

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314930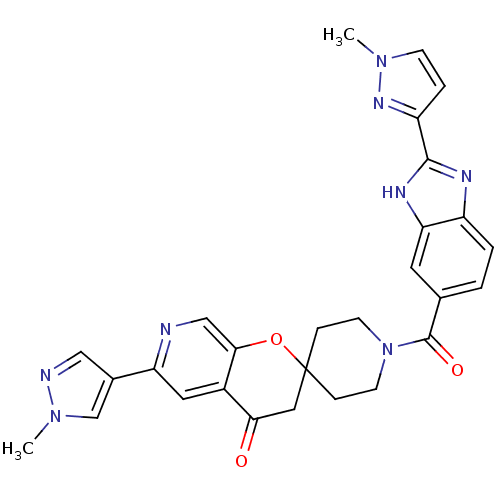 (1-(2-(1-methyl-1H-pyrazol-3-yl)-1H-benzo[d]imidazo...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314931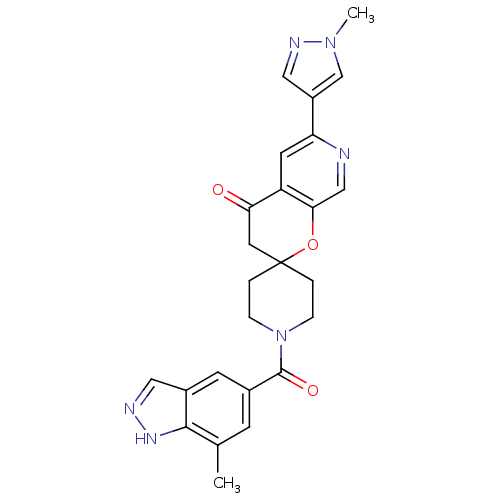 (1-(7-methyl-1H-indazole-5-carbonyl)-6'-(1-methyl-1...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50314932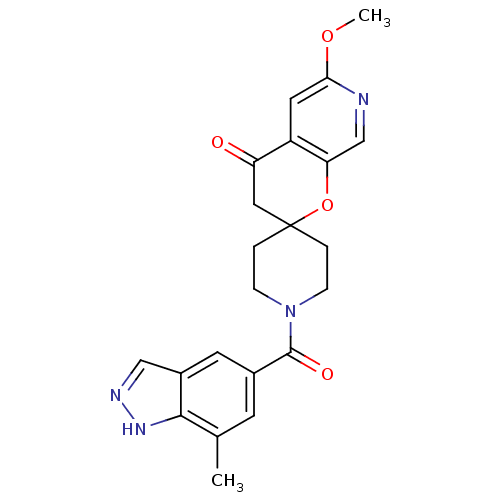 (6'-methoxy-1-(7-methyl-1H-indazole-5-carbonyl)spir...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.310 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Global Research and Development Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in CHO cells after 1 hr by fluorescence reader | Bioorg Med Chem Lett 20: 2383-8 (2010) Article DOI: 10.1016/j.bmcl.2009.04.091 BindingDB Entry DOI: 10.7270/Q22N52DW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152913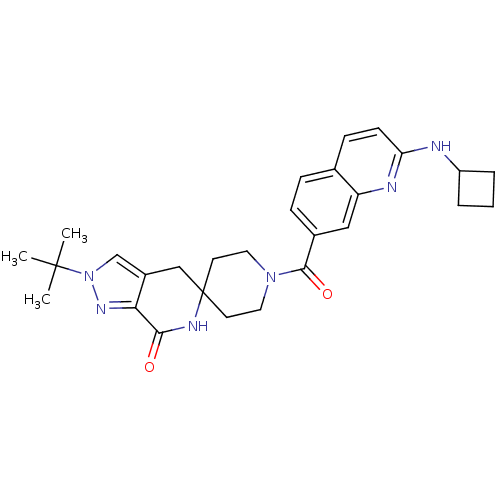 (US8993586, 109) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM459211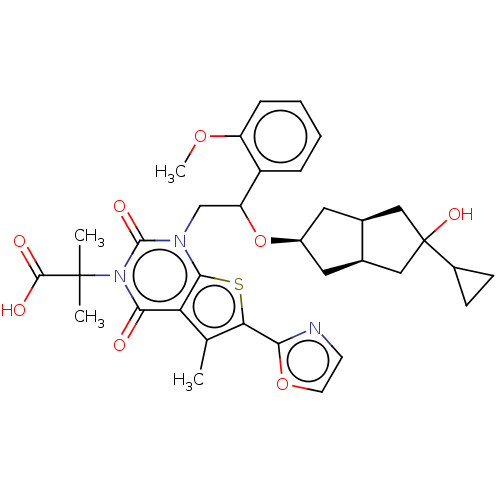 (2-[1-[2-[[(3aR,6aS)-5-cyclopropyl-5-hydroxy-2,3,3a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.13 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50426521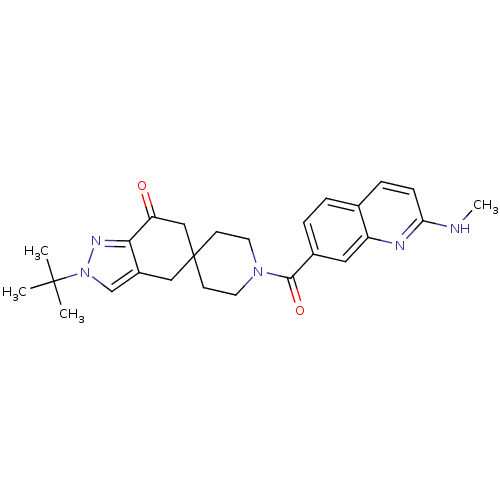 (CHEMBL2323626) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 | ACS Med Chem Lett 4: 16-7 (2013) Article DOI: 10.1021/ml3004044 BindingDB Entry DOI: 10.7270/Q2833TBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439642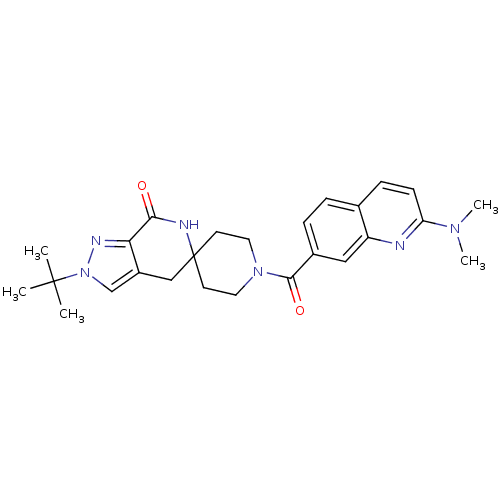 (CHEMBL2419589 | US8993586, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50069756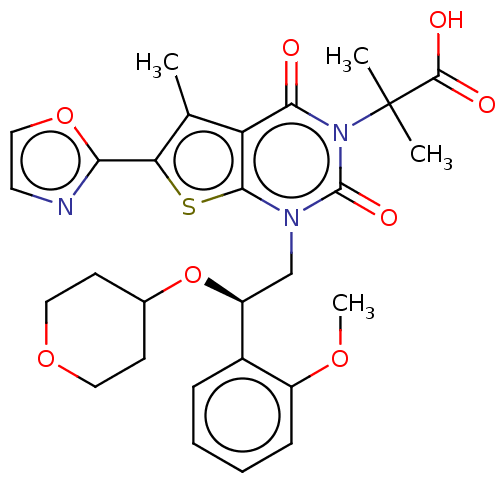 (CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen, Inc. Curated by ChEMBL | Assay Description In vitro inhibition of human recombinant His-tagged ACC2 assessed as malonyl-CoA level | J Med Chem 58: 525-36 (2015) Article DOI: 10.1021/jm500695e BindingDB Entry DOI: 10.7270/Q2DR2X6J | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439643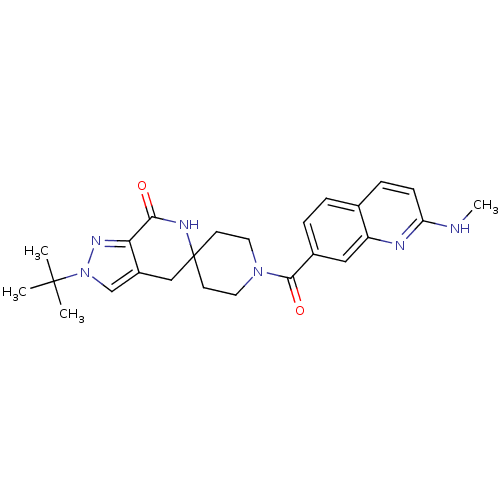 (CHEMBL2419598 | US8993586, 76) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152927 (US8993586, 125) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439646 (CHEMBL2419600 | US8993586, 110) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152926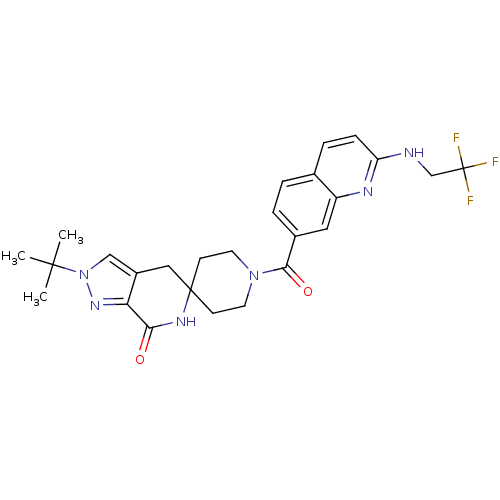 (US8993586, 124) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.72 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM459201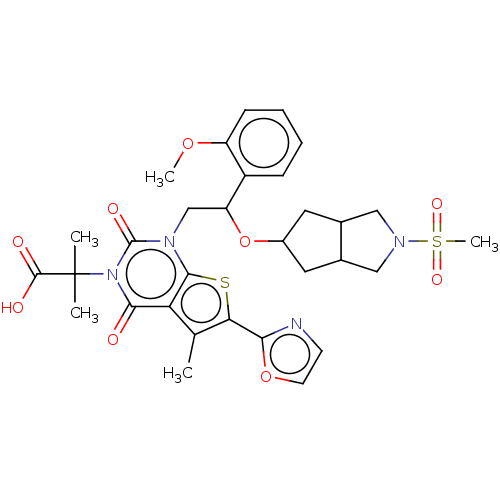 (2-[1-[2-(2-methoxyphenyl)-2-[(2-methylsulfonyl-3,3...