 Found 60 hits of ic50 data for polymerid = 50000820,50000821
Found 60 hits of ic50 data for polymerid = 50000820,50000821 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596365
(CHEMBL5179810)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(NC(C)=O)ccc1O)Cc1ccccc1N(C)C | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516960
(CHEMBL4435544)Show SMILES O=c1ccc2ccc(OCCCCCCCn3cc(CNc4c5CCCCc5nc5ccccc45)nn3)cc2o1 Show InChI InChI=1S/C32H35N5O3/c38-31-17-15-23-14-16-25(20-30(23)40-31)39-19-9-3-1-2-8-18-37-22-24(35-36-37)21-33-32-26-10-4-6-12-28(26)34-29-13-7-5-11-27(29)32/h4,6,10,12,14-17,20,22H,1-3,5,7-9,11,13,18-19,21H2,(H,33,34) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) after 2 mins |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596366
(CHEMBL5204683)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(NC(C)=O)ccc1O)Cc1ccccc1OC | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516959

(CHEMBL4471876)Show SMILES Cc1cc(=O)oc2cc(OCCCCCn3cc(CNc4c5CCCCc5nc5cc(Cl)ccc45)nn3)ccc12 Show InChI InChI=1S/C31H32ClN5O3/c1-20-15-30(38)40-29-17-23(10-12-24(20)29)39-14-6-2-5-13-37-19-22(35-36-37)18-33-31-25-7-3-4-8-27(25)34-28-16-21(32)9-11-26(28)31/h9-12,15-17,19H,2-8,13-14,18H2,1H3,(H,33,34) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) after 2 mins |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596377
(CHEMBL5181578)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(F)ccc1O)Cc1ccccc1N(C)C | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM8960

((+/-)-2-[(1-benzylpiperidin-4-yl)methyl]-5,6-dimet...)Show SMILES COc1cc2CC(CC3CCN(Cc4ccccc4)CC3)C(=O)c2cc1OC Show InChI InChI=1S/C24H29NO3/c1-27-22-14-19-13-20(24(26)21(19)15-23(22)28-2)12-17-8-10-25(11-9-17)16-18-6-4-3-5-7-18/h3-7,14-15,17,20H,8-13,16H2,1-2H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596341

(CHEMBL5176418)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(ccc1O)N(C)C)Cc1ccccc1N(C)C | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596380

(CHEMBL5173121)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(ccc1O)[N+]([O-])=O)Cc1ccccc1N(C)C | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM8961

(1,2,3,4-tetrahydro-9-acridinamine | 1,2,3,4-tetrah...)Show InChI InChI=1S/C13H14N2/c14-13-9-5-1-3-7-11(9)15-12-8-4-2-6-10(12)13/h1,3,5,7H,2,4,6,8H2,(H2,14,15) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase |
J Med Chem 48: 7496-9 (2005)
Article DOI: 10.1021/jm058041z
BindingDB Entry DOI: 10.7270/Q20864WZ |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516976

