 Found 34 hits of ic50 data for polymerid = 5692
Found 34 hits of ic50 data for polymerid = 5692 Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50306253

(1-(2-(pyridin-3-yl)acetyl)-2,3,9,9a-tetrahydro-1H-...)Show InChI InChI=1S/C18H17N3O2/c22-16(9-12-3-2-7-19-10-12)21-8-6-13-4-1-5-14-17(13)15(21)11-20-18(14)23/h1-5,7,10,15H,6,8-9,11H2,(H,20,23) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 50 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of PARP3 |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM206061
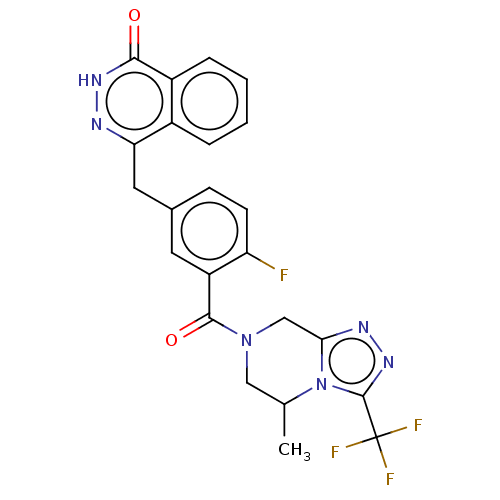
(US9255106, S3)Show SMILES CC1CN(Cc2nnc(n12)C(F)(F)F)C(=O)c1cc(Cc2n[nH]c(=O)c3ccccc23)ccc1F | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
| US Patent
| n/a | n/a | 66.9 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
US Patent
| Assay Description
In order to test the selectivity of substituents on piperazinotriazole ring within the PARP family, the selectivity of compound S3 and positive compo... |
US Patent US9255106 (2016)
BindingDB Entry DOI: 10.7270/Q2610Z5N |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50306256
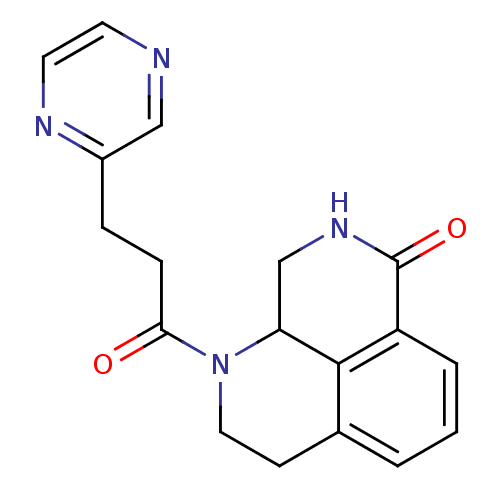
(1-(3-(pyrazin-2-yl)propanoyl)-2,3,9,9a-tetrahydro-...)Show InChI InChI=1S/C18H18N4O2/c23-16(5-4-13-10-19-7-8-20-13)22-9-6-12-2-1-3-14-17(12)15(22)11-21-18(14)24/h1-3,7-8,10,15H,4-6,9,11H2,(H,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 70 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM-Merck Research Laboratories Rome
Curated by ChEMBL
| Assay Description
Inhibition of PARP3 |
Bioorg Med Chem Lett 20: 448-52 (2010)
Article DOI: 10.1016/j.bmcl.2009.12.002
BindingDB Entry DOI: 10.7270/Q2GT5N91 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM27566

