

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM14722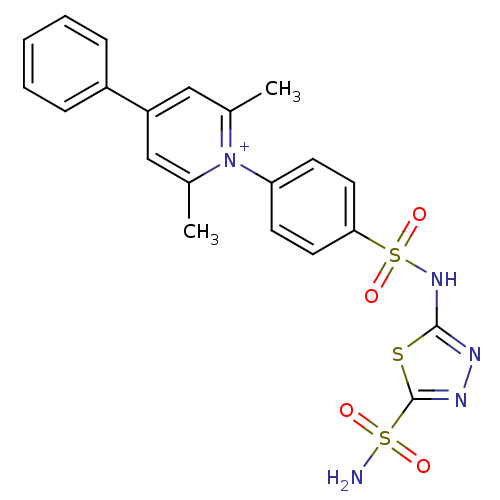 (1-N-[5-Sulfamoyl-1,3,4-thiadiazol-2-yl-(aminosulfo...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 2 | -48.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10886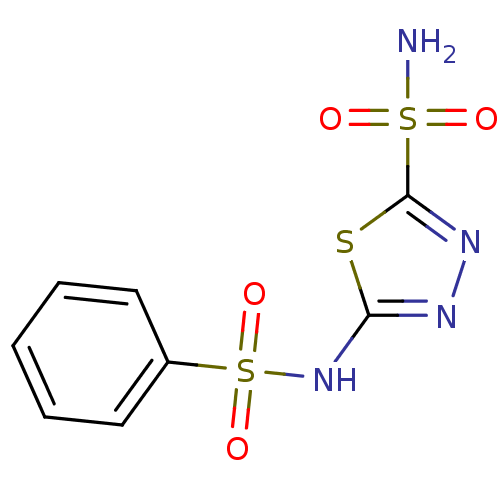 (2-N-benzene-1,3,4-thiadiazole-2,5-disulfonamide | ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 12 | -44.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM85658 (EZA4) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 13 | -44.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM13058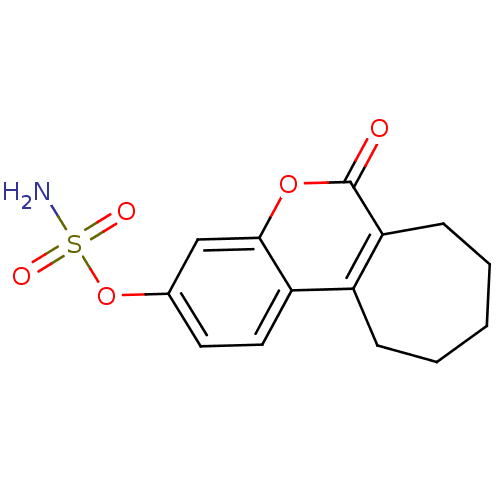 (6-oxo-6,7,8,9,10,11-hexahydrocyclohepta[c]chromen-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 24 | -43.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem Lett 18: 4282-6 (2008) Article DOI: 10.1016/j.bmcl.2008.06.105 BindingDB Entry DOI: 10.7270/Q27D2SF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM50141779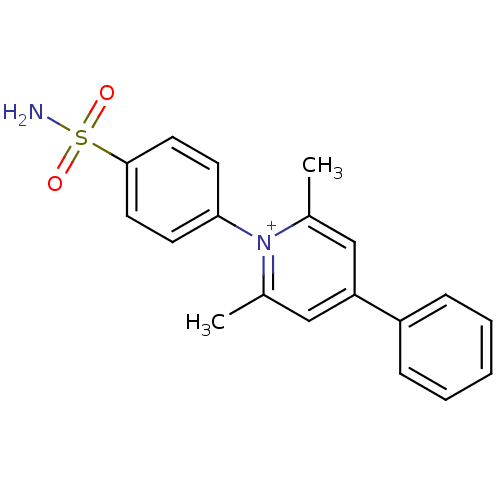 (2,6-Dimethyl-4-phenyl-1-(4-sulfamoyl-phenyl)-pyrid...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | -42.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM85563 (2-[4-(4-Bromophenylsulfonyl)-Phenyl}-thiazole-4-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 50 | -41.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Università degli Studi | Assay Description Inhibition assay using human Carbonic anhydrase I and II and bovine carbonic anhydrase IV. | J Enzym Inhib 16: 351-8 (2001) BindingDB Entry DOI: 10.7270/Q2K9363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM85566 (2-[4-(4-Bromophenylsulfonyl)-Phenyl}-thiazole-4-yl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 54 | -41.