(CHEMBL4518438)Show SMILES Cc1nn(CC(=O)N\N=C\c2ccccc2)c(=O)n1-c1ccc(cc1)C(=O)N\N=C\c1ccccc1 Show InChI InChI=1S/C26H23N7O3/c1-19-31-32(18-24(34)29-27-16-20-8-4-2-5-9-20)26(36)33(19)23-14-12-22(13-15-23)25(35)30-28-17-21-10-6-3-7-11-21/h2-17H,18H2,1H3,(H,29,34)(H,30,35)/b27-16+,28-17+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 47 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Ellman's method |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50459975
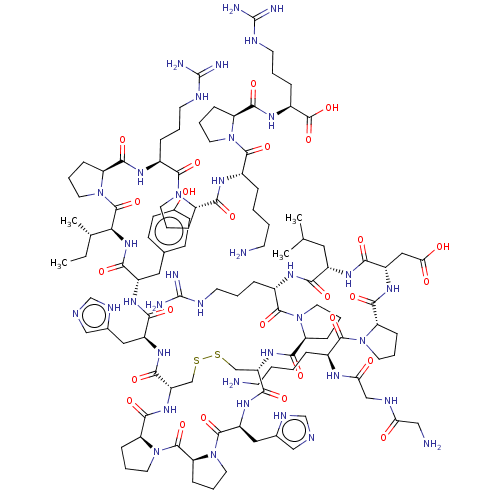
(CHEMBL4228909)Show SMILES CC[C@H](C)[C@H](NC(=O)[C@H](Cc1ccc(O)cc1)NC(=O)[C@H](Cc1cnc[nH]1)NC(=O)[C@@H]1CSSC[C@H](NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCNC(N)=N)NC(=O)[C@H](CC(C)C)NC(=O)[C@H](CC(O)=O)NC(=O)[C@@H]2CCCN2C(=O)[C@H](CCCCN)NC(=O)CNC(=O)CN)C(=O)N[C@@H](Cc2cnc[nH]2)C(=O)N2CCC[C@H]2C(=O)N2CCC[C@H]2C(=O)N1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCCN)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CCCNC(N)=N)C(O)=O |r| Show InChI InChI=1S/C112H175N37O26S2/c1-5-62(4)89(108(173)148-46-18-30-84(148)97(162)133-70(23-11-39-125-111(118)119)105(170)144-42-13-25-79(144)96(161)132-68(21-7-9-37-114)103(168)145-43-14-26-80(145)98(163)134-71(109(174)175)24-12-40-126-112(120)121)142-93(158)73(49-63-32-34-66(150)35-33-63)136-91(156)74(50-64-54-122-59-128-64)137-94(159)77-57-176-177-58-78(95(160)139-76(51-65-55-123-60-129-65)106(171)149-47-19-31-85(149)107(172)147-45-17-29-83(147)101(166)140-77)141-100(165)82-28-16-44-146(82)104(169)69(22-10-38-124-110(116)117)131-90(155)72(48-61(2)3)135-92(157)75(52-88(153)154)138-99(164)81-27-15-41-143(81)102(167)67(20-6-8-36-113)130-87(152)56-127-86(151)53-115/h32-35,54-55,59-62,67-85,89,150H,5-31,36-53,56-58,113-115H2,1-4H3,(H,122,128)(H,123,129)(H,127,151)(H,130,152)(H,131,155)(H,132,161)(H,133,162)(H,134,163)(H,135,157)(H,136,156)(H,137,159)(H,138,164)(H,139,160)(H,140,166)(H,141,165)(H,142,158)(H,153,154)(H,174,175)(H4,116,117,124)(H4,118,119,125)(H4,120,121,126)/t62-,67-,68-,69-,70-,71-,72-,73-,74-,75-,76-,77-,78-,79-,80-,81-,82-,83-,84-,85-,89-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| KEGG
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
Peptides International, Inc.
Curated by ChEMBL
| Assay Description
Inhibition of nAChR epsilon (unknown origin) |
Bioorg Med Chem 26: 2738-2758 (2018)
Article DOI: 10.1016/j.bmc.2017.09.029
BindingDB Entry DOI: 10.7270/Q2Q52S8Q |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516974
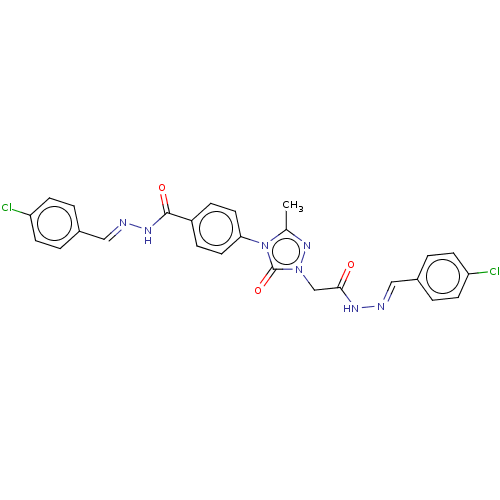
(CHEMBL4516351)Show SMILES Cc1nn(CC(=O)N\N=C\c2ccc(Cl)cc2)c(=O)n1-c1ccc(cc1)C(=O)N\N=C\c1ccc(Cl)cc1 Show InChI InChI=1S/C26H21Cl2N7O3/c1-17-33-34(16-24(36)31-29-14-18-2-8-21(27)9-3-18)26(38)35(17)23-12-6-20(7-13-23)25(37)32-30-15-19-4-10-22(28)11-5-19/h2-15H,16H2,1H3,(H,31,36)(H,32,37)/b29-14+,30-15+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 57 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Ellman's method |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516977
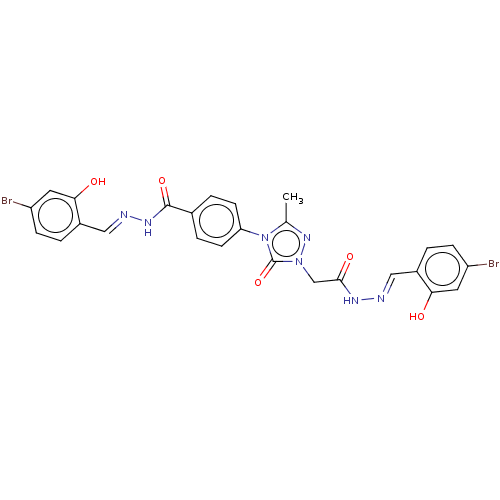
(CHEMBL4518992)Show SMILES Cc1nn(CC(=O)N\N=C\c2ccc(Br)cc2O)c(=O)n1-c1ccc(cc1)C(=O)N\N=C\c1ccc(Br)cc1O Show InChI InChI=1S/C26H21Br2N7O5/c1-15-33-34(14-24(38)31-29-12-17-2-6-19(27)10-22(17)36)26(40)35(15)21-8-4-16(5-9-21)25(39)32-30-13-18-3-7-20(28)11-23(18)37/h2-13,36-37H,14H2,1H3,(H,31,38)(H,32,39)/b29-12+,30-13+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Ellman's method |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596378
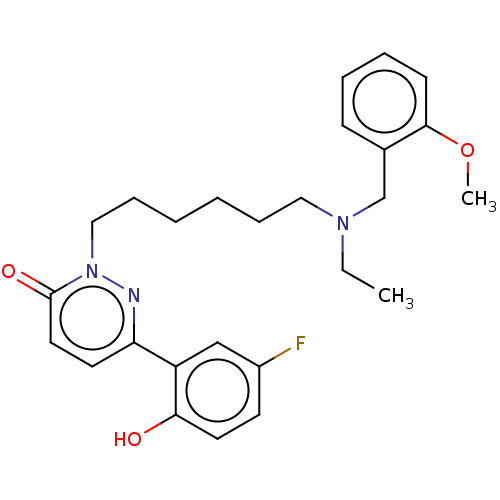
(CHEMBL5207035)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(F)ccc1O)Cc1ccccc1OC | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 58 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516975
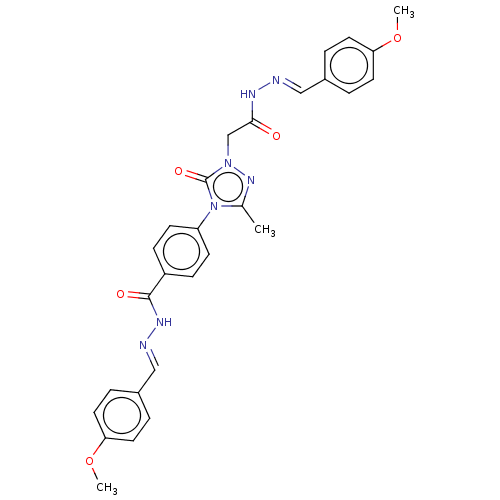
(CHEMBL4514263)Show SMILES COc1ccc(\C=N\NC(=O)Cn2nc(C)n(-c3ccc(cc3)C(=O)N\N=C\c3ccc(OC)cc3)c2=O)cc1 Show InChI InChI=1S/C28H27N7O5/c1-19-33-34(18-26(36)31-29-16-20-4-12-24(39-2)13-5-20)28(38)35(19)23-10-8-22(9-11-23)27(37)32-30-17-21-6-14-25(40-3)15-7-21/h4-17H,18H2,1-3H3,(H,31,36)(H,32,37)/b29-16+,30-17+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Ellman's method |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596367