(4-({3-[(4-cyclopropanecarbonylpiperazin-1-yl)carbo...)Show SMILES Fc1ccc(Cc2n[nH]c(=O)c3ccccc23)cc1C(=O)N1CCN(CC1)C(=O)C1CC1 Show InChI InChI=1S/C24H23FN4O3/c25-20-8-5-15(14-21-17-3-1-2-4-18(17)22(30)27-26-21)13-19(20)24(32)29-11-9-28(10-12-29)23(31)16-6-7-16/h1-5,8,13,16H,6-7,9-12,14H2,(H,27,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| US Patent
| n/a | n/a | 320 | n/a | n/a | n/a | n/a | n/a | n/a |
Shanghai Institute of Materia Medica, Chinese Academy of Sciences
US Patent
| Assay Description
In order to test the selectivity of substituents on piperazinotriazole ring within the PARP family, the selectivity of compound S3 and positive compo... |
US Patent US9255106 (2016)
BindingDB Entry DOI: 10.7270/Q2610Z5N |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103558
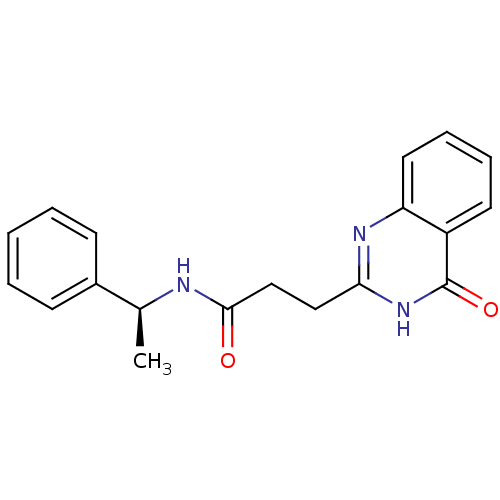
(ME0328)Show SMILES C[C@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C19H19N3O2/c1-13(14-7-3-2-4-8-14)20-18(23)12-11-17-21-16-10-6-5-9-15(16)19(24)22-17/h2-10,13H,11-12H2,1H3,(H,20,23)(H,21,22,24)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 890 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
| Assay Description
Experiments todetermine IC50 values were conducted with compound concentrationsin the range between 10 nM and 450 μM with a DMSO concentrationof... |
ACS Chem Biol 8: 1698-703 (2013)
Article DOI: 10.1021/cb4002014
BindingDB Entry DOI: 10.7270/Q2668BS1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103558

(ME0328)Show SMILES C[C@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C19H19N3O2/c1-13(14-7-3-2-4-8-14)20-18(23)12-11-17-21-16-10-6-5-9-15(16)19(24)22-17/h2-10,13H,11-12H2,1H3,(H,20,23)(H,21,22,24)/t13-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444033