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Università degli Studi | Assay Description Inhibition assay using human Carbonic anhydrase I and II and bovine carbonic anhydrase IV. | J Enzym Inhib 16: 351-8 (2001) BindingDB Entry DOI: 10.7270/Q2K9363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM85562 (2-[4-(4-Chlorophenylsulfonyl)-Phenyl}-thiazole-4-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 65 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Università degli Studi | Assay Description Inhibition assay using human Carbonic anhydrase I and II and bovine carbonic anhydrase IV. | J Enzym Inhib 16: 351-8 (2001) BindingDB Entry DOI: 10.7270/Q2K9363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM85565 (2-[4-(4-Chlorophenylsulfonyl)-Phenyl}-thiazole-4-y...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 66 | -41.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Università degli Studi | Assay Description Inhibition assay using human Carbonic anhydrase I and II and bovine carbonic anhydrase IV. | J Enzym Inhib 16: 351-8 (2001) BindingDB Entry DOI: 10.7270/Q2K9363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 70 | -40.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Università degli Studi | Assay Description Inhibition assay using human Carbonic anhydrase I and II and bovine carbonic anhydrase IV. | J Enzym Inhib 16: 351-8 (2001) BindingDB Entry DOI: 10.7270/Q2K9363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 74 | -40.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem Lett 18: 4282-6 (2008) Article DOI: 10.1016/j.bmcl.2008.06.105 BindingDB Entry DOI: 10.7270/Q27D2SF1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM85561 (2-[4-(Phenylsulfonyl)-Phenyl}-thiazole-4-yl-methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 83 | -40.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Università degli Studi | Assay Description Inhibition assay using human Carbonic anhydrase I and II and bovine carbonic anhydrase IV. | J Enzym Inhib 16: 351-8 (2001) BindingDB Entry DOI: 10.7270/Q2K9363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM85564 (2-[4-(Phenylsulfonyl)-Phenyl}-thiazole-4-yl-methyl...