(CHEMBL5190924)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(NC(C)=O)ccc1O)Cc1cccc(F)c1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 71 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516963
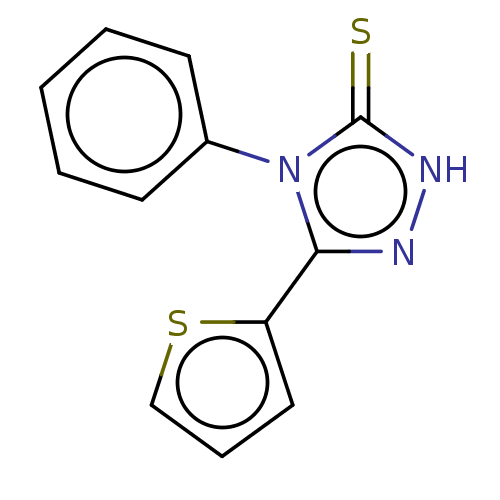
(CHEMBL2376519)Show InChI InChI=1S/C12H9N3S2/c16-12-14-13-11(10-7-4-8-17-10)15(12)9-5-2-1-3-6-9/h1-8H,(H,14,16) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 82 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596382
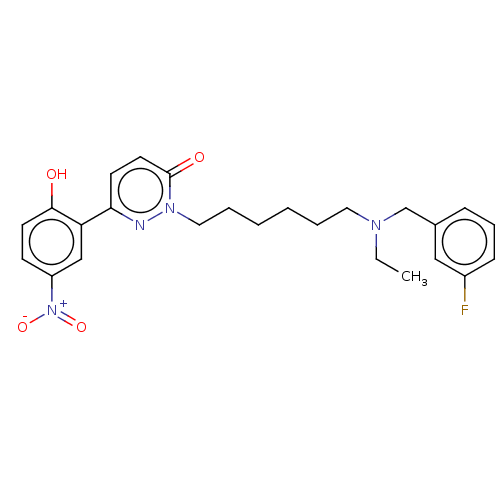
(CHEMBL5188135)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(ccc1O)[N+]([O-])=O)Cc1cccc(F)c1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 83 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596360
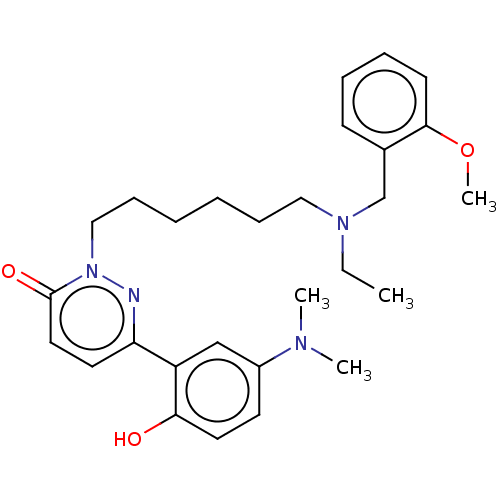
(CHEMBL5200573)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(ccc1O)N(C)C)Cc1ccccc1OC | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 87 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516982
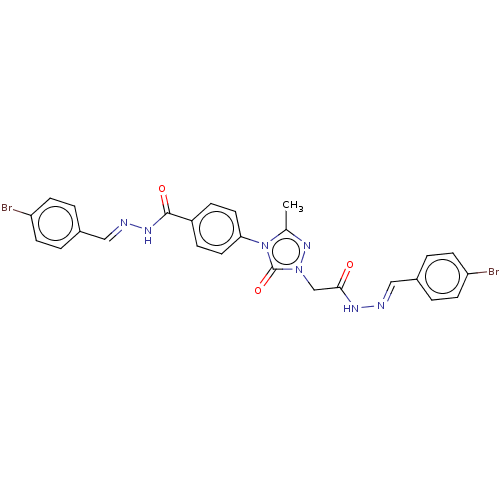
(CHEMBL4447626)Show SMILES Cc1nn(CC(=O)N\N=C\c2ccc(Br)cc2)c(=O)n1-c1ccc(cc1)C(=O)N\N=C\c1ccc(Br)cc1 Show InChI InChI=1S/C26H21Br2N7O3/c1-17-33-34(16-24(36)31-29-14-18-2-8-21(27)9-3-18)26(38)35(17)23-12-6-20(7-13-23)25(37)32-30-15-19-4-10-22(28)11-5-19/h2-15H,16H2,1H3,(H,31,36)(H,32,37)/b29-14+,30-15+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 88 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Ellman's method |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596381
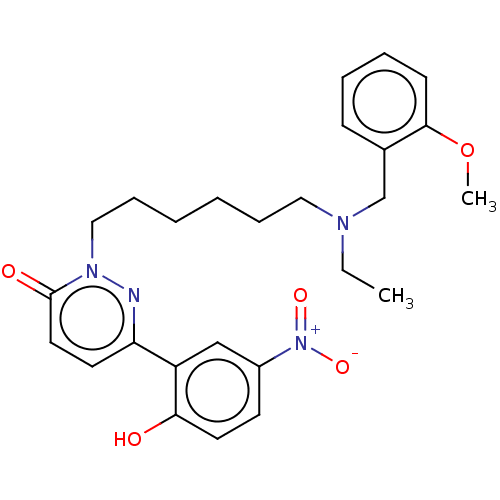
(CHEMBL5179648)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(ccc1O)[N+]([O-])=O)Cc1ccccc1OC | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 91 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516950
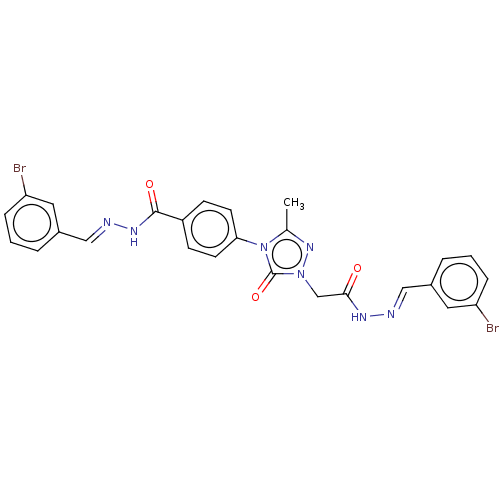
(CHEMBL4544630)Show SMILES Cc1nn(CC(=O)N\N=C\c2cccc(Br)c2)c(=O)n1-c1ccc(cc1)C(=O)N\N=C\c1cccc(Br)c1 Show InChI InChI=1S/C26H21Br2N7O3/c1-17-33-34(16-24(36)31-29-14-18-4-2-6-21(27)12-18)26(38)35(17)23-10-8-20(9-11-23)25(37)32-30-15-19-5-3-7-22(28)13-19/h2-15H,16H2,1H3,(H,31,36)(H,32,37)/b29-14+,30-15+ | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 97 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) by Ellman's method |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50177174
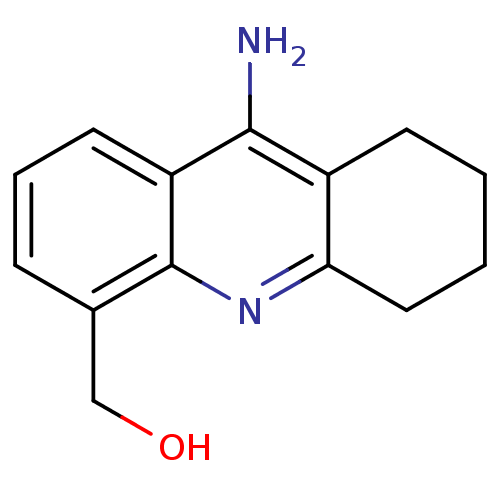
(9-amino-5,6,7,8-tetrahydroacridin-4yl)methano | CH...)Show InChI InChI=1S/C14H16N2O/c15-13-10-5-1-2-7-12(10)16-14-9(8-17)4-3-6-11(13)14/h3-4,6,17H,1-2,5,7-8H2,(H2,15,16) | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 125 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Würzburg
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase |
J Med Chem 48: 7496-9 (2005)
Article DOI: 10.1021/jm058041z
BindingDB Entry DOI: 10.7270/Q20864WZ |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596379
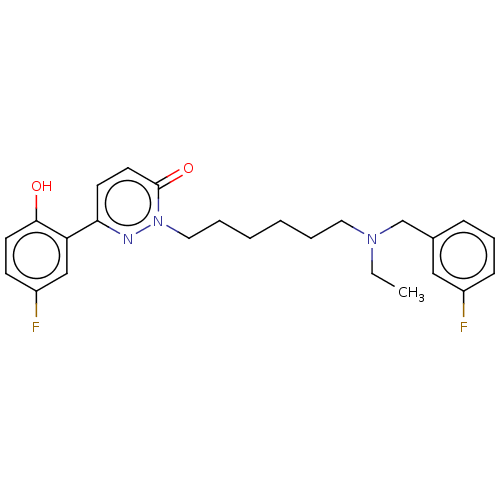
(CHEMBL5196896)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(F)ccc1O)Cc1cccc(F)c1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 407 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50596361
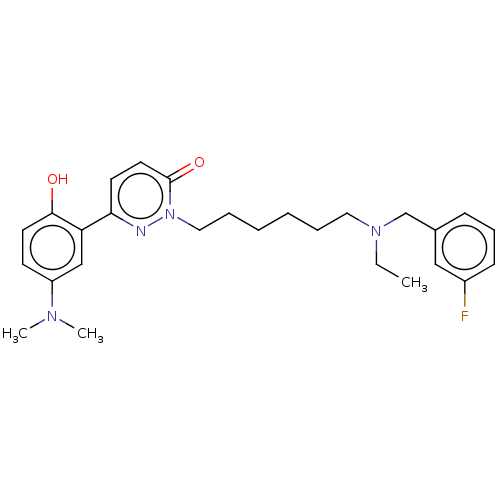
(CHEMBL5206933)Show SMILES CCN(CCCCCCn1nc(ccc1=O)-c1cc(ccc1O)N(C)C)Cc1cccc(F)c1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 676 | n/a | n/a | n/a | n/a | n/a | n/a |
TBA
| |
Citation and Details
Article DOI: 10.1016/j.ejmech.2021.114098
BindingDB Entry DOI: 10.7270/Q20R9TFC |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524696