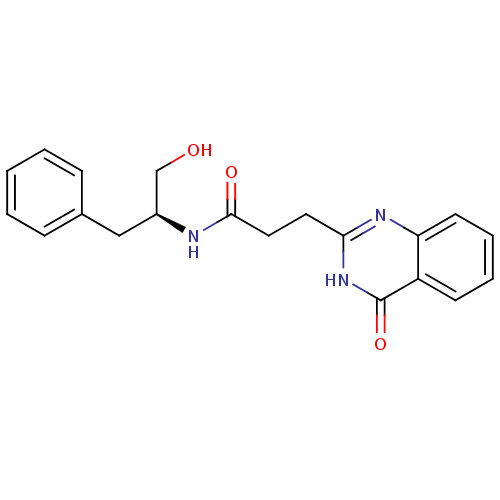
(CHEMBL3092553)Show SMILES OC[C@H](Cc1ccccc1)NC(=O)CCc1nc2ccccc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H21N3O3/c24-13-15(12-14-6-2-1-3-7-14)21-19(25)11-10-18-22-17-9-5-4-8-16(17)20(26)23-18/h1-9,15,24H,10-13H2,(H,21,25)(H,22,23,26)/t15-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103556
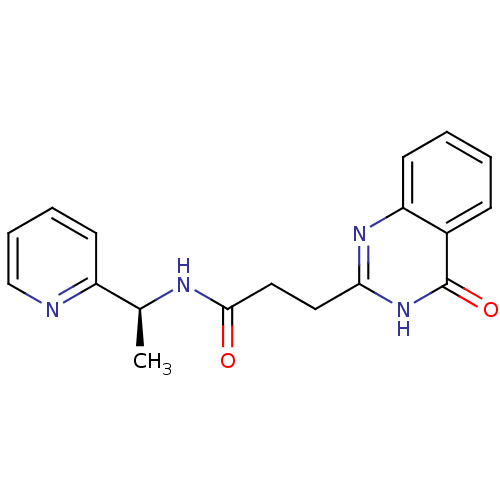
(ME0355)Show SMILES C[C@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccn1 |r| Show InChI InChI=1S/C18H18N4O2/c1-12(14-7-4-5-11-19-14)20-17(23)10-9-16-21-15-8-3-2-6-13(15)18(24)22-16/h2-8,11-12H,9-10H2,1H3,(H,20,23)(H,21,22,24)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50316226
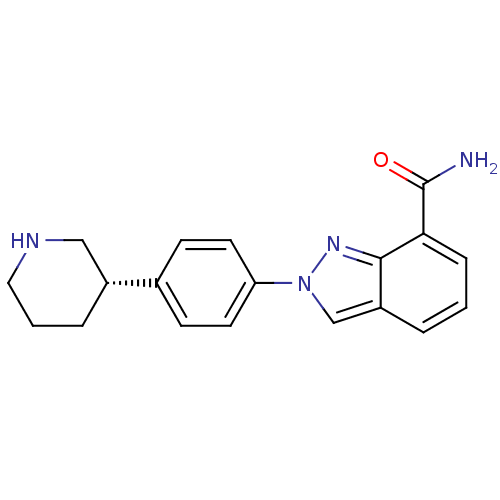
((S)-2-(4-(piperidin-3-yl)phenyl)-2H-indazole-7-car...)Show SMILES NC(=O)c1cccc2cn(nc12)-c1ccc(cc1)[C@@H]1CCCNC1 |r| Show InChI InChI=1S/C19H20N4O/c20-19(24)17-5-1-3-15-12-23(22-18(15)17)16-8-6-13(7-9-16)14-4-2-10-21-11-14/h1,3,5-9,12,14,21H,2,4,10-11H2,(H2,20,24)/t14-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
IRBM/Merck Research Labs Rome
Curated by ChEMBL
| Assay Description
Inhibition of human PARP3 by trichloroacetic acid precipitation assay |
J Med Chem 52: 7170-85 (2009)
Article DOI: 10.1021/jm901188v
BindingDB Entry DOI: 10.7270/Q2DN457M |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103556

(ME0355)Show SMILES C[C@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccn1 |r| Show InChI InChI=1S/C18H18N4O2/c1-12(14-7-4-5-11-19-14)20-17(23)10-9-16-21-15-8-3-2-6-13(15)18(24)22-16/h2-8,11-12H,9-10H2,1H3,(H,20,23)(H,21,22,24)/t12-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
