,-2.51,;-1.98,-1.74,;-1.98,-.2,;-3.32,.57,;-.65,-2.51,;-.65,-4.05,;.68,-4.82,;2.02,-4.05,;3.35,-4.82,;4.69,-4.05,;4.69,-2.51,;5.83,-1.48,;7.32,-1.87,;5.2,-.07,;5.97,1.26,;3.67,-.23,;2.58,.86,;1.1,.46,;.01,1.55,;.41,3.04,;1.89,3.44,;2.98,2.35,;-.68,4.13,;-2.17,3.73,;-3.26,4.82,;-4.75,4.42,;3.35,-1.74,;2.02,-2.51,;.68,-1.74,)| Show InChI InChI=1S/C25H30N6O3/c1-29(2)22-11-4-16(14-27-22)19-9-10-20-23(28-19)24-21(15-26-20)30(3)25(33)31(24)17-5-7-18(8-6-17)34-13-12-32/h4,9-11,14-15,17-18,32H,5-8,12-13H2,1-3H3/t17-,18- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.102 | -57.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127481
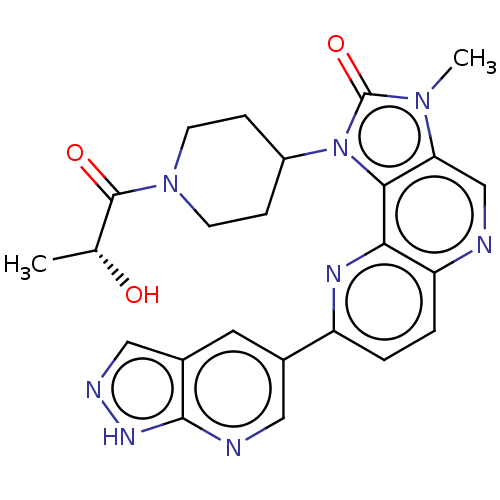
(US8791131, 258)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O |r| Show InChI InChI=1S/C24H24N8O3/c1-13(33)23(34)31-7-5-16(6-8-31)32-21-19(30(2)24(32)35)12-25-18-4-3-17(28-20(18)21)14-9-15-11-27-29-22(15)26-10-14/h3-4,9-13,16,33H,5-8H2,1-2H3,(H,26,27,29)/t13-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.110 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127483
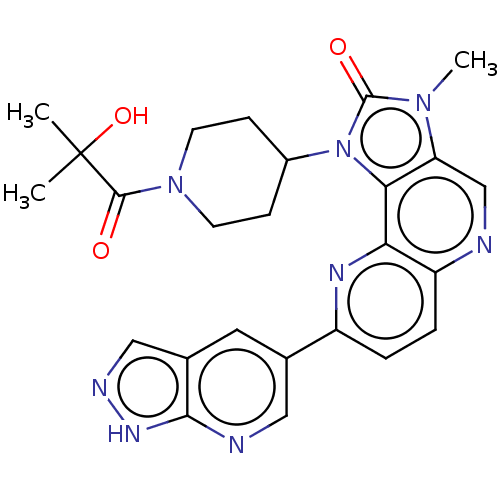
(US8791131, 261)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCN(CC2)C(=O)C(C)(C)O)c1=O)-c1cnc2[nH]ncc2c1 Show InChI InChI=1S/C25H26N8O3/c1-25(2,36)23(34)32-8-6-16(7-9-32)33-21-19(31(3)24(33)35)13-26-18-5-4-17(29-20(18)21)14-10-15-12-28-30-22(15)27-11-14/h4-5,10-13,16,36H,6-9H2,1-3H3,(H,27,28,30) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.114 | -56.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127489
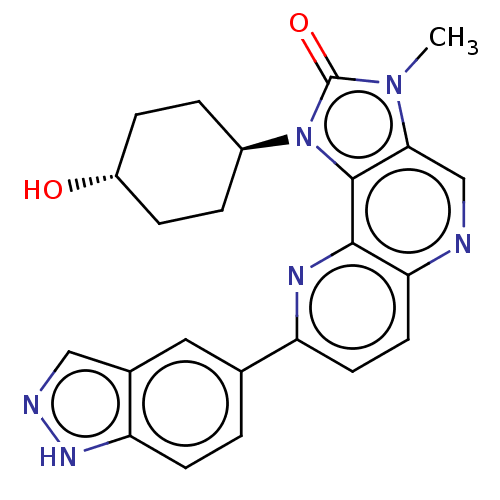
(US8791131, 267)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1ccc2[nH]ncc2c1 |r,wU:13.14,wD:16.18,(7.27,-2.02,;5.78,-1.62,;4.64,-2.65,;4.64,-4.19,;3.31,-4.96,;1.97,-4.19,;.64,-4.96,;-.69,-4.19,;-.69,-2.65,;.64,-1.88,;1.97,-2.65,;3.31,-1.88,;3.63,-.37,;2.86,.96,;1.32,.96,;.55,2.29,;1.32,3.63,;.55,4.96,;2.86,3.63,;3.63,2.29,;5.16,-.21,;5.93,1.12,;-2.23,-2.65,;-2.23,-4.19,;-3.57,-4.96,;-4.9,-4.19,;-6.37,-4.67,;-7.27,-3.42,;-6.37,-2.17,;-4.9,-2.65,;-3.57,-1.88,)| Show InChI InChI=1S/C23H22N6O2/c1-28-20-12-24-19-9-8-17(13-2-7-18-14(10-13)11-25-27-18)26-21(19)22(20)29(23(28)31)15-3-5-16(30)6-4-15/h2,7-12,15-16,30H,3-6H2,1H3,(H,25,27)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.145 | -56.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127482
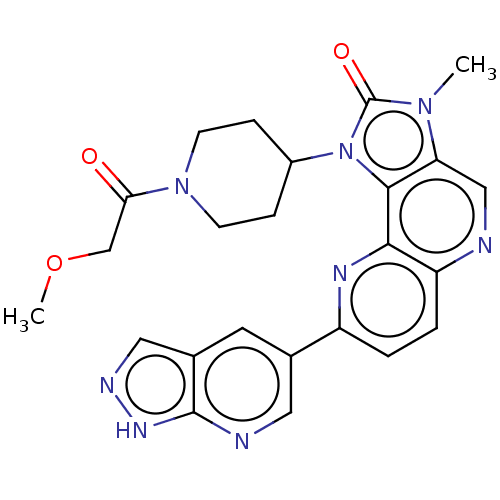
(US8791131, 260)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C24H24N8O3/c1-30-19-12-25-18-4-3-17(14-9-15-11-27-29-23(15)26-10-14)28-21(18)22(19)32(24(30)34)16-5-7-31(8-6-16)20(33)13-35-2/h3-4,9-12,16H,5-8,13H2,1-2H3,(H,26,27,29) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.167 | -55.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428109
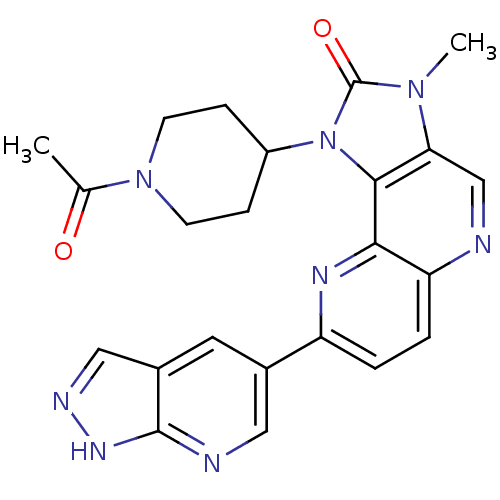
(CHEMBL2331668 | US8791131, 259)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-7-5-16(6-8-30)31-21-19(29(2)23(31)33)12-24-18-4-3-17(27-20(18)21)14-9-15-11-26-28-22(15)25-10-14/h3-4,9-12,16H,5-8H2,1-2H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.191 | -55.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127468
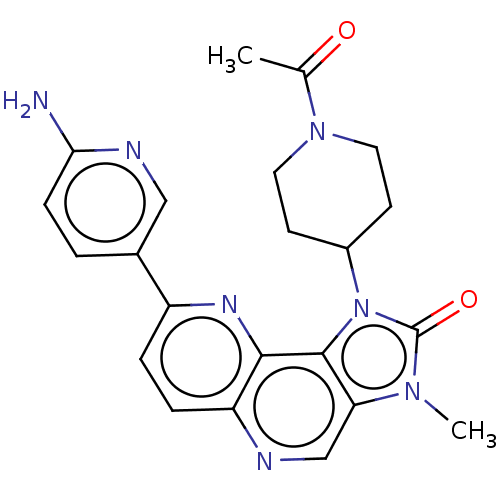