(CHEMBL4571835)Show InChI InChI=1S/C17H23N3O2/c1-6-20(5)17(21)22-15-11-14(12(2)19(3)4)10-13-8-7-9-18-16(13)15/h7-12H,6H2,1-5H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516970
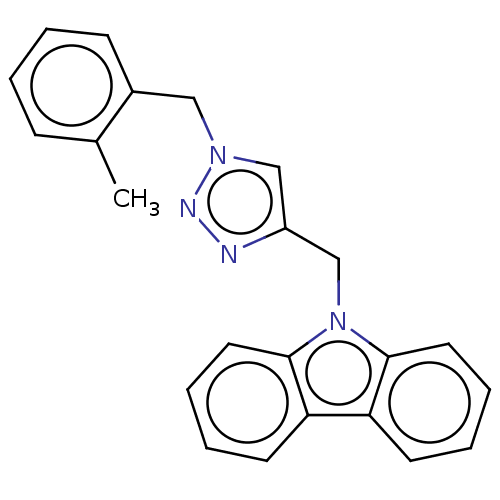
(CHEMBL4456791)Show InChI InChI=1S/C23H20N4/c1-17-8-2-3-9-18(17)14-26-15-19(24-25-26)16-27-22-12-6-4-10-20(22)21-11-5-7-13-23(21)27/h2-13,15H,14,16H2,1H3 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of human AChE using acetylthiocholine iodide as substrate after 10 secs measured at 30 s intervals for two minutes by Ellman's method |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516985
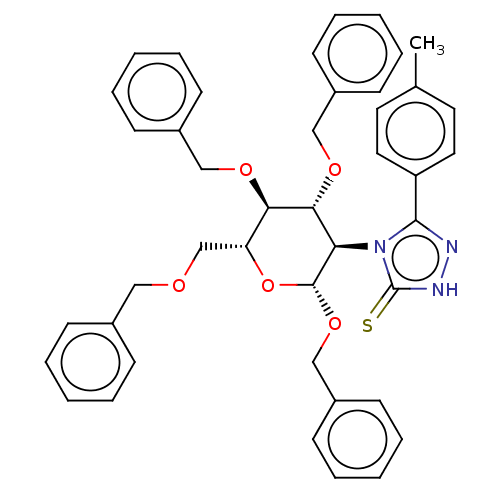
(CHEMBL4544034)Show SMILES Cc1ccc(cc1)-c1n[nH]c(=S)n1[C@H]1[C@H](OCc2ccccc2)O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C43H43N3O5S/c1-31-22-24-36(25-23-31)41-44-45-43(52)46(41)38-40(49-28-34-18-10-4-11-19-34)39(48-27-33-16-8-3-9-17-33)37(30-47-26-32-14-6-2-7-15-32)51-42(38)50-29-35-20-12-5-13-21-35/h2-25,37-40,42H,26-30H2,1H3,(H,45,52)/t37-,38-,39-,40-,42-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.10E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM10404