| Assay Description
Experiments todetermine IC50 values were conducted with compound concentrationsin the range between 10 nM and 450 μM with a DMSO concentrationof... |
ACS Chem Biol 8: 1698-703 (2013)
Article DOI: 10.1021/cb4002014
BindingDB Entry DOI: 10.7270/Q2668BS1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444034
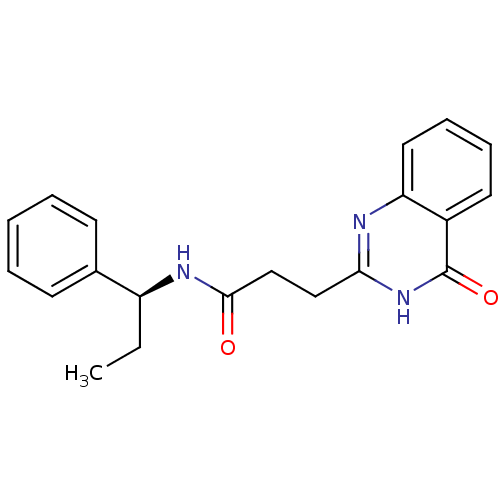
(CHEMBL3092544)Show SMILES CC[C@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C20H21N3O2/c1-2-16(14-8-4-3-5-9-14)22-19(24)13-12-18-21-17-11-7-6-10-15(17)20(25)23-18/h3-11,16H,2,12-13H2,1H3,(H,22,24)(H,21,23,25)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444027
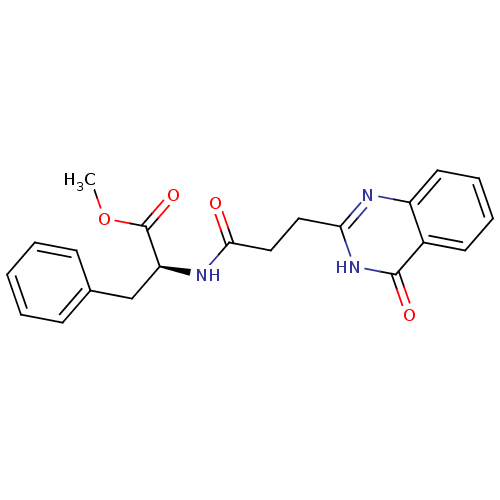
(CHEMBL3092523)Show SMILES COC(=O)[C@H](Cc1ccccc1)NC(=O)CCc1nc2ccccc2c(=O)[nH]1 |r| Show InChI InChI=1S/C21H21N3O4/c1-28-21(27)17(13-14-7-3-2-4-8-14)23-19(25)12-11-18-22-16-10-6-5-9-15(16)20(26)24-18/h2-10,17H,11-13H2,1H3,(H,23,25)(H,22,24,26)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 2.20E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444028
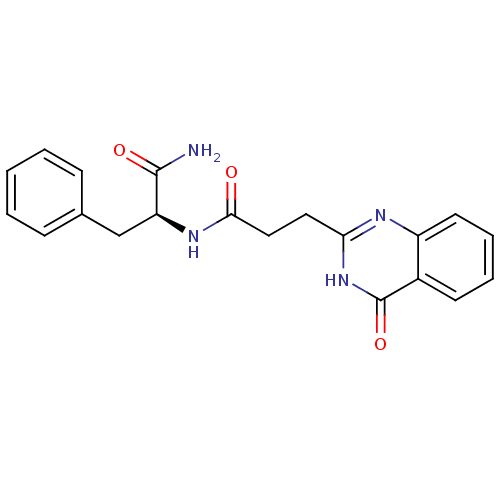
(CHEMBL3092522)Show SMILES NC(=O)[C@H](Cc1ccccc1)NC(=O)CCc1nc2ccccc2c(=O)[nH]1 |r| Show InChI InChI=1S/C20H20N4O3/c21-19(26)16(12-13-6-2-1-3-7-13)23-18(25)11-10-17-22-15-9-5-4-8-14(15)20(27)24-17/h1-9,16H,10-12H2,(H2,21,26)(H,23,25)(H,22,24,27)/t16-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.80E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103554
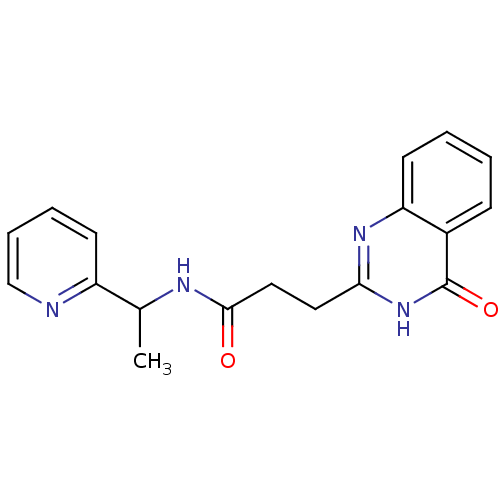