(US8791131, 242)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(N)nc2)n(C)c1=O Show InChI InChI=1S/C22H23N7O2/c1-13(30)28-9-7-15(8-10-28)29-21-18(27(2)22(29)31)12-24-17-5-4-16(26-20(17)21)14-3-6-19(23)25-11-14/h3-6,11-12,15H,7-10H2,1-2H3,(H2,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.252 | -54.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127435
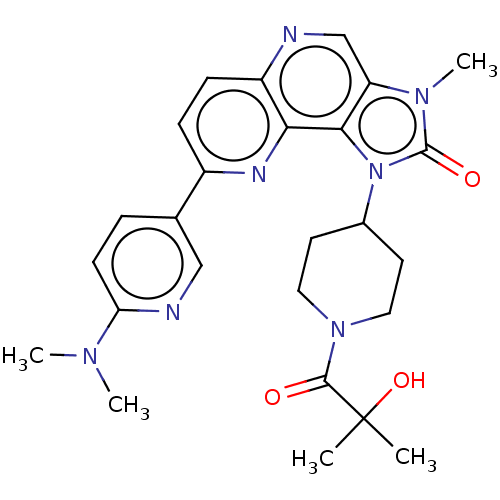
(US8791131, 209)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)C(C)(C)O)c3c2n1 Show InChI InChI=1S/C26H31N7O3/c1-26(2,36)24(34)32-12-10-17(11-13-32)33-23-20(31(5)25(33)35)15-27-19-8-7-18(29-22(19)23)16-6-9-21(28-14-16)30(3)4/h6-9,14-15,17,36H,10-13H2,1-5H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.264 | -54.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127455
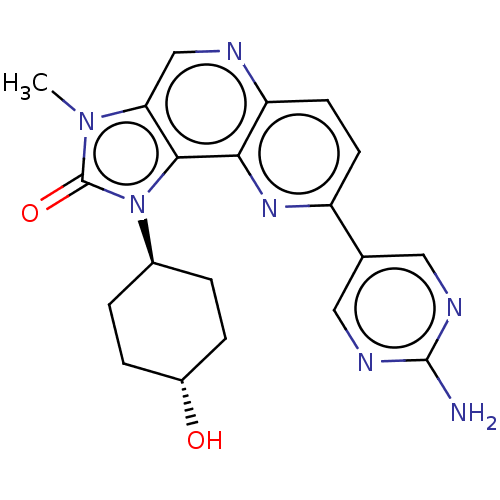
(US8791131, 229)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cnc(N)nc1 |r,wU:13.14,wD:16.18,(6.65,-1.53,;5.16,-1.13,;4.02,-2.16,;4.02,-3.7,;2.69,-4.47,;1.35,-3.7,;.02,-4.47,;-1.32,-3.7,;-1.32,-2.16,;.02,-1.39,;1.35,-2.16,;2.69,-1.39,;3.01,.12,;1.92,1.2,;.43,.81,;-.66,1.89,;-.26,3.38,;-1.35,4.47,;1.23,3.78,;2.31,2.69,;4.54,.28,;5.31,1.61,;-2.65,-1.39,;-2.65,.15,;-3.98,.92,;-5.32,.15,;-6.65,.92,;-5.32,-1.39,;-3.98,-2.16,)| Show InChI InChI=1S/C20H21N7O2/c1-26-16-10-22-15-7-6-14(11-8-23-19(21)24-9-11)25-17(15)18(16)27(20(26)29)12-2-4-13(28)5-3-12/h6-10,12-13,28H,2-5H2,1H3,(H2,21,23,24)/t12-,13- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.273 | -54.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127431
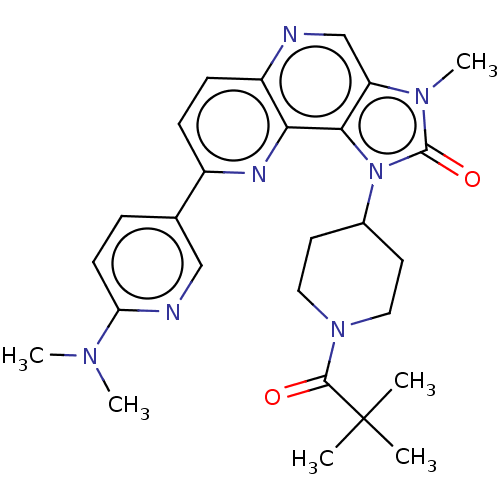
(US8791131, 205)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)C(C)(C)C)c3c2n1 Show InChI InChI=1S/C27H33N7O2/c1-27(2,3)25(35)33-13-11-18(12-14-33)34-24-21(32(6)26(34)36)16-28-20-9-8-19(30-23(20)24)17-7-10-22(29-15-17)31(4)5/h7-10,15-16,18H,11-14H2,1-6H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.281 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127485
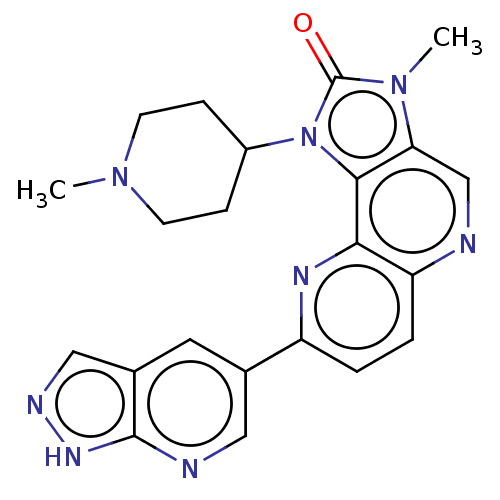
(US8791131, 263)Show SMILES CN1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3[nH]ncc3c2)n(C)c1=O Show InChI InChI=1S/C22H22N8O/c1-28-7-5-15(6-8-28)30-20-18(29(2)22(30)31)12-23-17-4-3-16(26-19(17)20)13-9-14-11-25-27-21(14)24-10-13/h3-4,9-12,15H,5-8H2,1-2H3,(H,24,25,27) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.282 | -54.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127430
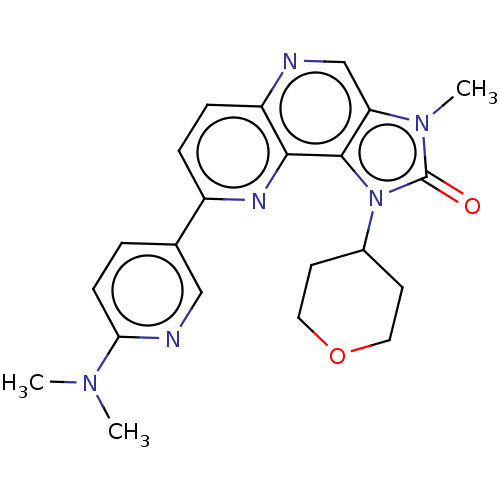
(US8791131, 204)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2n1 Show InChI InChI=1S/C22H24N6O2/c1-26(2)19-7-4-14(12-24-19)16-5-6-17-20(25-16)21-18(13-23-17)27(3)22(29)28(21)15-8-10-30-11-9-15/h4-7,12-13,15H,8-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.298 | -54.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127411
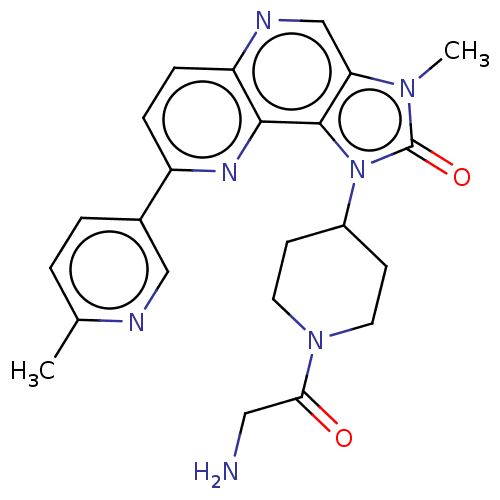
(US8791131, 180)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CN)c3c2n1 Show InChI InChI=1S/C23H25N7O2/c1-14-3-4-15(12-25-14)17-5-6-18-21(27-17)22-19(13-26-18)28(2)23(32)30(22)16-7-9-29(10-8-16)20(31)11-24/h3-6,12-13,16H,7-11,24H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.320 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127484
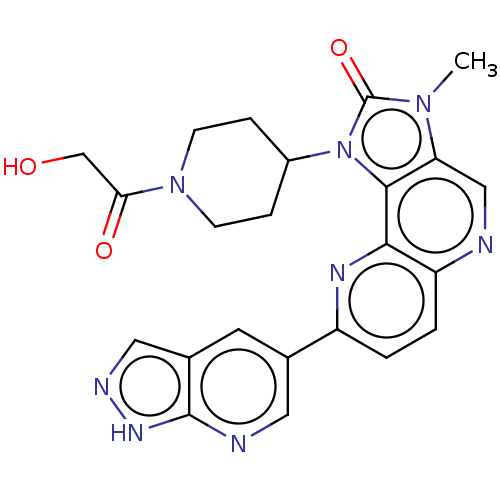