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | PubMed | 87 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Università degli Studi | Assay Description Inhibition assay using human Carbonic anhydrase I and II and bovine carbonic anhydrase IV. | J Enzym Inhib 16: 351-8 (2001) BindingDB Entry DOI: 10.7270/Q2K9363Z | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM85659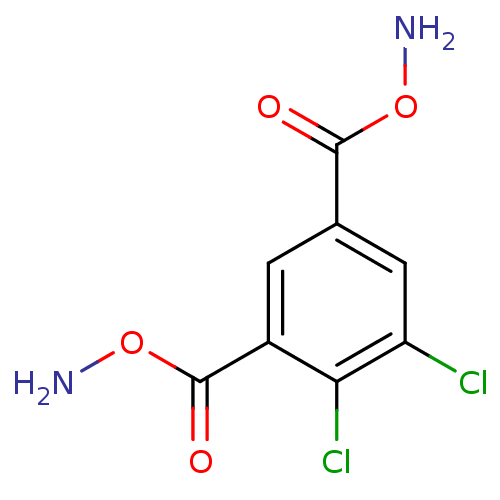 (DCP5) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 120 | -38.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM85660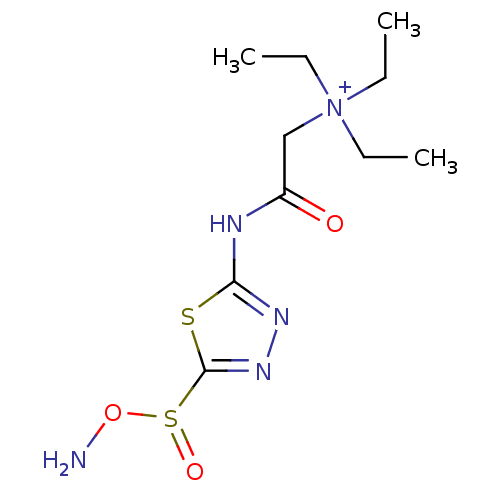 (Sulfonamide, 6) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 170 | -38.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 220 | -37.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10881 (CHEMBL288100 | MZA3 | Methazolamide | Methazolamid...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 240 | -37.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 20 |
Università degli Studi di Firenze | Assay Description The initial rates of 4-nitrophenylacetate hydrolysis catalysed by different CA isozymes were monitored spectrophotometrically at 400nm with a Cary 3 ... | J Enzyme Inhib Med Chem 19: 269-73 (2004) Article DOI: 10.1080/14756360410001689559 BindingDB Entry DOI: 10.7270/Q20C4TBB | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 578 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 580 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10882 (6-ethoxy-1,3-benzothiazole-2-sulfonamide | CHEMBL1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | 840 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM25896 (EMD 486019 | [2-(cycloheptylmethyl)-1,1-dioxo--ben...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank PC cid PC sid PDB UniChem | Article PubMed | 842 | -34.