(STO1131 | US8551988, 39)Show InChI InChI=1S/C18H18N4O2/c1-12(14-7-4-5-11-19-14)20-17(23)10-9-16-21-15-8-3-2-6-13(15)18(24)22-16/h2-8,11-12H,9-10H2,1H3,(H,20,23)(H,21,22,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 6.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
| Assay Description
Experiments todetermine IC50 values were conducted with compound concentrationsin the range between 10 nM and 450 μM with a DMSO concentrationof... |
ACS Chem Biol 8: 1698-703 (2013)
Article DOI: 10.1021/cb4002014
BindingDB Entry DOI: 10.7270/Q2668BS1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444029
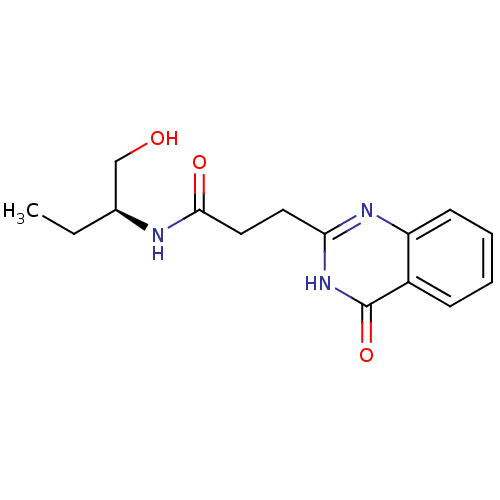
(CHEMBL3092521)Show SMILES CC[C@@H](CO)NC(=O)CCc1nc2ccccc2c(=O)[nH]1 |r| Show InChI InChI=1S/C15H19N3O3/c1-2-10(9-19)16-14(20)8-7-13-17-12-6-4-3-5-11(12)15(21)18-13/h3-6,10,19H,2,7-9H2,1H3,(H,16,20)(H,17,18,21)/t10-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 9.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50434127
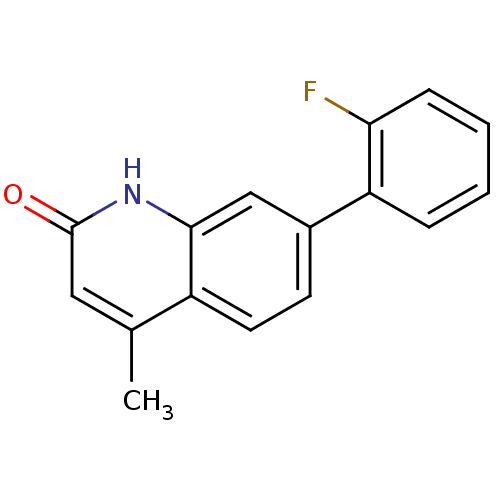
(CHEMBL2381633)Show InChI InChI=1S/C16H12FNO/c1-10-8-16(19)18-15-9-11(6-7-12(10)15)13-4-2-3-5-14(13)17/h2-9H,1H3,(H,18,19) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University
Curated by ChEMBL
| Assay Description
Inhibition of PARP3 (unknown origin) |
J Med Chem 56: 4497-508 (2013)
Article DOI: 10.1021/jm400211f
BindingDB Entry DOI: 10.7270/Q23R0V82 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50434157
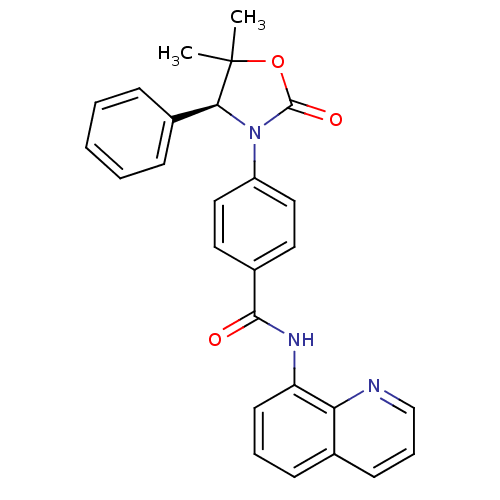
(CHEMBL2381958 | US9340549, 78)Show SMILES CC1(C)OC(=O)N([C@H]1c1ccccc1)c1ccc(cc1)C(=O)Nc1cccc2cccnc12 |r| Show InChI InChI=1S/C27H23N3O3/c1-27(2)24(19-8-4-3-5-9-19)30(26(32)33-27)21-15-13-20(14-16-21)25(31)29-22-12-6-10-18-11-7-17-28-23(18)22/h3-17,24H,1-2H3,(H,29,31)/t24-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Amgen Inc.