(US8791131, 262)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCN(CC2)C(=O)CO)c1=O)-c1cnc2[nH]ncc2c1 Show InChI InChI=1S/C23H22N8O3/c1-29-18-11-24-17-3-2-16(13-8-14-10-26-28-22(14)25-9-13)27-20(17)21(18)31(23(29)34)15-4-6-30(7-5-15)19(33)12-32/h2-3,8-11,15,32H,4-7,12H2,1H3,(H,25,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.320 | -54.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127377
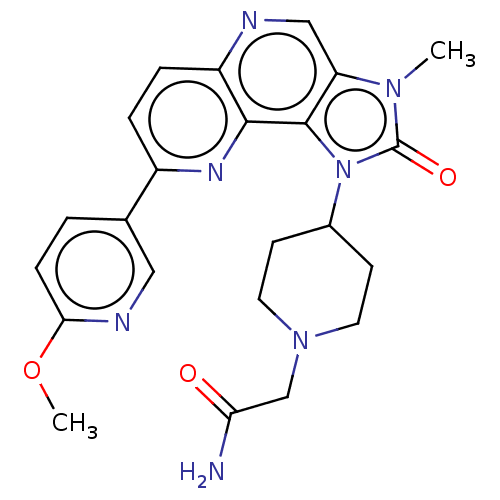
(US8791131, 139)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC(N)=O)CC4)c3c2n1 Show InChI InChI=1S/C23H25N7O3/c1-28-18-12-25-17-5-4-16(14-3-6-20(33-2)26-11-14)27-21(17)22(18)30(23(28)32)15-7-9-29(10-8-15)13-19(24)31/h3-6,11-12,15H,7-10,13H2,1-2H3,(H2,24,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.339 | -54.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127490
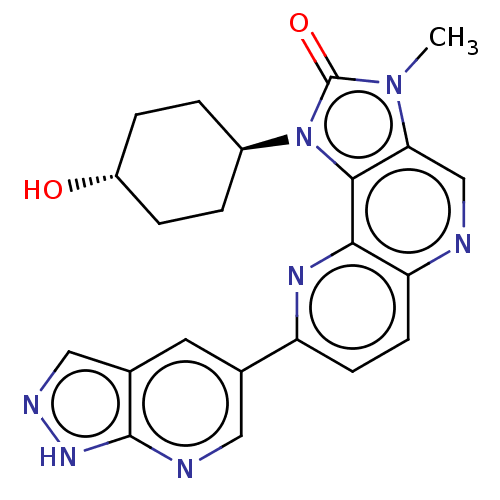
(US8791131, 268)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@H](O)CC2)c1=O)-c1cnc2[nH]ncc2c1 |r,wU:13.14,wD:16.18,(7.27,-2.02,;5.78,-1.62,;4.64,-2.65,;4.64,-4.19,;3.31,-4.96,;1.97,-4.19,;.64,-4.96,;-.69,-4.19,;-.69,-2.65,;.64,-1.88,;1.97,-2.65,;3.31,-1.88,;3.63,-.37,;2.86,.96,;1.32,.96,;.55,2.29,;1.32,3.63,;.55,4.96,;2.86,3.63,;3.63,2.29,;5.16,-.21,;5.93,1.12,;-2.23,-2.65,;-2.23,-4.19,;-3.57,-4.96,;-4.9,-4.19,;-6.37,-4.67,;-7.27,-3.42,;-6.37,-2.17,;-4.9,-2.65,;-3.57,-1.88,)| Show InChI InChI=1S/C22H21N7O2/c1-28-18-11-23-17-7-6-16(12-8-13-10-25-27-21(13)24-9-12)26-19(17)20(18)29(22(28)31)14-2-4-15(30)5-3-14/h6-11,14-15,30H,2-5H2,1H3,(H,24,25,27)/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.366 | -53.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127421
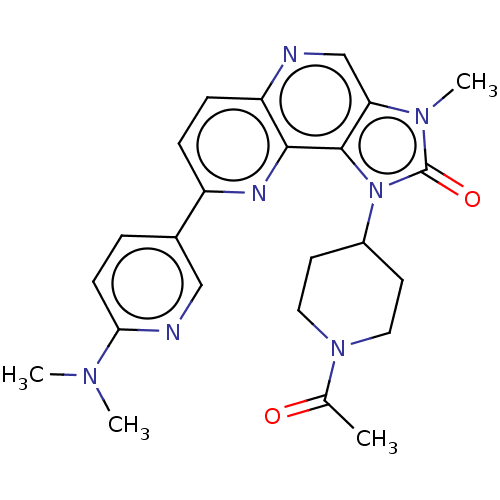
(US8791131, 195)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(C)=O)c3c2n1 Show InChI InChI=1S/C24H27N7O2/c1-15(32)30-11-9-17(10-12-30)31-23-20(29(4)24(31)33)14-25-19-7-6-18(27-22(19)23)16-5-8-21(26-13-16)28(2)3/h5-8,13-14,17H,9-12H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.391 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127440
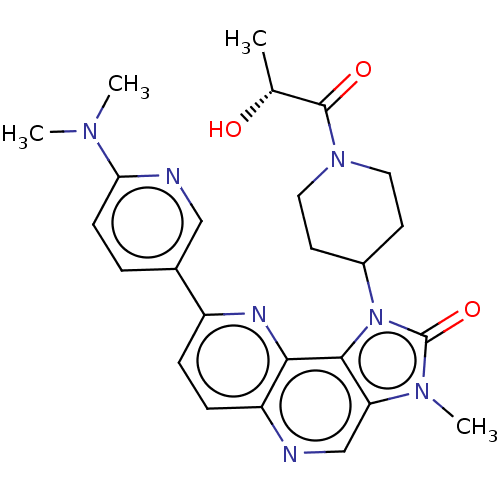
(US8791131, 214)Show SMILES C[C@@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(nc2)N(C)C)n(C)c1=O |r| Show InChI InChI=1S/C25H29N7O3/c1-15(33)24(34)31-11-9-17(10-12-31)32-23-20(30(4)25(32)35)14-26-19-7-6-18(28-22(19)23)16-5-8-21(27-13-16)29(2)3/h5-8,13-15,17,33H,9-12H2,1-4H3/t15-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.397 | -53.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127464
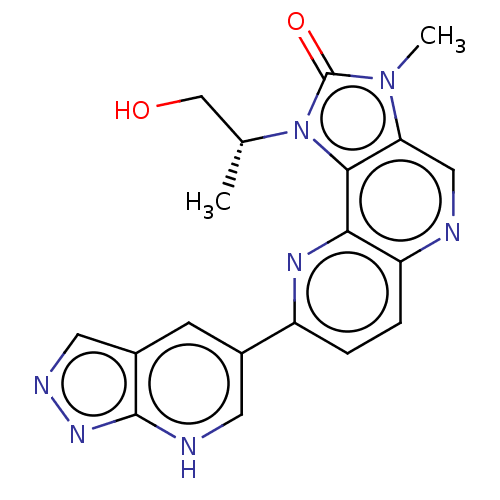
(US8791131, 238)Show SMILES C[C@H](CO)n1c2c(cnc3ccc(nc23)-c2c[nH]c3nncc3c2)n(C)c1=O |r| Show InChI InChI=1S/C19H18N7O2/c1-10(9-27)26-17-15(25(2)19(26)28)8-20-14-4-3-13(23-16(14)17)11-5-12-7-22-24-18(12)21-6-11/h3-8,10,21,24,27H,9H2,1-2H3/t10-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.438 | -53.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428115
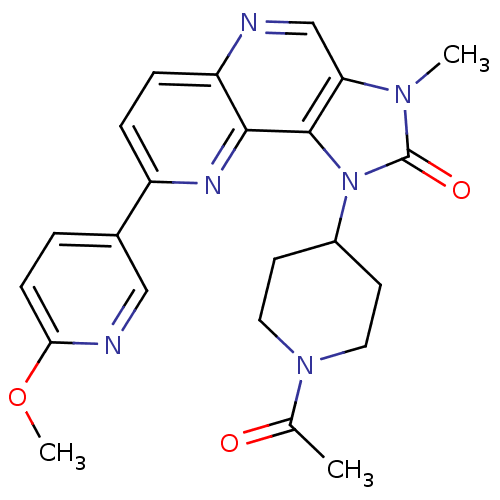
(CHEMBL2331661 | US8791131, 136)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(C)=O)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14(30)28-10-8-16(9-11-28)29-22-19(27(2)23(29)31)13-24-18-6-5-17(26-21(18)22)15-4-7-20(32-3)25-12-15/h4-7,12-13,16H,8-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.478 | -53.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428118