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem Lett 18: 4282-6 (2008) Article DOI: 10.1016/j.bmcl.2008.06.105 BindingDB Entry DOI: 10.7270/Q27D2SF1 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM19461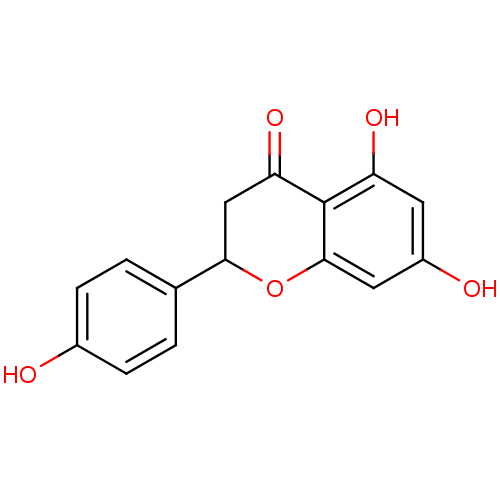 (α-CA inhibitor, 5 | 5,7-dihydroxy-2-(4-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239159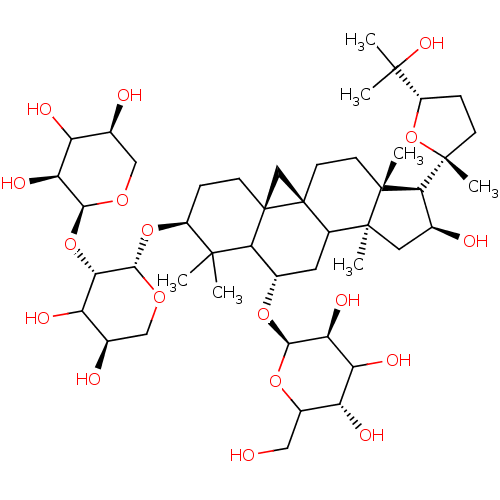 (α-CA inhibitor, 7) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.41E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239160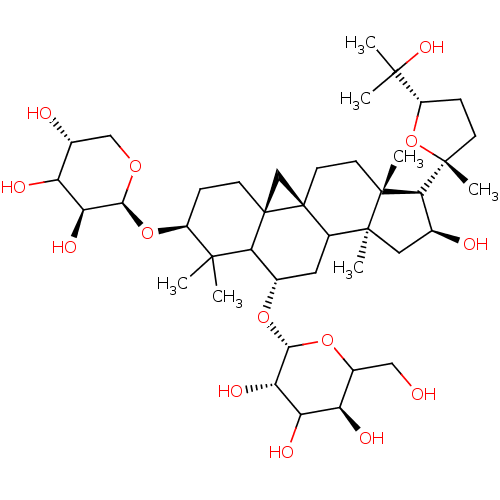 (α-CA inhibitor, 8) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 2.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239157 (α-CA inhibitor, 2) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.76E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239161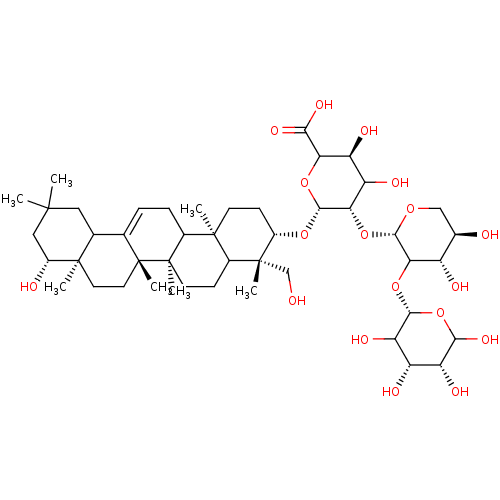 (α-CA inhibitor, 9) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 3.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM23417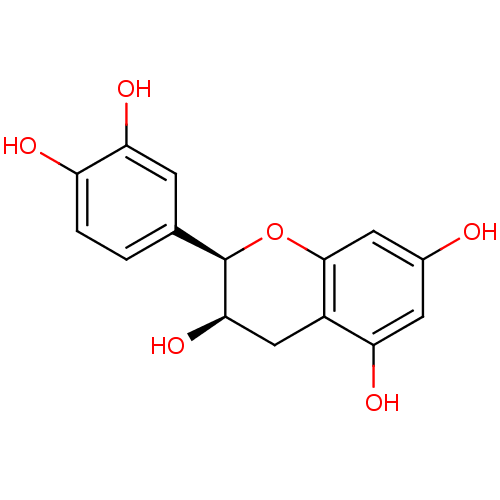 (α-CA inhibitor, 4 | (-)-Epicatechin | (2R,3R)...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 3.98E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM239162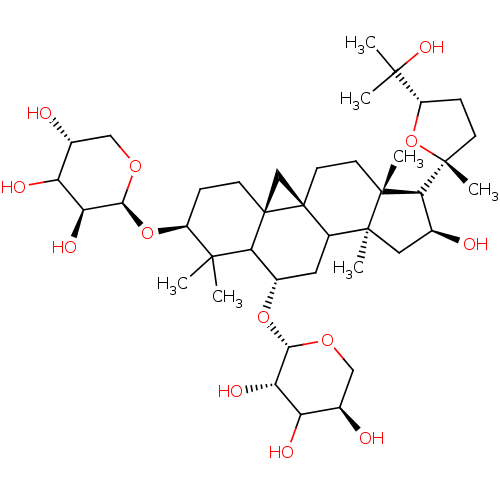 (α-CA inhibitor, 10) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 4.36E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ege University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 28: 412-7 (2013) Article DOI: 10.3109/14756366.2011.651464 BindingDB Entry DOI: 10.7270/Q2W66JQ2 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM7459 (2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-4H-chromen-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 4.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10887 (Sulfamate 7 | Topiramate (TPM) | US11535599, Examp...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 4.90E+3 | -30.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 25 |