Curated by ChEMBL
| Assay Description
Inhibition of PARP3 (unknown origin) using histone as substrate after 1 hr by luminescence assay |
J Med Chem 56: 4320-42 (2013)
Article DOI: 10.1021/jm4000038
BindingDB Entry DOI: 10.7270/Q2QF8V74 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50318567
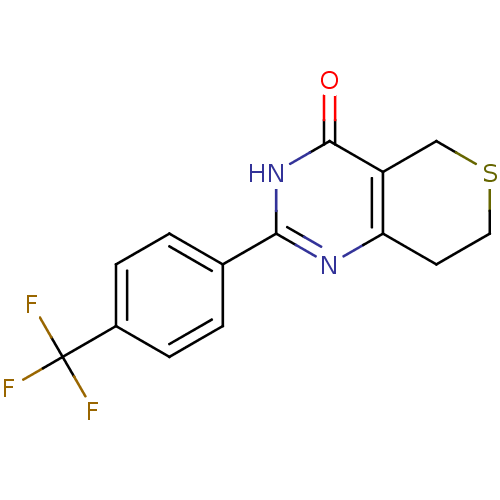
(2-(4-(Trifluoromethyl)phenyl)-7,8-dihydro-5H-thiop...)Show InChI InChI=1S/C14H11F3N2OS/c15-14(16,17)9-3-1-8(2-4-9)12-18-11-5-6-21-7-10(11)13(20)19-12/h1-4H,5-7H2,(H,18,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
MMDB
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Nanyang Technological University
Curated by ChEMBL
| Assay Description
Inhibition of PARP3 (unknown origin) |
J Med Chem 56: 4497-508 (2013)
Article DOI: 10.1021/jm400211f
BindingDB Entry DOI: 10.7270/Q23R0V82 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444030
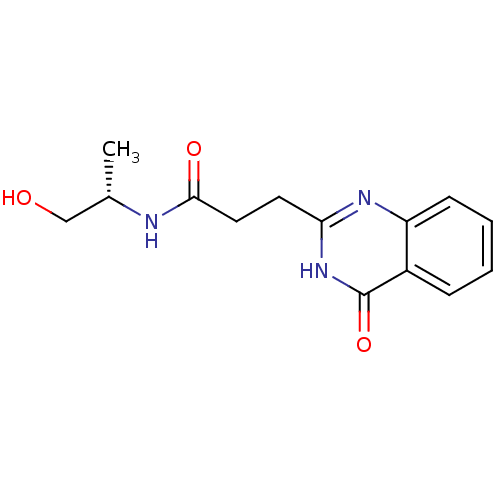
(CHEMBL3092520)Show SMILES C[C@@H](CO)NC(=O)CCc1nc2ccccc2c(=O)[nH]1 |r| Show InChI InChI=1S/C14H17N3O3/c1-9(8-18)15-13(19)7-6-12-16-11-5-3-2-4-10(11)14(20)17-12/h2-5,9,18H,6-8H2,1H3,(H,15,19)(H,16,17,20)/t9-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.11E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50104286