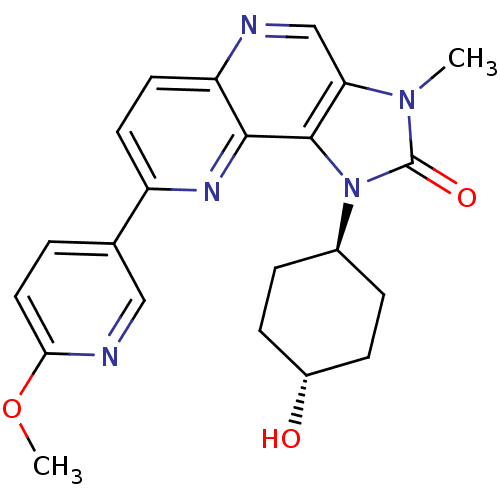
(CHEMBL2331659 | US8791131, 134)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@H](O)CC4)c3c2n1 |r,wU:20.20,wD:23.24,(32.03,-7.87,;33.36,-7.1,;34.69,-7.87,;34.69,-9.41,;36.03,-10.18,;37.36,-9.41,;37.36,-7.86,;36.02,-7.1,;38.7,-10.18,;38.69,-11.72,;40.02,-12.49,;41.35,-11.71,;42.69,-12.48,;44.03,-11.7,;44.01,-10.15,;45.14,-9.12,;46.65,-9.43,;44.51,-7.72,;45.28,-6.39,;42.99,-7.89,;42.18,-6.59,;40.64,-6.65,;39.83,-5.35,;40.55,-3.98,;39.73,-2.68,;42.09,-3.93,;42.9,-5.23,;42.68,-9.39,;41.35,-10.17,;40.02,-9.41,)| Show InChI InChI=1S/C22H23N5O3/c1-26-18-12-23-17-9-8-16(13-3-10-19(30-2)24-11-13)25-20(17)21(18)27(22(26)29)14-4-6-15(28)7-5-14/h3,8-12,14-15,28H,4-7H2,1-2H3/t14-,15- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127419
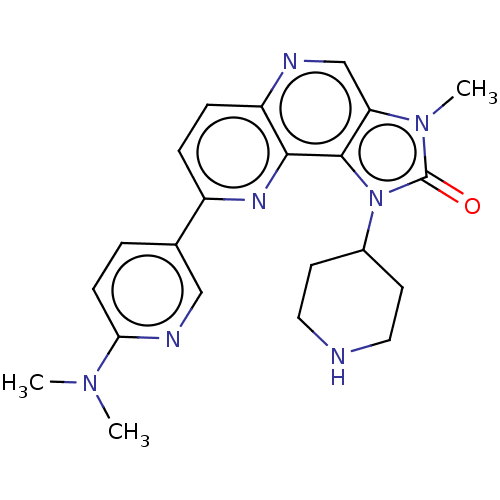
(US8791131, 191)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCNCC4)c3c2n1 Show InChI InChI=1S/C22H25N7O/c1-27(2)19-7-4-14(12-25-19)16-5-6-17-20(26-16)21-18(13-24-17)28(3)22(30)29(21)15-8-10-23-11-9-15/h4-7,12-13,15,23H,8-11H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428119
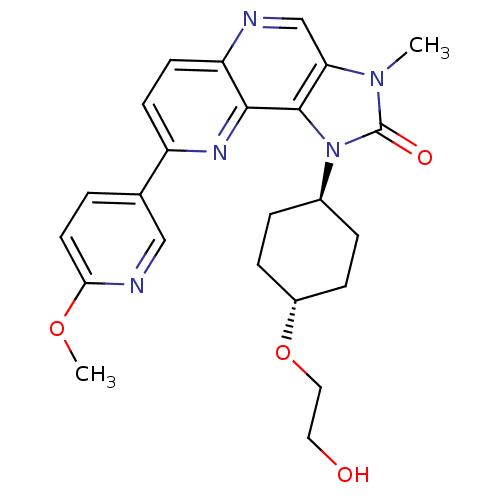
(CHEMBL2331658 | US8791131, 133)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@@H](CC4)OCCO)c3c2n1 |r,wU:20.20,wD:23.27,(17.65,-10.2,;18.98,-9.43,;20.31,-10.2,;20.31,-11.74,;21.64,-12.51,;22.98,-11.74,;22.98,-10.19,;21.64,-9.43,;24.32,-12.51,;24.31,-14.05,;25.64,-14.81,;26.97,-14.04,;28.31,-14.81,;29.65,-14.03,;29.63,-12.48,;30.76,-11.45,;32.27,-11.76,;30.13,-10.05,;30.9,-8.71,;28.61,-10.22,;27.8,-8.92,;26.26,-8.97,;25.44,-7.67,;26.17,-6.31,;27.71,-6.25,;28.52,-7.56,;25.35,-5,;26.07,-3.64,;25.25,-2.34,;25.98,-.97,;28.3,-11.72,;26.97,-12.49,;25.63,-11.73,)| Show InChI InChI=1S/C24H27N5O4/c1-28-20-14-25-19-9-8-18(15-3-10-21(32-2)26-13-15)27-22(19)23(20)29(24(28)31)16-4-6-17(7-5-16)33-12-11-30/h3,8-10,13-14,16-17,30H,4-7,11-12H2,1-2H3/t16-,17- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.5 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127442
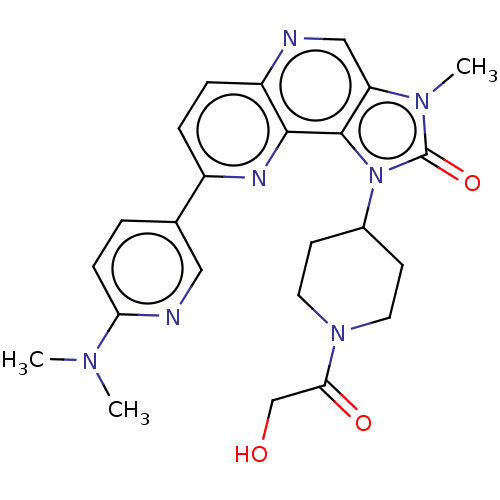
(US8791131, 216)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C24H27N7O3/c1-28(2)20-7-4-15(12-26-20)17-5-6-18-22(27-17)23-19(13-25-18)29(3)24(34)31(23)16-8-10-30(11-9-16)21(33)14-32/h4-7,12-13,16,32H,8-11,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.505 | -53.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127374
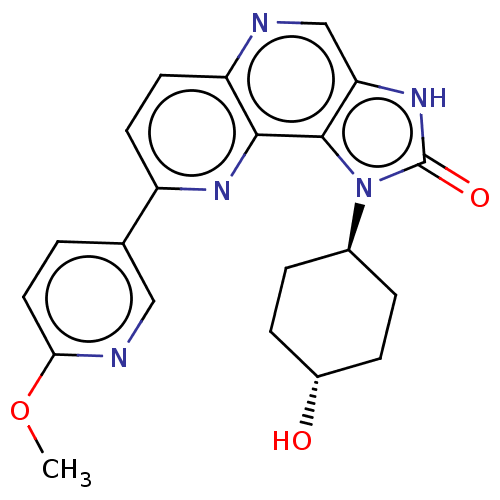
(US8791131, 132)Show SMILES COc1ccc(cn1)-c1ccc2ncc3[nH]c(=O)n([C@H]4CC[C@H](O)CC4)c3c2n1 |r,wU:19.19,wD:22.23,(-6.65,.15,;-5.31,.92,;-3.98,.15,;-3.98,-1.39,;-2.64,-2.16,;-1.31,-1.39,;-1.31,.15,;-2.64,.92,;.02,-2.16,;.02,-3.7,;1.36,-4.47,;2.69,-3.7,;4.02,-4.47,;5.36,-3.7,;5.36,-2.16,;6.5,-1.13,;5.88,.28,;6.65,1.61,;4.34,.12,;3.26,1.2,;1.77,.81,;.68,1.89,;1.08,3.38,;-.01,4.47,;2.56,3.78,;3.65,2.69,;4.02,-1.39,;2.69,-2.16,;1.36,-1.39,)| Show InChI InChI=1S/C21H21N5O3/c1-29-18-9-2-12(10-23-18)15-7-8-16-19(24-15)20-17(11-22-16)25-21(28)26(20)13-3-5-14(27)6-4-13/h2,7-11,13-14,27H,3-6H2,1H3,(H,25,28)/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.510 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