((1S,12S,14R)-9-methoxy-4-methyl-11-oxa-4-azatetrac...)Show SMILES COc1ccc2CN(C)CC[C@@]34C=C[C@H](O)C[C@@H]3Oc1c24 |r,c:12| Show InChI InChI=1S/C17H21NO3/c1-18-8-7-17-6-5-12(19)9-14(17)21-16-13(20-2)4-3-11(10-18)15(16)17/h3-6,12,14,19H,7-10H2,1-2H3/t12-,14-,17-/m0/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| DrugBank
Article
PubMed
| n/a | n/a | 2.66E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516966
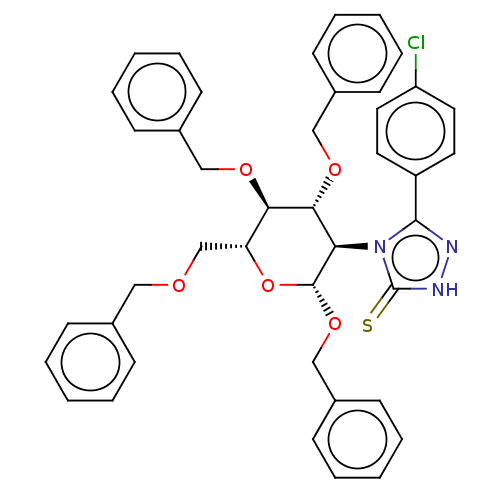
(CHEMBL4452517)Show SMILES Clc1ccc(cc1)-c1n[nH]c(=S)n1[C@H]1[C@H](OCc2ccccc2)O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C42H40ClN3O5S/c43-35-23-21-34(22-24-35)40-44-45-42(52)46(40)37-39(49-27-32-17-9-3-10-18-32)38(48-26-31-15-7-2-8-16-31)36(29-47-25-30-13-5-1-6-14-30)51-41(37)50-28-33-19-11-4-12-20-33/h1-24,36-39,41H,25-29H2,(H,45,52)/t36-,37-,38-,39-,41-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.72E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516965
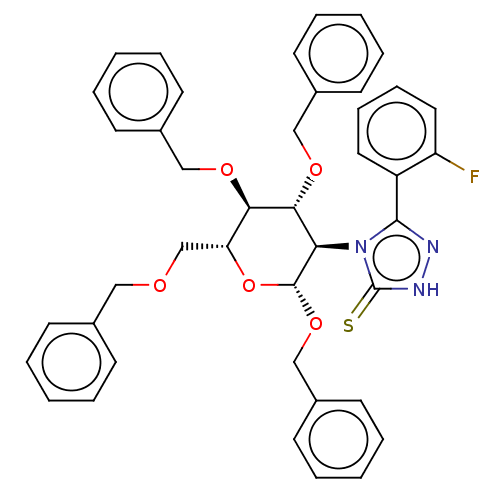
(CHEMBL4458943)Show SMILES Fc1ccccc1-c1n[nH]c(=S)n1[C@H]1[C@H](OCc2ccccc2)O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C42H40FN3O5S/c43-35-24-14-13-23-34(35)40-44-45-42(52)46(40)37-39(49-27-32-19-9-3-10-20-32)38(48-26-31-17-7-2-8-18-31)36(29-47-25-30-15-5-1-6-16-30)51-41(37)50-28-33-21-11-4-12-22-33/h1-24,36-39,41H,25-29H2,(H,45,52)/t36-,37-,38-,39-,41-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.06E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516964