Universita degli Studi di Firenze | Assay Description An Applied Photophysics stopped-flow instrument has been used for assaying the CA-catalyzed CO2 hydration activity. Phenol red has been used as indic... | Bioorg Med Chem Lett 18: 4282-6 (2008) Article DOI: 10.1016/j.bmcl.2008.06.105 BindingDB Entry DOI: 10.7270/Q27D2SF1 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM23416 (α-CA inhibitor, 3 | (+)-Catechin | (2R,3S)-2-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 5.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10880 (AZA | AZA2 | AZM acetazolamide | Acerazolamide, AA...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank PDB Article PubMed | 5.64E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM7458 (5,7-dihydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 9.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM26188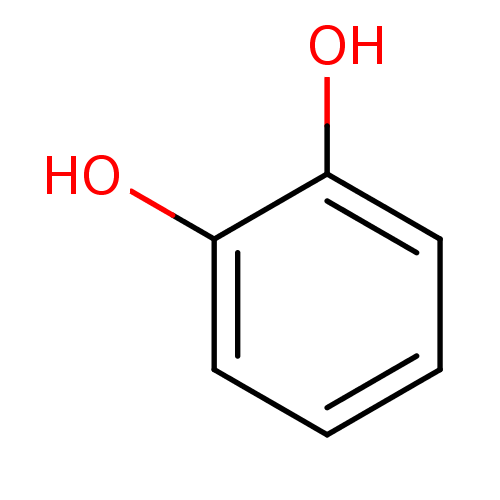 (α-CA inhibitor, 12 | 1,2-Dihydroxybenzene, XI...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.19E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM235706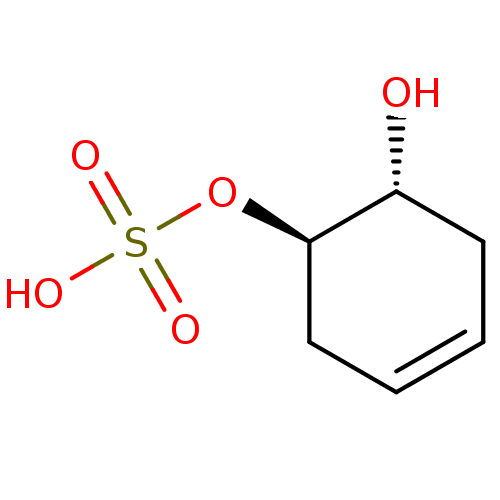 (trans-(1R(S),6R(S))-6-Hydroxycyclohex-3-enyl hydro...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.37E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM7460 (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chrome...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM26187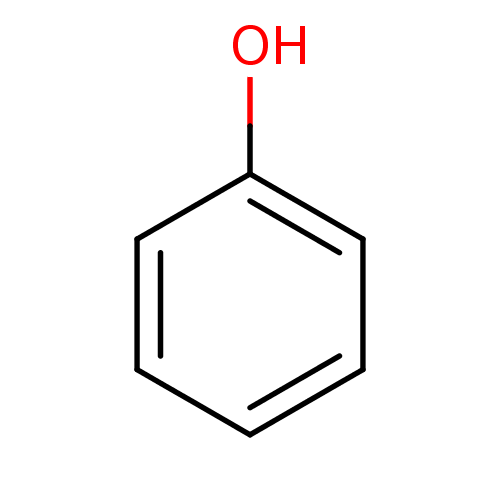 (α-CA inhibitor, 11 | CHEMBL14060 | US9688816,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.43E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM26658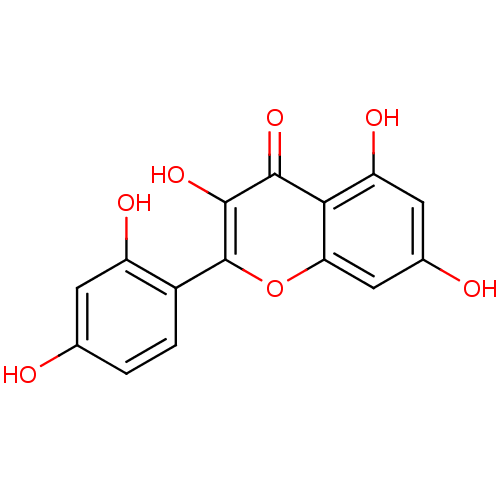 (2-(2,4-dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.57E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM235708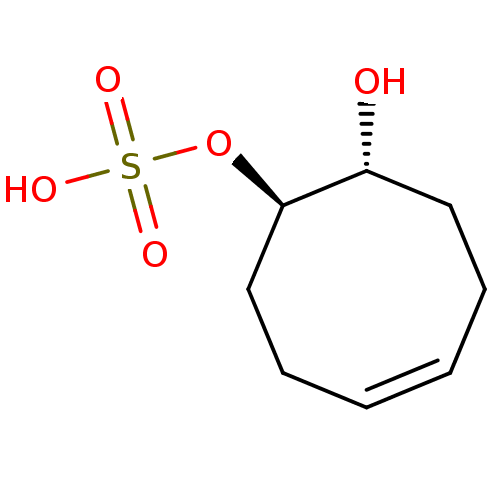 (trans-(1R(S),8R(S),Z)-8-Hydroxycyclooct-4-enyl hyd...