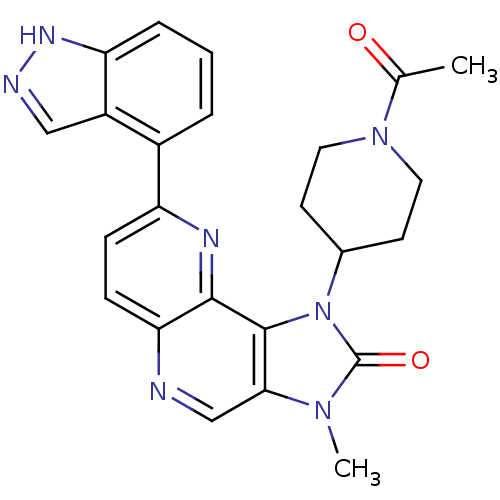
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428110
(CHEMBL2331667 | US8791131, 254)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C24H23N7O2/c1-14(32)30-10-8-15(9-11-30)31-23-21(29(2)24(31)33)13-25-20-7-6-18(27-22(20)23)16-4-3-5-19-17(16)12-26-28-19/h3-7,12-13,15H,8-11H2,1-2H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.513 | -53.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
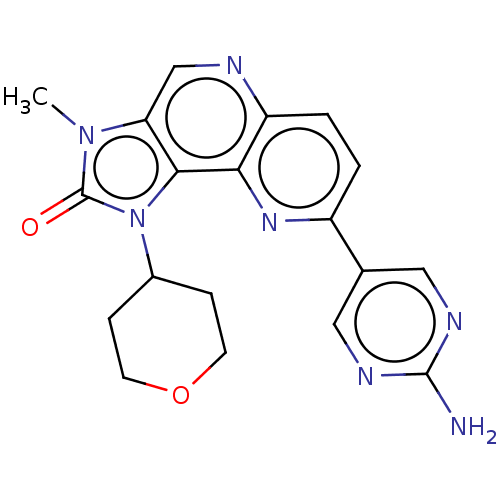
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127436
(US8791131, 210)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCOCC2)c1=O)-c1cnc(N)nc1 Show InChI InChI=1S/C19H19N7O2/c1-25-15-10-21-14-3-2-13(11-8-22-18(20)23-9-11)24-16(14)17(15)26(19(25)27)12-4-6-28-7-5-12/h2-3,8-10,12H,4-7H2,1H3,(H2,20,22,23) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.529 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
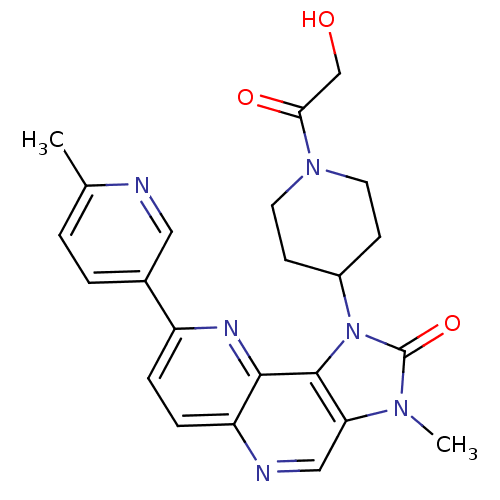
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428113
(CHEMBL2331663 | US8791131, 172)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(=O)CO)c3c2n1 Show InChI InChI=1S/C23H24N6O3/c1-14-3-4-15(11-24-14)17-5-6-18-21(26-17)22-19(12-25-18)27(2)23(32)29(22)16-7-9-28(10-8-16)20(31)13-30/h3-6,11-12,16,30H,7-10,13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.532 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127363
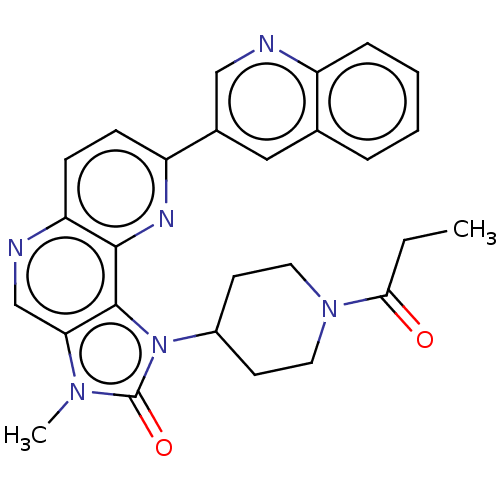
(US8791131, 121)Show SMILES CCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3ccccc3c2)n(C)c1=O Show InChI InChI=1S/C27H26N6O2/c1-3-24(34)32-12-10-19(11-13-32)33-26-23(31(2)27(33)35)16-29-22-9-8-21(30-25(22)26)18-14-17-6-4-5-7-20(17)28-15-18/h4-9,14-16,19H,3,10-13H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.539 | -52.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127381
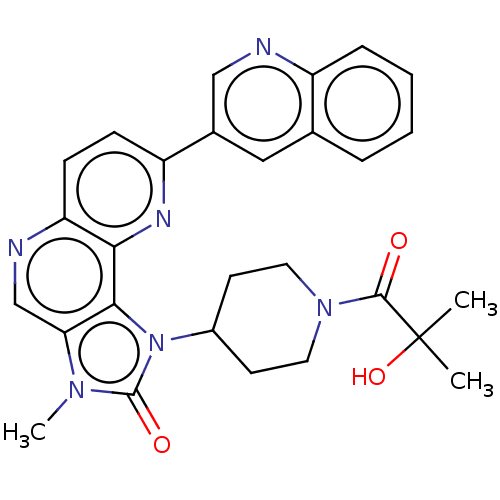
(US8791131, 143)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCN(CC2)C(=O)C(C)(C)O)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C28H28N6O3/c1-28(2,37)26(35)33-12-10-19(11-13-33)34-25-23(32(3)27(34)36)16-30-22-9-8-21(31-24(22)25)18-14-17-6-4-5-7-20(17)29-15-18/h4-9,14-16,19,37H,10-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.568 | -52.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127480
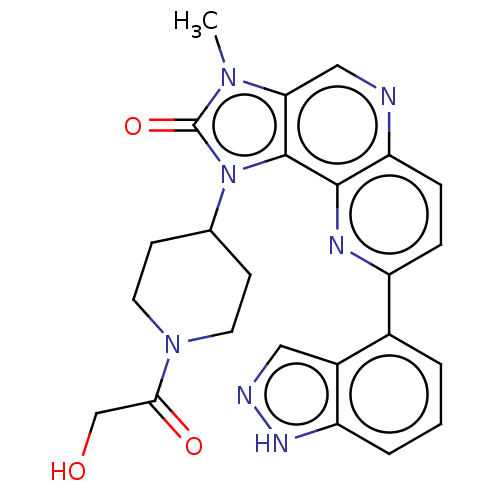
(US8791131, 256)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCN(CC2)C(=O)CO)c1=O)-c1cccc2[nH]ncc12 Show InChI InChI=1S/C24H23N7O3/c1-29-20-12-25-19-6-5-17(15-3-2-4-18-16(15)11-26-28-18)27-22(19)23(20)31(24(29)34)14-7-9-30(10-8-14)21(33)13-32/h2-6,11-12,14,32H,7-10,13H2,1H3,(H,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.575 | -52.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127373
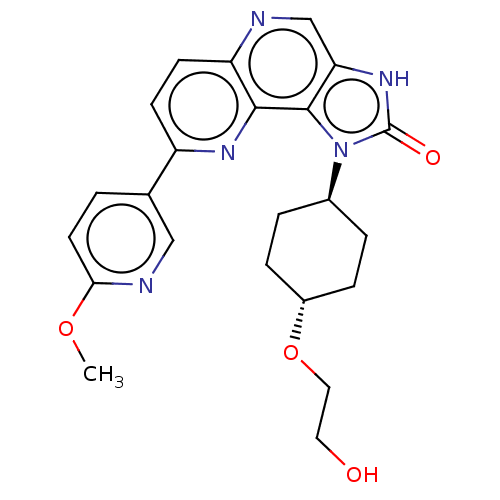