(CHEMBL4573754)Show SMILES S=c1[nH]nc(-c2cccs2)n1[C@H]1[C@H](OCc2ccccc2)O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C40H39N3O5S2/c49-40-42-41-38(34-22-13-23-50-34)43(40)35-37(46-26-31-18-9-3-10-19-31)36(45-25-30-16-7-2-8-17-30)33(28-44-24-29-14-5-1-6-15-29)48-39(35)47-27-32-20-11-4-12-21-32/h1-23,33,35-37,39H,24-28H2,(H,42,49)/t33-,35-,36-,37-,39-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 6.37E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50448370
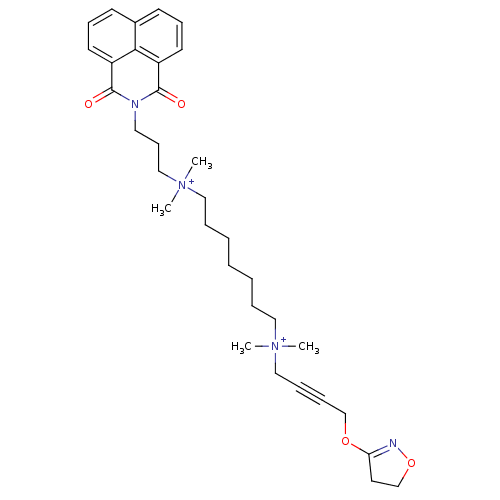
(CHEMBL3120178)Show SMILES C[N+](C)(CCCCCCC[N+](C)(C)CC#CCOC1=NOCC1)CCCN1C(=O)c2cccc3cccc(C1=O)c23 |t:18| Show InChI InChI=1S/C33H46N4O4/c1-36(2,23-10-11-25-40-30-19-26-41-34-30)21-8-6-5-7-9-22-37(3,4)24-14-20-35-32(38)28-17-12-15-27-16-13-18-29(31(27)28)33(35)39/h12-13,15-18H,5-9,14,19-26H2,1-4H3/q+2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 7.94E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay |
Eur J Med Chem 75: 222-32 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.032
BindingDB Entry DOI: 10.7270/Q2K075RG |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516989
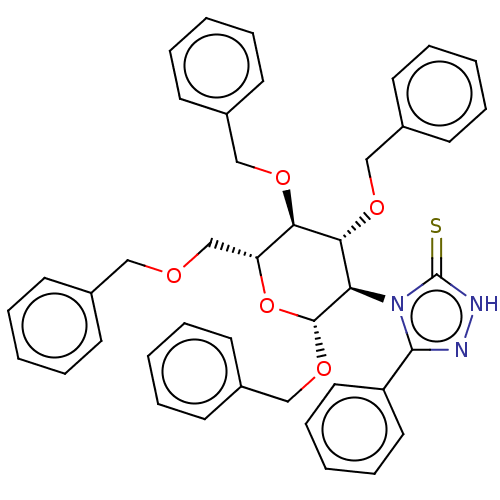
(CHEMBL4590975)Show SMILES S=c1[nH]nc(-c2ccccc2)n1[C@H]1[C@H](OCc2ccccc2)O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C42H41N3O5S/c51-42-44-43-40(35-24-14-5-15-25-35)45(42)37-39(48-28-33-20-10-3-11-21-33)38(47-27-32-18-8-2-9-19-32)36(30-46-26-31-16-6-1-7-17-31)50-41(37)49-29-34-22-12-4-13-23-34/h1-25,36-39,41H,26-30H2,(H,44,51)/t36-,37-,38-,39-,41-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524701
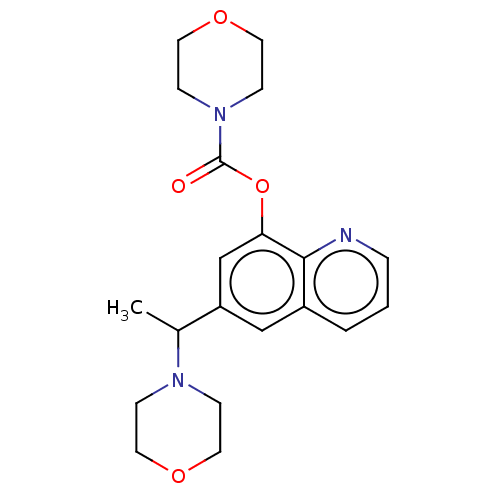
(CHEMBL4594074)Show InChI InChI=1S/C20H25N3O4/c1-15(22-5-9-25-10-6-22)17-13-16-3-2-4-21-19(16)18(14-17)27-20(24)23-7-11-26-12-8-23/h2-4,13-15H,5-12H2,1H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524708
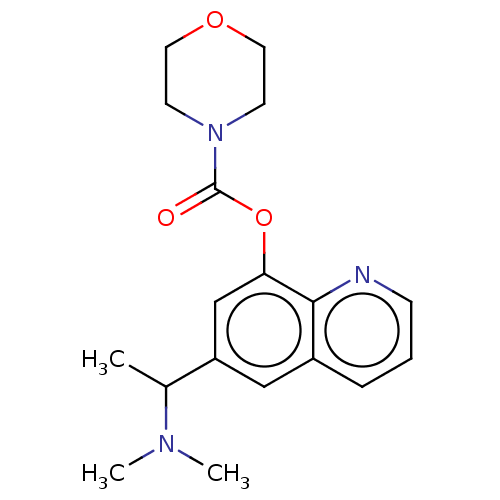
(CHEMBL4459614)Show InChI InChI=1S/C18H23N3O3/c1-13(20(2)3)15-11-14-5-4-6-19-17(14)16(12-15)24-18(22)21-7-9-23-10-8-21/h4-6,11-13H,7-10H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 8.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM11682

(2,3-dihydroxybutanedioic acid; 3-[(1S)-1-(dimethyl...)Show InChI InChI=1S/C14H22N2O2/c1-6-16(5)14(17)18-13-9-7-8-12(10-13)11(2)15(3)4/h7-11H,6H2,1-5H3/t11-/m0/s1 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
DrugBank
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50448375
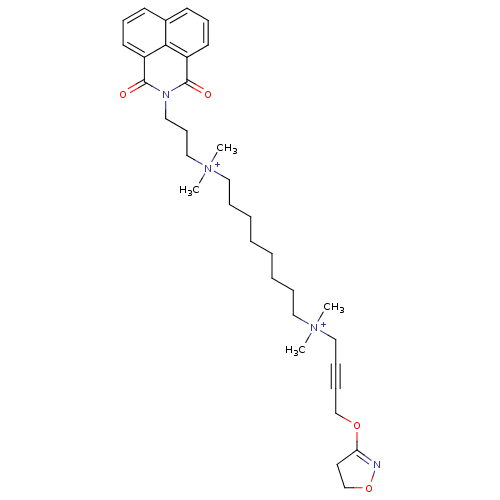
(CHEMBL3121479)Show SMILES C[N+](C)(CCCCCCCC[N+](C)(C)CC#CCOC1=NOCC1)CCCN1C(=O)c2cccc3cccc(C1=O)c23 |t:19| Show InChI InChI=1S/C34H48N4O4/c1-37(2,24-11-12-26-41-31-20-27-42-35-31)22-9-7-5-6-8-10-23-38(3,4)25-15-21-36-33(39)29-18-13-16-28-17-14-19-30(32(28)29)34(36)40/h13-14,16-19H,5-10,15,20-27H2,1-4H3/q+2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay |
Eur J Med Chem 75: 222-32 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.032
BindingDB Entry DOI: 10.7270/Q2K075RG |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50448374
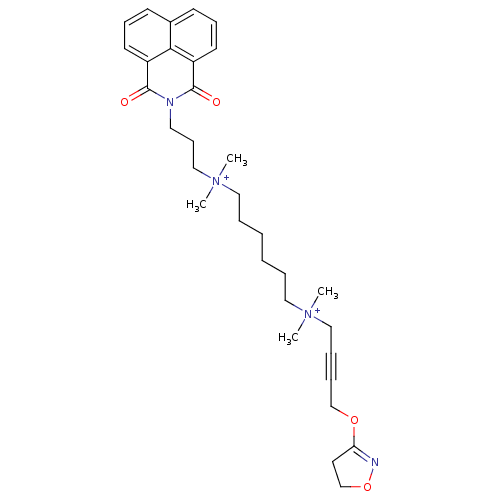
(CHEMBL3121478)Show SMILES C[N+](C)(CCCCCC[N+](C)(C)CC#CCOC1=NOCC1)CCCN1C(=O)c2cccc3cccc(C1=O)c23 |t:17| Show InChI InChI=1S/C32H44N4O4/c1-35(2,22-9-10-24-39-29-18-25-40-33-29)20-7-5-6-8-21-36(3,4)23-13-19-34-31(37)27-16-11-14-26-15-12-17-28(30(26)27)32(34)38/h11-12,14-17H,5-8,13,18-25H2,1-4H3/q+2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.02E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay |
Eur J Med Chem 75: 222-32 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.032
BindingDB Entry DOI: 10.7270/Q2K075RG |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Homo sapiens (Human)) | BDBM50516949
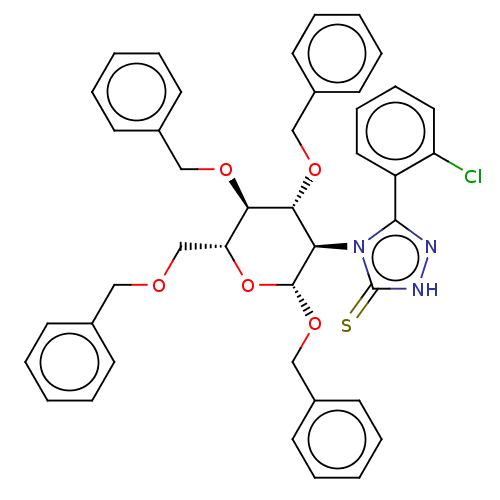
(CHEMBL4555431)Show SMILES Clc1ccccc1-c1n[nH]c(=S)n1[C@H]1[C@H](OCc2ccccc2)O[C@H](COCc2ccccc2)[C@@H](OCc2ccccc2)[C@@H]1OCc1ccccc1 |r| Show InChI InChI=1S/C42H40ClN3O5S/c43-35-24-14-13-23-34(35)40-44-45-42(52)46(40)37-39(49-27-32-19-9-3-10-20-32)38(48-26-31-17-7-2-8-18-31)36(29-47-25-30-15-5-1-6-16-30)51-41(37)50-28-33-21-11-4-12-22-33/h1-24,36-39,41H,25-29H2,(H,45,52)/t36-,37-,38-,39-,41-/m1/s1 | NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.23E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Wuhan Institute of Technology
Curated by ChEMBL
| Assay Description
Inhibition of AChE (unknown origin) |
Eur J Med Chem 180: 656-672 (2019)
Article DOI: 10.1016/j.ejmech.2019.07.059
BindingDB Entry DOI: 10.7270/Q22B92D9 |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524697