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM36071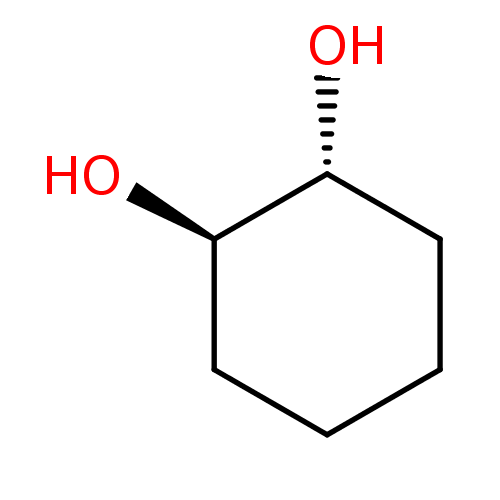 ((1R,2R)-trans-1,2-cyclohexanediol | trans-(1R(S),2...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE KEGG PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 1.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM235707 ((1R,2R)-cyclohexane-1,2-diol (5)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM10888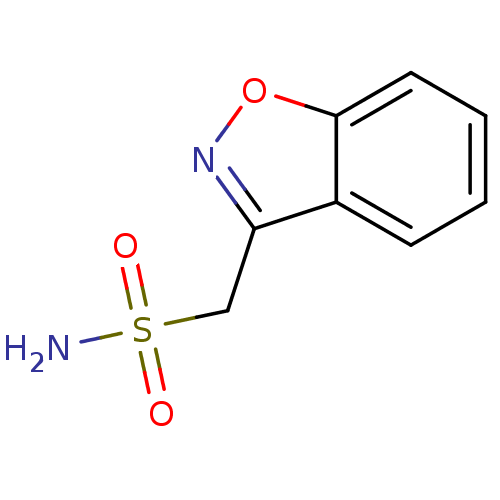 (1,2-benzoxazol-3-ylmethanesulfonamide | CHEMBL750 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | DrugBank Article PubMed | 3.84E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM235709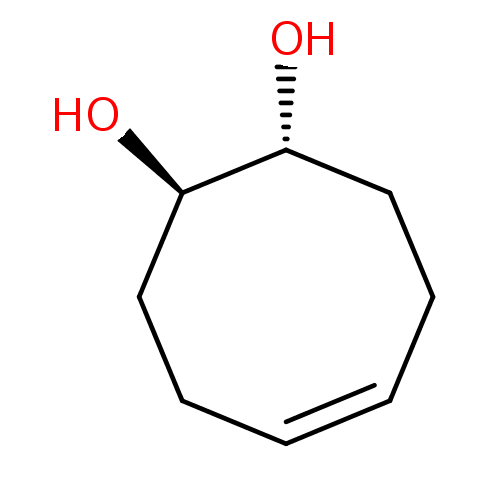 ((2R,3R)-1,2,3,4-tetrahydronaphthalene-2,3-diol (7)) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 5.38E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM235712 (trans-(2R(S),3R(S))-1,2,3,4-Tetrahydronaphthalene-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase PC cid PC sid UniChem | Article PubMed | 7.79E+4 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM235710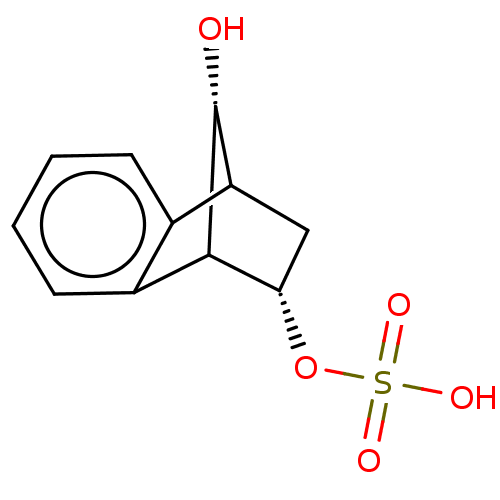 (9(R(S))-Hydroxy-1,2,3,4-tetrahydro-1,4-methanonaph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | KEGG PC cid PC sid UniChem | Article PubMed | 1.99E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM235711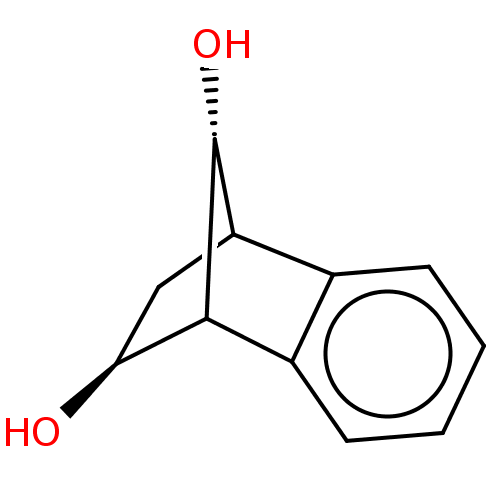 (9(R(S))-Hydroxy-1,2,3,4-tetrahydro-1,4-methanonaph...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 2.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Dumlupinar University | Assay Description CA activity was assayed by following the change in absorbance at 348 nm of 4-NPA to 4-nitrophenylate ion over a period of 3 min at 25°C using a spect... | J Enzyme Inhib Med Chem 27: 148-54 (2012) Article DOI: 10.3109/14756366.2011.629198 BindingDB Entry DOI: 10.7270/Q2M907JC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Homo sapiens (Human)) | BDBM26189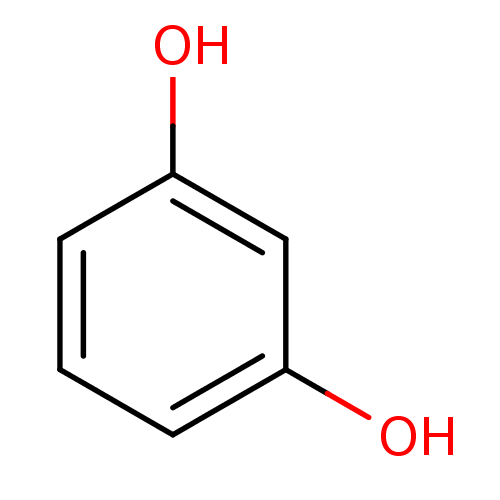 (α-CA inhibitor, 13 | 1,3-Dihydroxybenzene, XI...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | 6.34E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 7.4 | n/a |