(US8791131, 131)Show SMILES COc1ccc(cn1)-c1ccc2ncc3[nH]c(=O)n([C@H]4CC[C@@H](CC4)OCCO)c3c2n1 |r,wU:19.19,wD:22.26,(-6.65,-.2,;-5.31,.57,;-3.98,-.2,;-3.98,-1.74,;-2.64,-2.51,;-1.31,-1.74,;-1.31,-.2,;-2.64,.57,;.02,-2.51,;.02,-4.05,;1.36,-4.82,;2.69,-4.05,;4.02,-4.82,;5.36,-4.05,;5.36,-2.51,;6.5,-1.48,;5.88,-.07,;6.65,1.26,;4.34,-.23,;3.26,.86,;1.77,.46,;.68,1.55,;1.08,3.04,;2.56,3.44,;3.65,2.35,;-.01,4.13,;-1.5,3.73,;-2.59,4.82,;-4.08,4.42,;4.02,-1.74,;2.69,-2.51,;1.36,-1.74,)| Show InChI InChI=1S/C23H25N5O4/c1-31-20-9-2-14(12-25-20)17-7-8-18-21(26-17)22-19(13-24-18)27-23(30)28(22)15-3-5-16(6-4-15)32-11-10-29/h2,7-9,12-13,15-16,29H,3-6,10-11H2,1H3,(H,27,30)/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.644 | -52.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127378
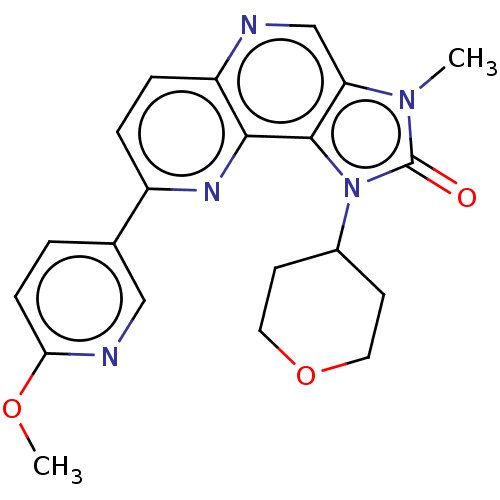
(US8791131, 140)Show SMILES COc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2n1 Show InChI InChI=1S/C21H21N5O3/c1-25-17-12-22-16-5-4-15(13-3-6-18(28-2)23-11-13)24-19(16)20(17)26(21(25)27)14-7-9-29-10-8-14/h3-6,11-12,14H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.668 | -52.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
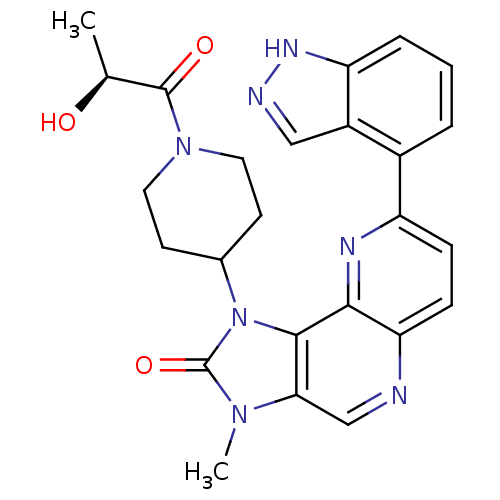
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428108
(CHEMBL2331669 | US8791131, 255)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cccc3[nH]ncc23)n(C)c1=O |r| Show InChI InChI=1S/C25H25N7O3/c1-14(33)24(34)31-10-8-15(9-11-31)32-23-21(30(2)25(32)35)13-26-20-7-6-18(28-22(20)23)16-4-3-5-19-17(16)12-27-29-19/h3-7,12-15,33H,8-11H2,1-2H3,(H,27,29)/t14-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.721 | -52.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127422
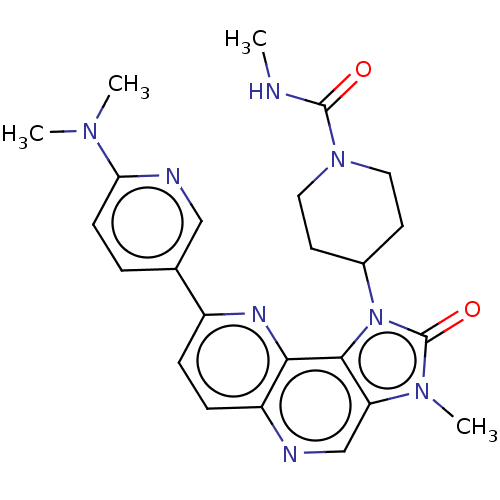
(US8791131, 196)Show SMILES CNC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(nc2)N(C)C)n(C)c1=O Show InChI InChI=1S/C24H28N8O2/c1-25-23(33)31-11-9-16(10-12-31)32-22-19(30(4)24(32)34)14-26-18-7-6-17(28-21(18)22)15-5-8-20(27-13-15)29(2)3/h5-8,13-14,16H,9-12H2,1-4H3,(H,25,33) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.760 | -52.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127432
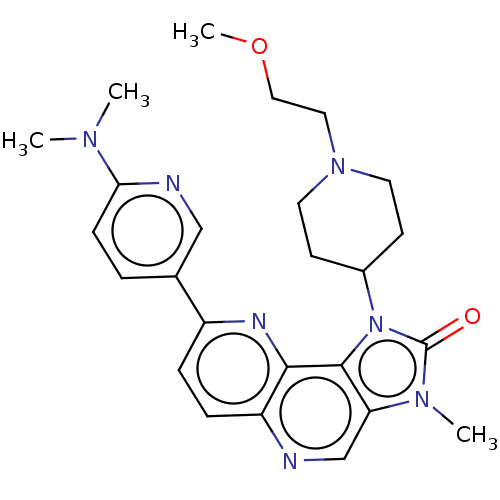
(US8791131, 206)Show SMILES COCCN1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(nc2)N(C)C)n(C)c1=O Show InChI InChI=1S/C25H31N7O2/c1-29(2)22-8-5-17(15-27-22)19-6-7-20-23(28-19)24-21(16-26-20)30(3)25(33)32(24)18-9-11-31(12-10-18)13-14-34-4/h5-8,15-16,18H,9-14H2,1-4H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.802 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127364
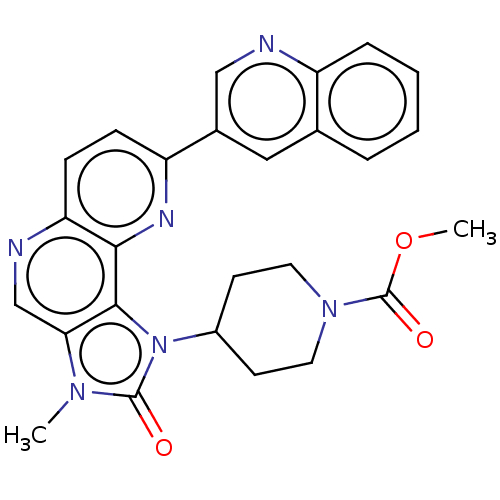
(US8791131, 122)Show SMILES COC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3ccccc3c2)n(C)c1=O Show InChI InChI=1S/C26H24N6O3/c1-30-22-15-28-21-8-7-20(17-13-16-5-3-4-6-19(16)27-14-17)29-23(21)24(22)32(25(30)33)18-9-11-31(12-10-18)26(34)35-2/h3-8,13-15,18H,9-12H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.806 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127477
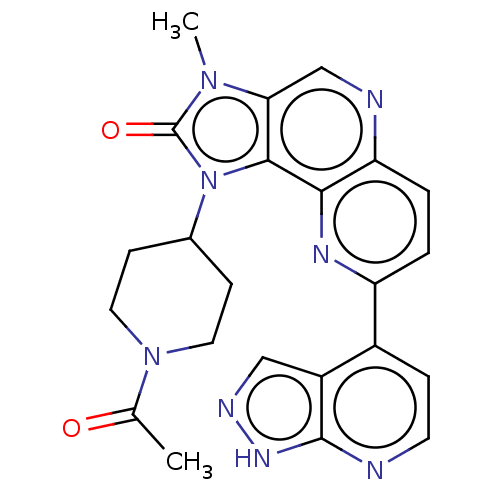
(US8791131, 251)Show SMILES CC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccnc3[nH]ncc23)n(C)c1=O Show InChI InChI=1S/C23H22N8O2/c1-13(32)30-9-6-14(7-10-30)31-21-19(29(2)23(31)33)12-25-18-4-3-17(27-20(18)21)15-5-8-24-22-16(15)11-26-28-22/h3-5,8,11-12,14H,6-7,9-10H2,1-2H3,(H,24,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.811 | -51.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127478