(CHEMBL4470950)Show InChI InChI=1S/C18H25N3O2/c1-6-21(7-2)18(22)23-16-12-15(13(3)20(4)5)11-14-9-8-10-19-17(14)16/h8-13H,6-7H2,1-5H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.36E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524707

(CHEMBL4468053)Show InChI InChI=1S/C16H21N3O2/c1-11(18(2)3)13-9-12-7-6-8-17-15(12)14(10-13)21-16(20)19(4)5/h6-11H,1-5H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.63E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524705

(CHEMBL4452617)Show InChI InChI=1S/C19H25N3O2/c1-4-21(3)19(23)24-17-13-16(14(2)22-10-5-6-11-22)12-15-8-7-9-20-18(15)17/h7-9,12-14H,4-6,10-11H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 1.81E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50448369
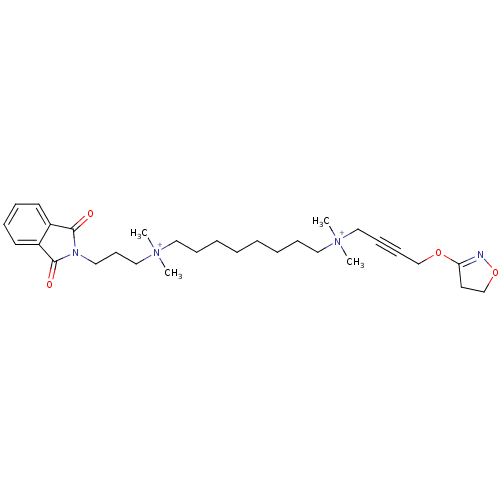
(CHEMBL3121476)Show SMILES C[N+](C)(CCCCCCCC[N+](C)(C)CC#CCOC1=NOCC1)CCCN1C(=O)c2ccccc2C1=O |t:19| Show InChI InChI=1S/C30H46N4O4/c1-33(2,22-13-14-24-37-28-18-25-38-31-28)20-11-7-5-6-8-12-21-34(3,4)23-15-19-32-29(35)26-16-9-10-17-27(26)30(32)36/h9-10,16-17H,5-8,11-12,15,18-25H2,1-4H3/q+2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.95E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay |
Eur J Med Chem 75: 222-32 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.032
BindingDB Entry DOI: 10.7270/Q2K075RG |
More data for this
Ligand-Target Pair | |
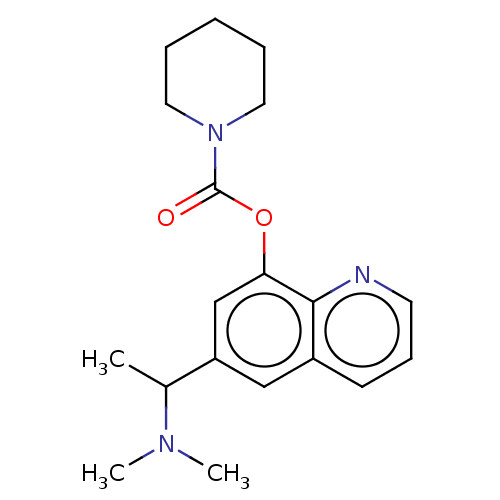
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524698
(CHEMBL4452986)Show InChI InChI=1S/C19H25N3O2/c1-14(21(2)3)16-12-15-8-7-9-20-18(15)17(13-16)24-19(23)22-10-5-4-6-11-22/h7-9,12-14H,4-6,10-11H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.06E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
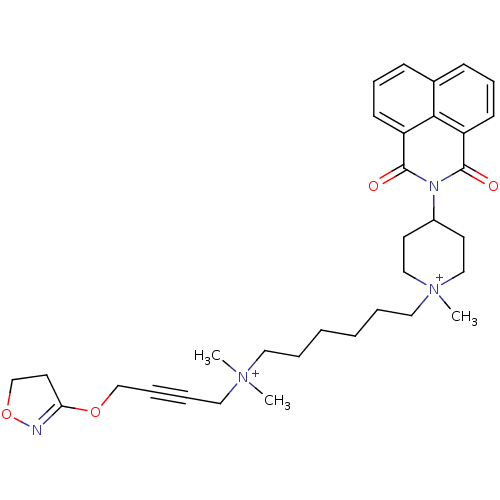
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50448371
(CHEMBL3121480)Show SMILES C[N+](C)(CCCCCC[N+]1(C)CCC(CC1)N1C(=O)c2cccc3cccc(C1=O)c23)CC#CCOC1=NOCC1 |t:40,(26.01,-22.81,;26.76,-24.15,;27.54,-22.83,;25.23,-24.16,;24.45,-22.84,;22.91,-22.84,;22.13,-21.52,;20.59,-21.53,;19.81,-20.19,;18.27,-20.2,;19.03,-18.86,;17.49,-18.87,;15.95,-18.88,;15.19,-20.22,;15.97,-21.55,;17.51,-21.54,;13.65,-20.23,;12.88,-18.89,;13.64,-17.57,;11.35,-18.91,;10.58,-17.58,;9.05,-17.57,;8.28,-18.91,;9.04,-20.25,;8.28,-21.58,;9.06,-22.91,;10.59,-22.9,;11.35,-21.57,;12.9,-21.56,;13.67,-22.88,;10.58,-20.24,;27.53,-25.5,;29.07,-25.51,;30.61,-25.5,;32.15,-25.5,;32.92,-26.83,;34.46,-26.82,;35.38,-28.05,;36.84,-27.57,;36.83,-26.03,;35.37,-25.56,)| Show InChI InChI=1S/C33H44N4O4/c1-36(2,20-8-9-24-40-30-18-25-41-34-30)19-6-4-5-7-21-37(3)22-16-27(17-23-37)35-32(38)28-14-10-12-26-13-11-15-29(31(26)28)33(35)39/h10-15,27H,4-7,16-25H2,1-3H3/q+2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay |
Eur J Med Chem 75: 222-32 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.032
BindingDB Entry DOI: 10.7270/Q2K075RG |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524706