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 1.76 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152915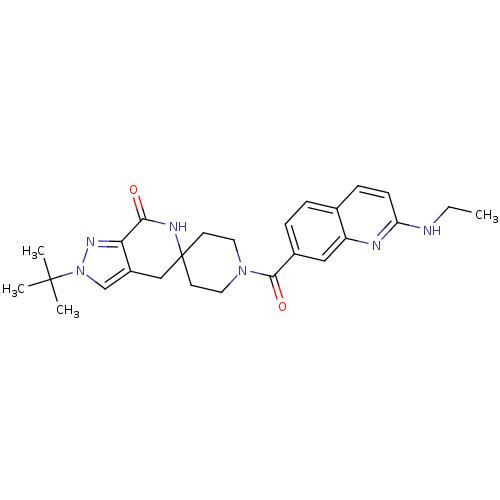 (US8993586, 112) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.80 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152877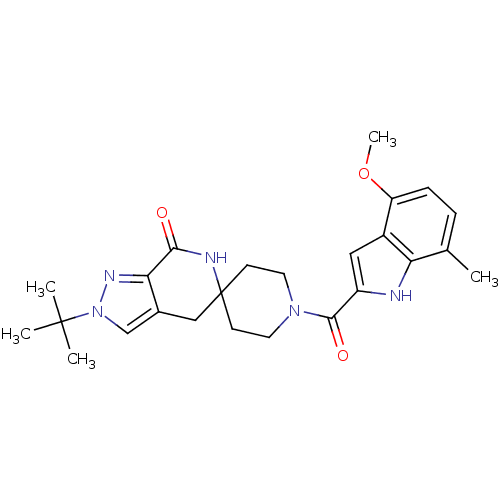 (US8993586, 56) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50462049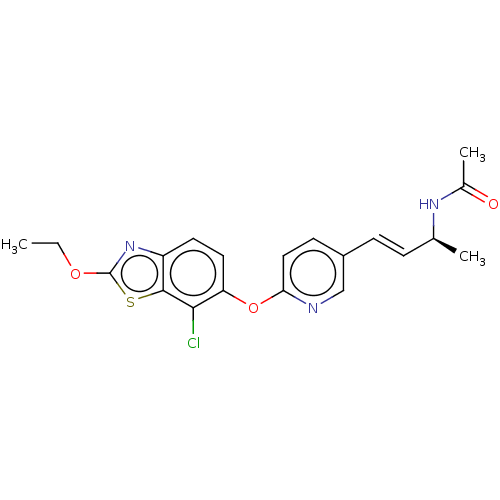 (CHEMBL4225405) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC2 assessed as reduction in conversion of acetyl-CoA to malonyl-CoA incubated for 1 to 3 hrs with substrate by MALD... | Bioorg Med Chem Lett 28: 2498-2503 (2018) Article DOI: 10.1016/j.bmcl.2018.05.055 BindingDB Entry DOI: 10.7270/Q20V8GFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307410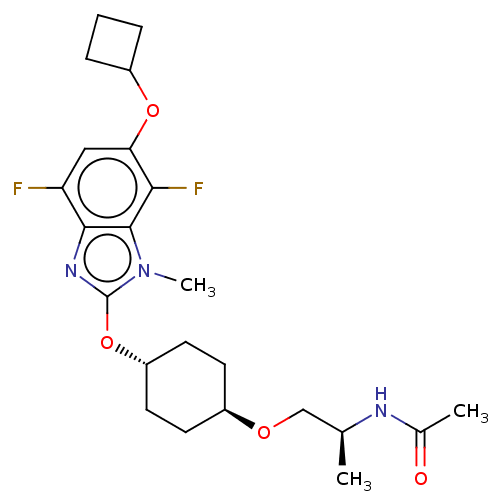 (US10150728, Example I-639) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307424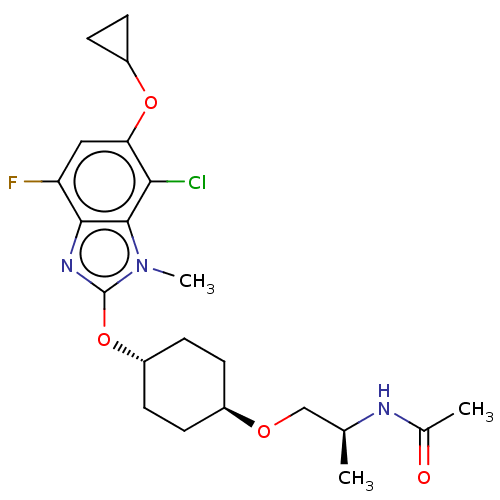 (US10150728, Example I-655) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152867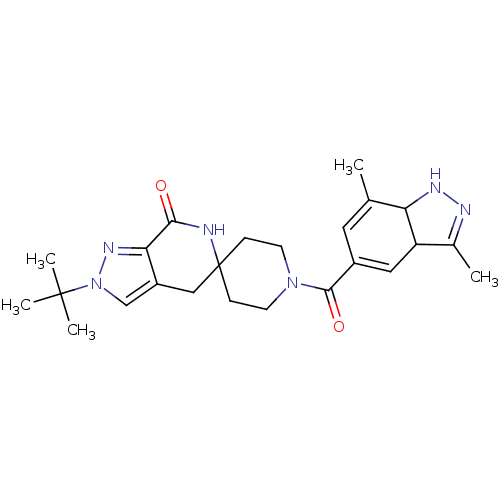 (US8993586, 45) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50462060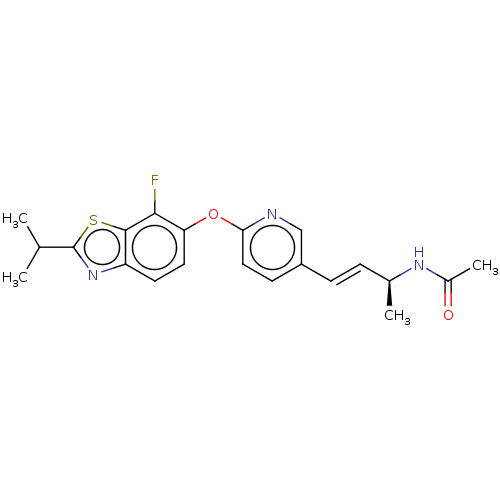 (CHEMBL4229067) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Shionogi & Co., Ltd Curated by ChEMBL | Assay Description Inhibition of recombinant human ACC2 assessed as reduction in conversion of acetyl-CoA to malonyl-CoA incubated for 1 to 3 hrs with substrate by MALD... | Bioorg Med Chem Lett 28: 2498-2503 (2018) Article DOI: 10.1016/j.bmcl.2018.05.055 BindingDB Entry DOI: 10.7270/Q20V8GFW | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152900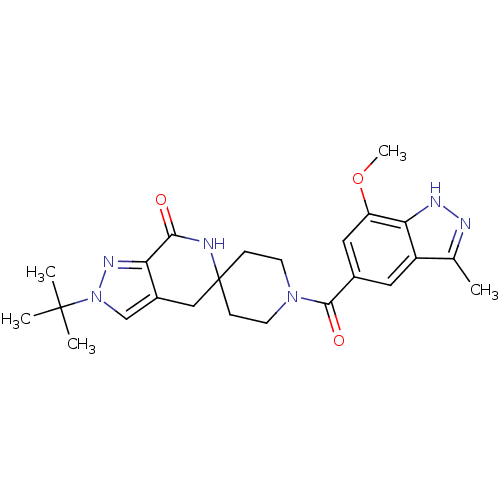 (US8993586, 87) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50426523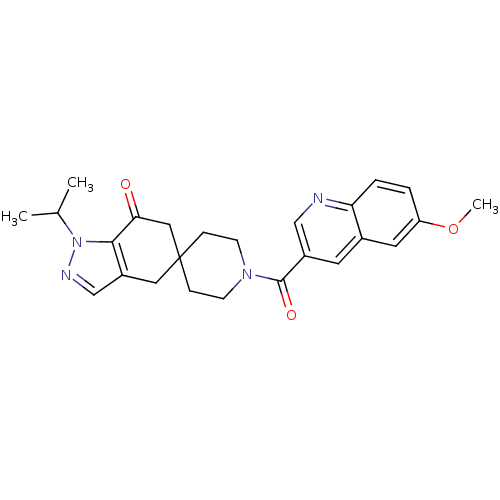 (CHEMBL2323628) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 | ACS Med Chem Lett 4: 16-7 (2013) Article DOI: 10.1021/ml3004044 BindingDB Entry DOI: 10.7270/Q2833TBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152882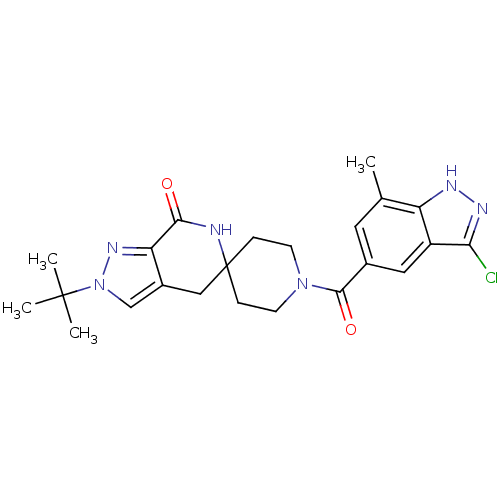 (US8993586, 62) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM459214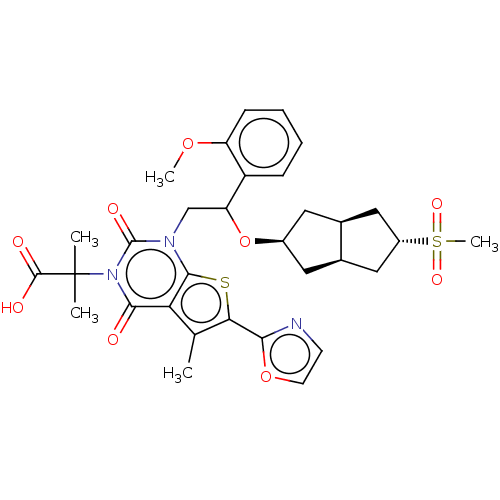 (2-[1-[2-[[(3aR,6aS)-5-methylsulfonyl-1,2,3,3a,4,5,...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.19 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152911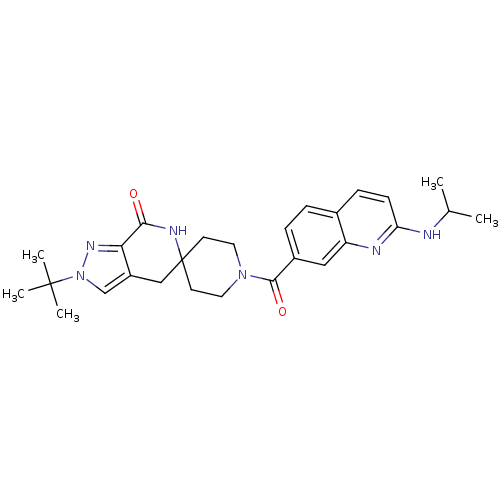 (US8993586, 107) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.20 