Ondokuz Mayis University | Assay Description Carbonic anhydrase activity was assayed by following the change in absorbance at 348 nm of 4-nitrophenylacetate (NPA) to 4-nitrophenylate ion over a ... | J Enzyme Inhib Med Chem 28: 283-8 (2013) Article DOI: 10.3109/14756366.2011.643303 BindingDB Entry DOI: 10.7270/Q2QR4W1F | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM13063 (4-(5-methyl-3-phenyl-1,2-oxazol-4-yl)benzene-1-sul...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 340 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Marburg | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 550-7 (2004) Article DOI: 10.1021/jm030912m BindingDB Entry DOI: 10.7270/Q2W957DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM11639 (4-[5-(4-methylphenyl)-3-(trifluoromethyl)-1H-pyraz...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | Article PubMed | n/a | n/a | 290 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Marburg | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 550-7 (2004) Article DOI: 10.1021/jm030912m BindingDB Entry DOI: 10.7270/Q2W957DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Carbonic anhydrase 4 (Bos taurus (bovine)) | BDBM13064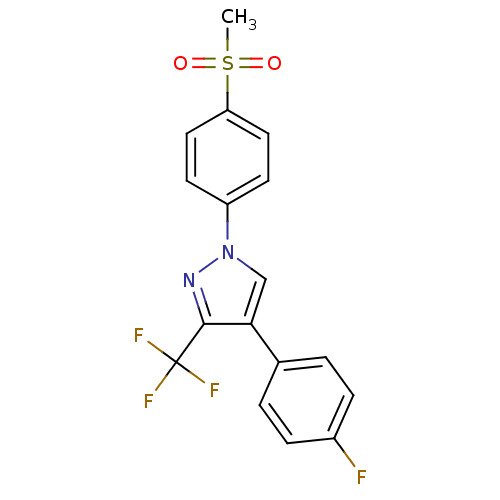 (4-(4-fluorophenyl)-1-(4-methanesulfonylphenyl)-3-(...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | 7.4 | 25 |
University of Marburg | Assay Description Initial rates of 4-nitrophenyl acetate hydrolysis catalyzed by different CA isozymes were monitored spectrophotometrically at 400 nm. A molar absorpt... | J Med Chem 47: 550-7 (2004) Article DOI: 10.1021/jm030912m BindingDB Entry DOI: 10.7270/Q2W957DZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 78 total ) | Next | Last >> |