(US8791131, 252)Show SMILES Cn1c2cnc3ccc(nc3c2n(C2CCN(CC2)C(=O)CO)c1=O)-c1ccnc2[nH]ncc12 Show InChI InChI=1S/C23H22N8O3/c1-29-18-11-25-17-3-2-16(14-4-7-24-22-15(14)10-26-28-22)27-20(17)21(18)31(23(29)34)13-5-8-30(9-6-13)19(33)12-32/h2-4,7,10-11,13,32H,5-6,8-9,12H2,1H3,(H,24,26,28) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.832 | -51.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127408

(US8791131, 176)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC(N)=O)CC4)c3c2n1 Show InChI InChI=1S/C23H25N7O2/c1-14-3-4-15(11-25-14)17-5-6-18-21(27-17)22-19(12-26-18)28(2)23(32)30(22)16-7-9-29(10-8-16)13-20(24)31/h3-6,11-12,16H,7-10,13H2,1-2H3,(H2,24,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.882 | -51.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428111
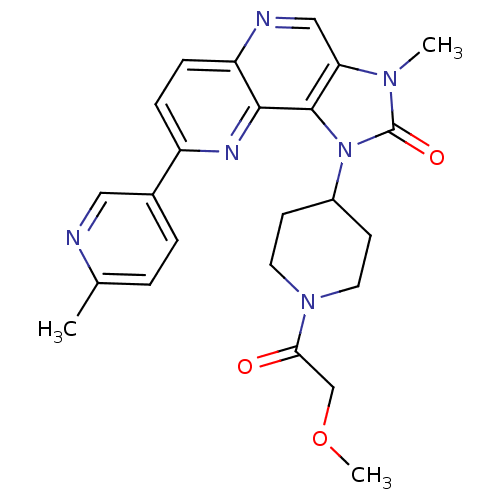
(CHEMBL2331666 | US8791131, 153)Show SMILES COCC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O Show InChI InChI=1S/C24H26N6O3/c1-15-4-5-16(12-25-15)18-6-7-19-22(27-18)23-20(13-26-19)28(2)24(32)30(23)17-8-10-29(11-9-17)21(31)14-33-3/h4-7,12-13,17H,8-11,14H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| US Patent
| 0.922 | -51.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50428107
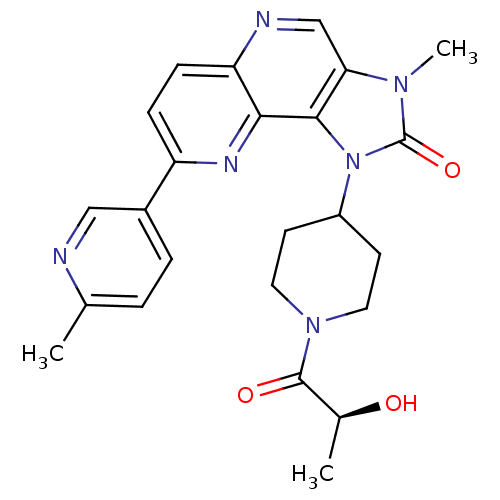
(CHEMBL2331664 | PF-04979064 | US8791131, 257)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(C)nc2)n(C)c1=O |r| Show InChI InChI=1S/C24H26N6O3/c1-14-4-5-16(12-25-14)18-6-7-19-21(27-18)22-20(13-26-19)28(3)24(33)30(22)17-8-10-29(11-9-17)23(32)15(2)31/h4-7,12-13,15,17,31H,8-11H2,1-3H3/t15-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| US Patent
| 0.958 | -51.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127404
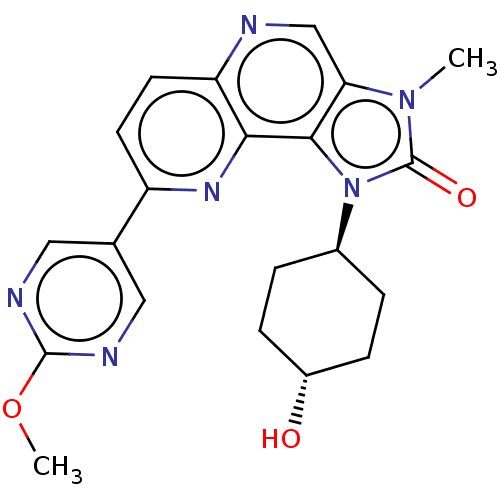
(US8791131, 169)Show SMILES COc1ncc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@H](O)CC4)c3c2n1 |r,wU:20.20,wD:23.24,(-7.32,.15,;-5.98,.92,;-4.65,.15,;-3.32,.92,;-1.98,.15,;-1.98,-1.39,;-3.32,-2.16,;-4.65,-1.39,;-.65,-2.16,;-.65,-3.7,;.68,-4.47,;2.02,-3.7,;3.35,-4.47,;4.69,-3.7,;4.69,-2.16,;5.83,-1.13,;7.32,-1.53,;5.2,.28,;5.97,1.61,;3.67,.12,;2.58,1.2,;1.1,.81,;.01,1.89,;.41,3.38,;-.68,4.47,;1.89,3.78,;2.98,2.69,;3.35,-1.39,;2.02,-2.16,;.68,-1.39,)| Show InChI InChI=1S/C21H22N6O3/c1-26-17-11-22-16-8-7-15(12-9-23-20(30-2)24-10-12)25-18(16)19(17)27(21(26)29)13-3-5-14(28)6-4-13/h7-11,13-14,28H,3-6H2,1-2H3/t13-,14- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 0.974 | -51.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127410

(US8791131, 179)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n([C@H]4CC[C@H](O)CC4)c3c2n1 |r,wU:19.19,wD:22.23,(-6.65,.92,;-5.32,.15,;-5.32,-1.39,;-3.98,-2.16,;-2.65,-1.39,;-2.65,.15,;-3.98,.92,;-1.32,-2.16,;-1.32,-3.7,;.02,-4.47,;1.35,-3.7,;2.69,-4.47,;4.02,-3.7,;4.02,-2.16,;5.16,-1.13,;6.65,-1.53,;4.54,.28,;5.31,1.61,;3.01,.12,;1.92,1.2,;.43,.81,;-.66,1.89,;-.26,3.38,;-1.35,4.47,;1.23,3.78,;2.31,2.69,;2.69,-1.39,;1.35,-2.16,;.02,-1.39,)| Show InChI InChI=1S/C22H23N5O2/c1-13-3-4-14(11-23-13)17-9-10-18-20(25-17)21-19(12-24-18)26(2)22(29)27(21)15-5-7-16(28)8-6-15/h3-4,9-12,15-16,28H,5-8H2,1-2H3/t15-,16- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.03 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127479
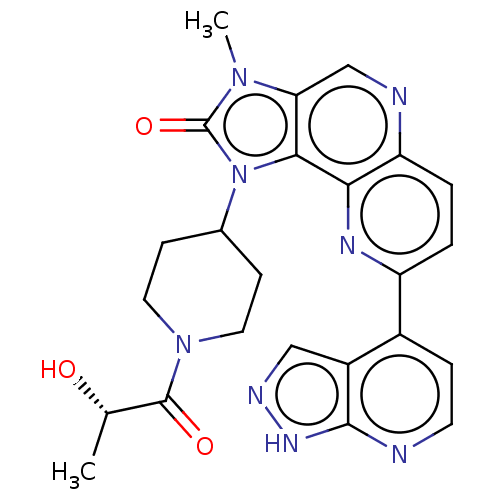