(CHEMBL4566337)Show InChI InChI=1S/C18H23N3O2/c1-13(20(2)3)15-11-14-7-6-8-19-17(14)16(12-15)23-18(22)21-9-4-5-10-21/h6-8,11-13H,4-5,9-10H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.86E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524703
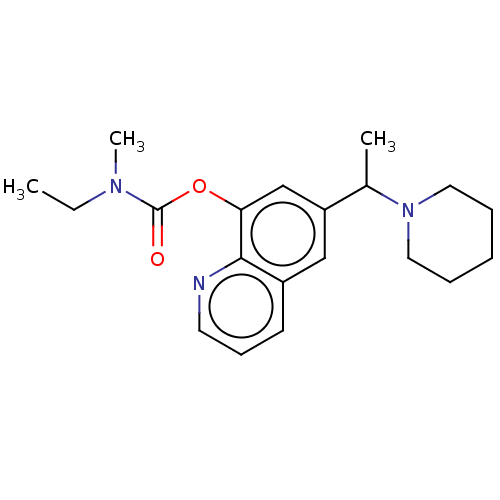
(CHEMBL4443818)Show InChI InChI=1S/C20H27N3O2/c1-4-22(3)20(24)25-18-14-17(13-16-9-8-10-21-19(16)18)15(2)23-11-6-5-7-12-23/h8-10,13-15H,4-7,11-12H2,1-3H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50448372
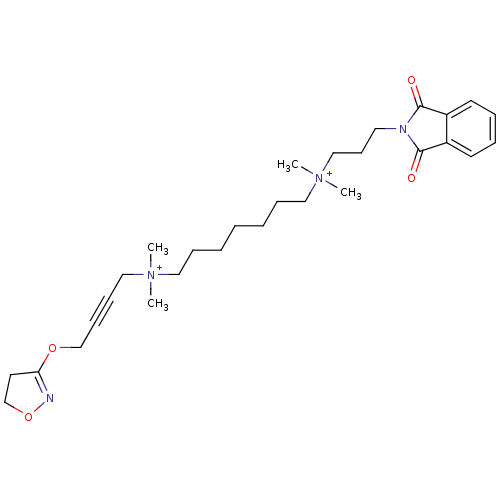
(CHEMBL3121475)Show SMILES C[N+](C)(CCCCCCC[N+](C)(C)CC#CCOC1=NOCC1)CCCN1C(=O)c2ccccc2C1=O |t:18| Show InChI InChI=1S/C29H44N4O4/c1-32(2,21-12-13-23-36-27-17-24-37-30-27)19-10-6-5-7-11-20-33(3,4)22-14-18-31-28(34)25-15-8-9-16-26(25)29(31)35/h8-9,15-16H,5-7,10-11,14,17-24H2,1-4H3/q+2 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.16E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Universit£ degli Studi di Milano
Curated by ChEMBL
| Assay Description
Inhibition of acetylcholinesterase in rat brain homogenate after 15 mins by Ellman assay |
Eur J Med Chem 75: 222-32 (2014)
Article DOI: 10.1016/j.ejmech.2014.01.032
BindingDB Entry DOI: 10.7270/Q2K075RG |
More data for this
Ligand-Target Pair | |
Acetylcholine receptor subunit epsilon
(Rattus norvegicus) | BDBM50524709
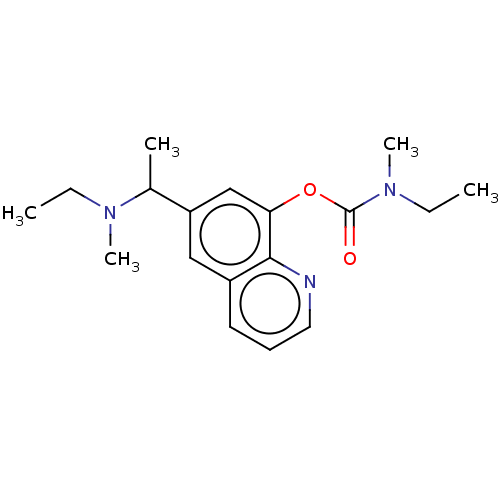
(CHEMBL4471688)Show InChI InChI=1S/C18H25N3O2/c1-6-20(4)13(3)15-11-14-9-8-10-19-17(14)16(12-15)23-18(22)21(5)7-2/h8-13H,6-7H2,1-5H3 | KEGG
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 4.07E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Zhejiang Academy of Medical Sciences
Curated by ChEMBL
| Assay Description
Inhibition of rat brain cortex AChE using acetylthiocholine iodide as substrate incubated for 15 mins by spectrophotometry |
Eur J Med Chem 177: 247-258 (2019)
Article DOI: 10.1016/j.ejmech.2019.05.051
BindingDB Entry DOI: 10.7270/Q24M980S |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data