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439642 (CHEMBL2419589 | US8993586, 105) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152912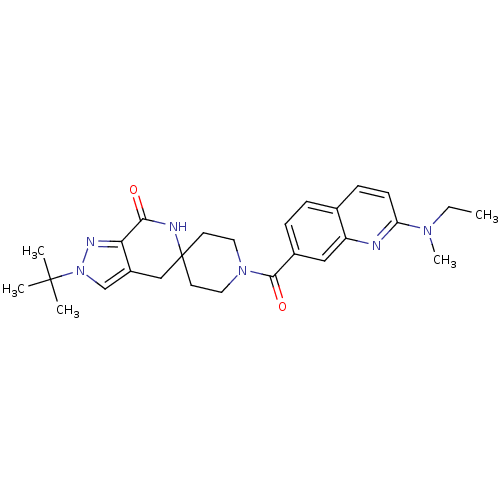 (US8993586, 108) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152889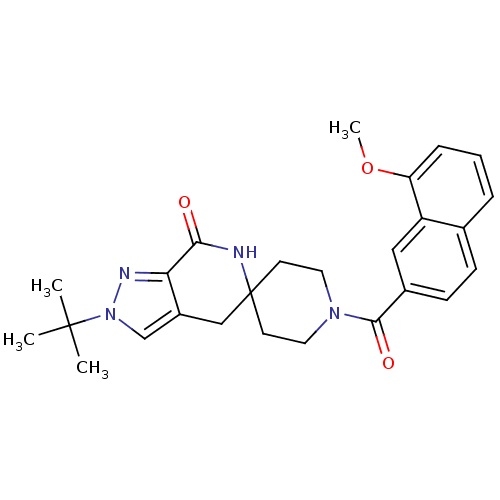 (US8993586, 70) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152885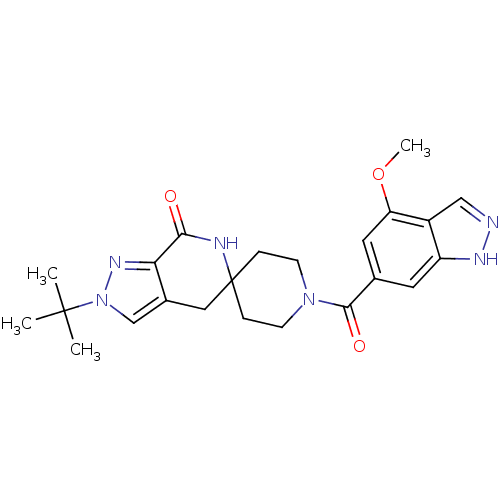 (US8993586, 66) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152922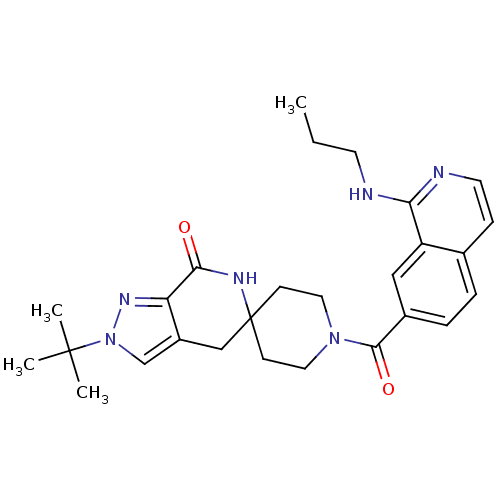 (US8993586, 119) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439641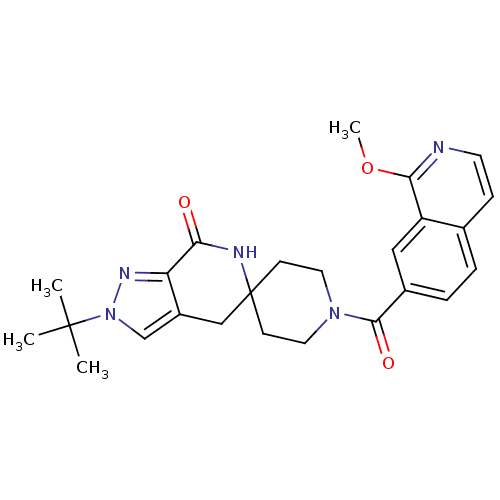 (CHEMBL2419597 | US8993586, 55) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152910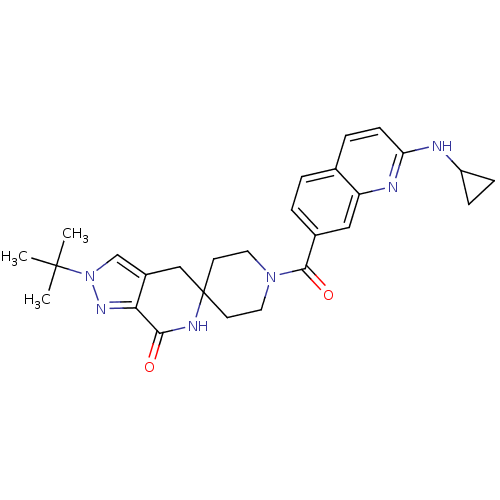 (US8993586, 106) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50069756 (CHEMBL3407547 | Firsocostat | ND-630 | NDI-010976 ...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.60 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC2 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152924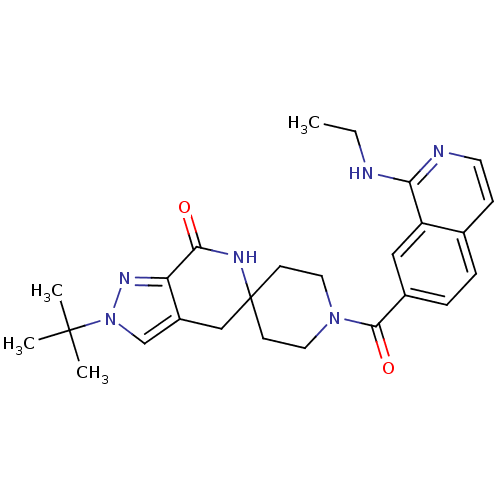 (US8993586, 121) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152921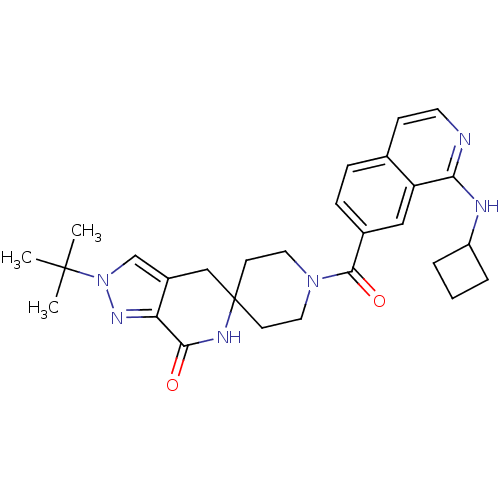 (US8993586, 118) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152869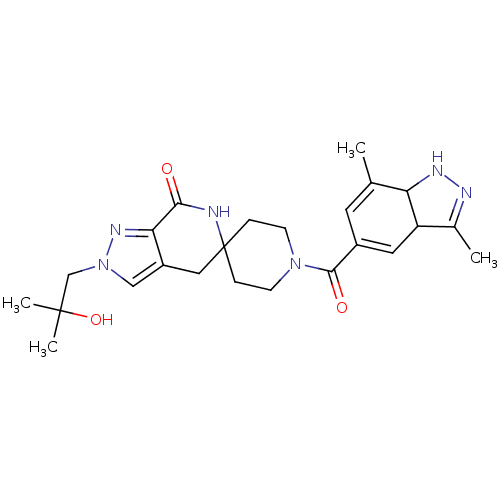 (US8993586, 47) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM459215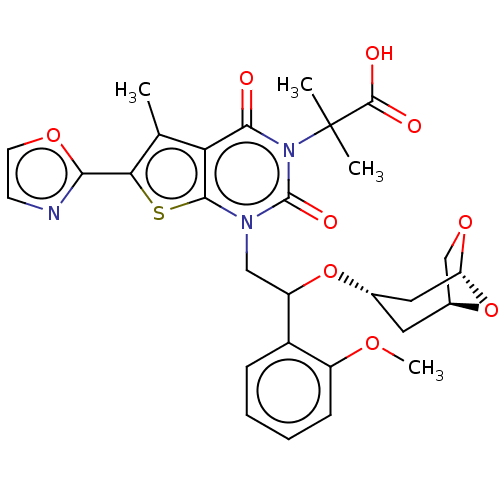 (2-[1-[2-[[(1S,3S,5R)-6,8-dioxabicyclo[3.2.1]oct-3-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 2.74 | n/a | n/a | n/a | n/a | n/a | n/a |
Sunshine Lake Pharma Co., Ltd. US Patent | Assay Description a. 4.5 μL/well of ACC1/ACC2 working solution (2.22 nM) was added to a 384-well reaction plate (PerkinElmer, 6007290). b. The comp... | US Patent US10759812 (2020) BindingDB Entry DOI: 10.7270/Q22N55B7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM152918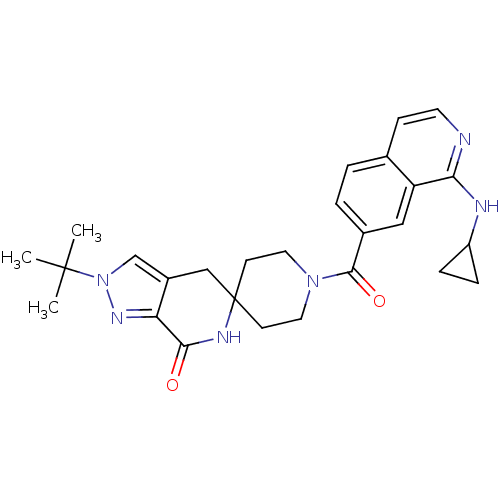 (US8993586, 115) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. US Patent | Assay Description Preparation of rhACC2. Human ACC2 inhibition was measured using purified recombinant human ACC2 (hrACC2). Briefly, a full length Cytomax clone of ACC... | US Patent US8993586 (2015) BindingDB Entry DOI: 10.7270/Q2N58K43 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50426524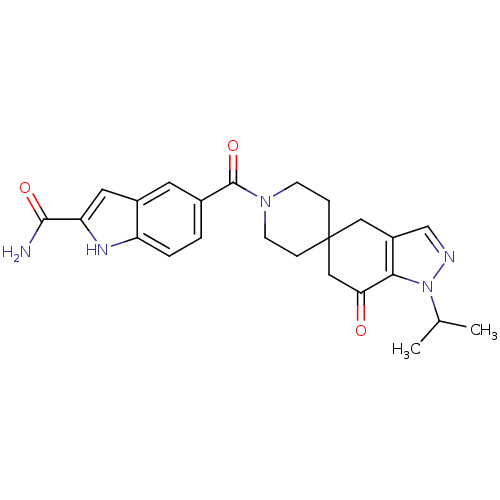 (CHEMBL2323629) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Therachem Research Medilab (India) Pvt. Ltd. Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 | ACS Med Chem Lett 4: 16-7 (2013) Article DOI: 10.1021/ml3004044 BindingDB Entry DOI: 10.7270/Q2833TBP | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307475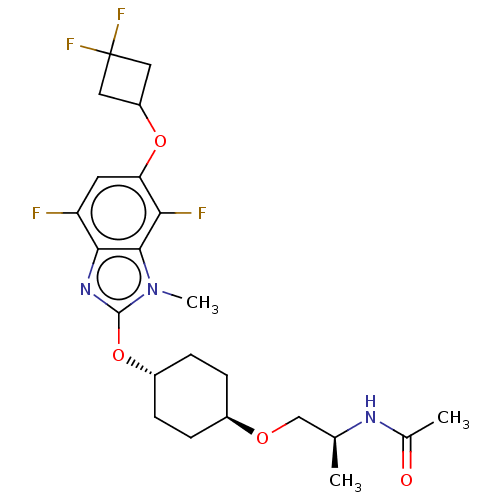 (US10150728, Example I-713) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50439644 (CHEMBL2419593 | US8993586, 86) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Pfizer Inc. Curated by ChEMBL | Assay Description Inhibition of human ACC2 using acetyl-CoA as substrate assessed as [14C]malonyl-CoA synthesis preincubated for 10 mins prior to substrate addition me... | J Med Chem 56: 7110-9 (2013) Article DOI: 10.1021/jm401033t BindingDB Entry DOI: 10.7270/Q2JW8G9D | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM306944 (US10150728, Example I-157) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50511117 (CHEMBL4557289) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Terns Pharmaceuticals Curated by ChEMBL | Assay Description Inhibition of ACC2 (unknown origin) | J Med Chem 63: 5031-5073 (2020) Article DOI: 10.1021/acs.jmedchem.9b01701 BindingDB Entry DOI: 10.7270/Q2DJ5JXG | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307449 (US10150728, Example I-683) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307463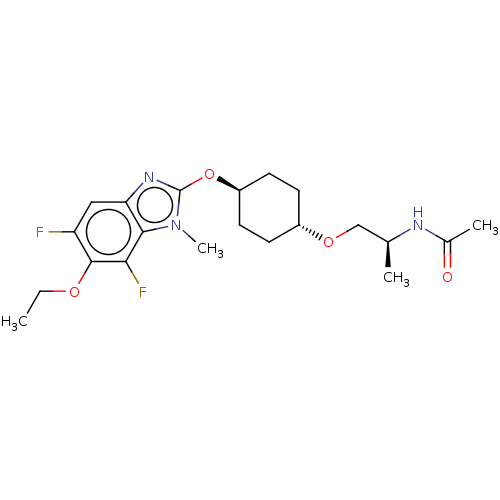 (US10150728, Example I-698) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM50205246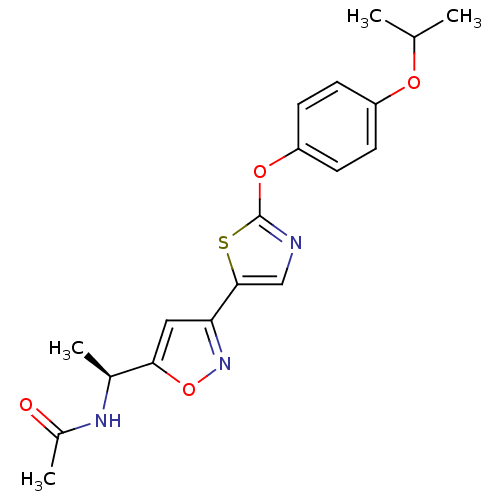 ((S)-(-)-N-(1-(3-(2-(4-isopropoxyphenoxy)thiazol-5-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Abbott Laboratories Curated by ChEMBL | Assay Description Inhibition of human recombinant ACC2 expressed in SF9 cells | J Med Chem 50: 1078-82 (2007) Article DOI: 10.1021/jm070035a BindingDB Entry DOI: 10.7270/Q24F1QCB | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307497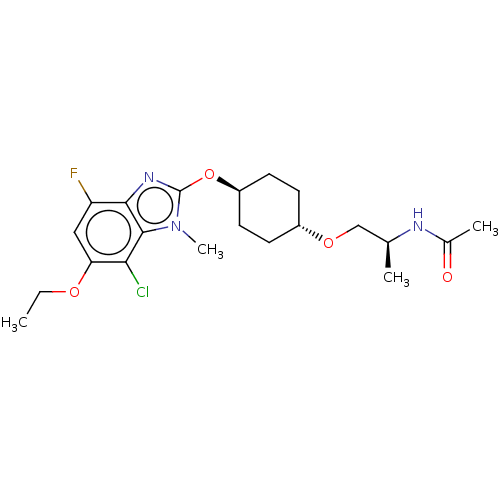 (US10150728, Example I-740) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM307385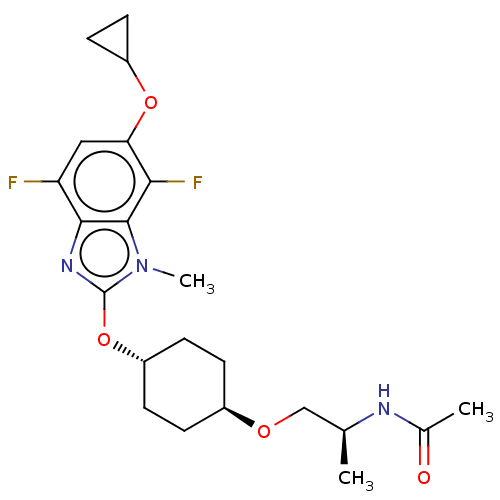 (US10150728, Example I-611) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Acetyl-CoA carboxylase 2 (Homo sapiens (Human)) | BDBM306965 (US10150728, Example I-178) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
SHIONOGI & CO., LTD. US Patent | Assay Description Recombinant human ACC1 and recombinant human ACC2, which were prepared by the method mentioned above, were preincubated with assay buffer solution (5... | US Patent US10150728 (2018) BindingDB Entry DOI: 10.7270/Q2WD42M7 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 4211 total ) | Next | Last >> |