(US8791131, 253)Show SMILES C[C@H](O)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccnc3[nH]ncc23)n(C)c1=O |r| Show InChI InChI=1S/C24H24N8O3/c1-13(33)23(34)31-9-6-14(7-10-31)32-21-19(30(2)24(32)35)12-26-18-4-3-17(28-20(18)21)15-5-8-25-22-16(15)11-27-29-22/h3-5,8,11-14,33H,6-7,9-10H2,1-2H3,(H,25,27,29)/t13-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.04 | -51.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127423
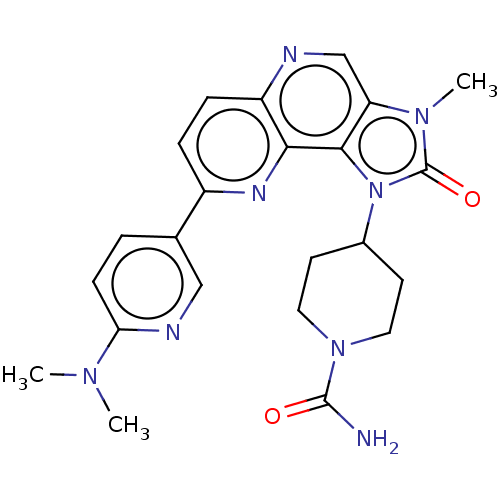
(US8791131, 197)Show SMILES CN(C)c1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCN(CC4)C(N)=O)c3c2n1 Show InChI InChI=1S/C23H26N8O2/c1-28(2)19-7-4-14(12-26-19)16-5-6-17-20(27-16)21-18(13-25-17)29(3)23(33)31(21)15-8-10-30(11-9-15)22(24)32/h4-7,12-13,15H,8-11H2,1-3H3,(H2,24,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.05 | -51.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127367
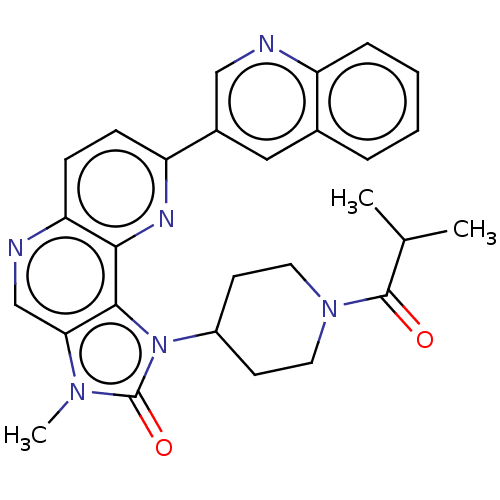
(US8791131, 125)Show SMILES CC(C)C(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2cnc3ccccc3c2)n(C)c1=O Show InChI InChI=1S/C28H28N6O2/c1-17(2)27(35)33-12-10-20(11-13-33)34-26-24(32(3)28(34)36)16-30-23-9-8-22(31-25(23)26)19-14-18-6-4-5-7-21(18)29-15-19/h4-9,14-17,20H,10-13H2,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.06 | -51.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127385

(US8791131, 147)Show SMILES Cn1c2cnc3ccc(nc3c2n([C@H]2CC[C@@H](CC2)OCCO)c1=O)-c1cnc2ccccc2c1 |r,wU:13.14,wD:16.21,(7.32,-1.87,;5.83,-1.48,;4.69,-2.51,;4.69,-4.05,;3.35,-4.82,;2.02,-4.05,;.68,-4.82,;-.65,-4.05,;-.65,-2.51,;.68,-1.74,;2.02,-2.51,;3.35,-1.74,;3.67,-.23,;2.58,.86,;1.1,.46,;.01,1.55,;.41,3.04,;1.89,3.44,;2.98,2.35,;-.68,4.13,;-2.17,3.73,;-3.26,4.82,;-4.75,4.42,;5.2,-.07,;5.97,1.26,;-1.98,-1.74,;-1.98,-.2,;-3.32,.57,;-4.65,-.2,;-5.98,.57,;-7.32,-.2,;-7.32,-1.74,;-5.98,-2.51,;-4.65,-1.74,;-3.32,-2.51,)| Show InChI InChI=1S/C27H27N5O3/c1-31-24-16-29-23-11-10-22(18-14-17-4-2-3-5-21(17)28-15-18)30-25(23)26(24)32(27(31)34)19-6-8-20(9-7-19)35-13-12-33/h2-5,10-11,14-16,19-20,33H,6-9,12-13H2,1H3/t19-,20- | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.08 | -51.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127426

(US8791131, 200)Show SMILES Cc1ccc(cn1)-c1ccc2ncc3n(C)c(=O)n(C4CCOCC4)c3c2n1 Show InChI InChI=1S/C21H21N5O2/c1-13-3-4-14(11-22-13)16-5-6-17-19(24-16)20-18(12-23-17)25(2)21(27)26(20)15-7-9-28-10-8-15/h3-6,11-12,15H,7-10H2,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.11 | -51.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM127380

(US8791131, 142)Show SMILES CNC(=O)N1CCC(CC1)n1c2c(cnc3ccc(nc23)-c2ccc(OC)nc2)n(C)c1=O Show InChI InChI=1S/C23H25N7O3/c1-24-22(31)29-10-8-15(9-11-29)30-21-18(28(2)23(30)32)13-25-17-6-5-16(27-20(17)21)14-4-7-19(33-3)26-12-14/h4-7,12-13,15H,8-11H2,1-3H3,(H,24,31) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| US Patent
| 1.15 | -51.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Pfizer Inc.
US Patent
| Assay Description
Compounds of the present invention were evaluated for potency against PI3-Kalpha using an in vitro kinase assay. PI3-Kalpha activity is measured in v... |
US Patent US8791131 (2014)
BindingDB Entry DOI: 10.7270/Q23B5XV8 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data