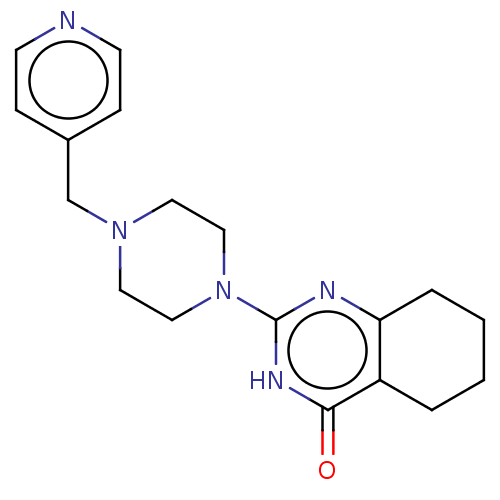
(CHEMBL3593715)Show InChI InChI=1S/C18H23N5O/c24-17-15-3-1-2-4-16(15)20-18(21-17)23-11-9-22(10-12-23)13-14-5-7-19-8-6-14/h5-8H,1-4,9-13H2,(H,20,21,24) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of full-length human ARTD3 using 500 nM NAD substrate after 1 hrs incubation |
Bioorg Med Chem 23: 4139-49 (2015)
Article DOI: 10.1016/j.bmc.2015.06.063
BindingDB Entry DOI: 10.7270/Q2W66NJ2 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444031
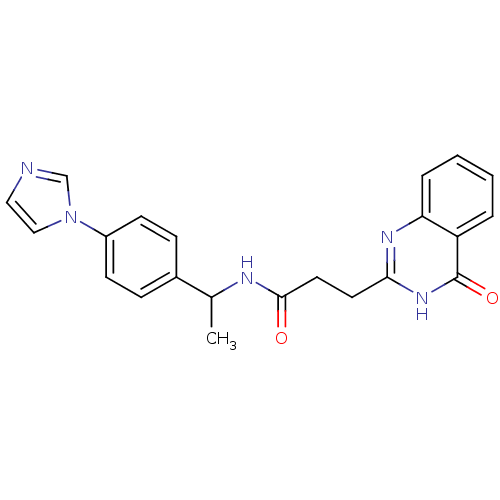
(CHEMBL3092518)Show SMILES CC(NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccc(cc1)-n1ccnc1 Show InChI InChI=1S/C22H21N5O2/c1-15(16-6-8-17(9-7-16)27-13-12-23-14-27)24-21(28)11-10-20-25-19-5-3-2-4-18(19)22(29)26-20/h2-9,12-15H,10-11H2,1H3,(H,24,28)(H,25,26,29) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.32E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444032
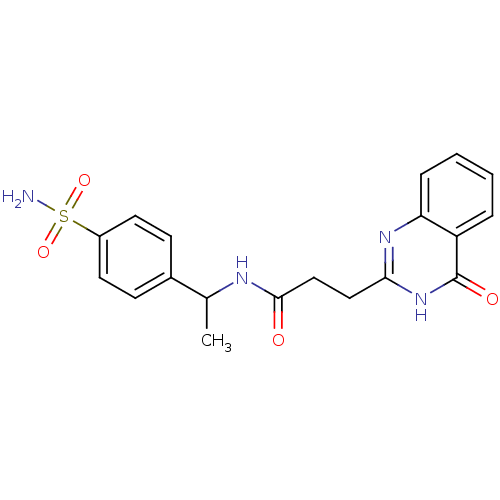
(CHEMBL3092517)Show SMILES CC(NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccc(cc1)S(N)(=O)=O Show InChI InChI=1S/C19H20N4O4S/c1-12(13-6-8-14(9-7-13)28(20,26)27)21-18(24)11-10-17-22-16-5-3-2-4-15(16)19(25)23-17/h2-9,12H,10-11H2,1H3,(H,21,24)(H2,20,26,27)(H,22,23,25) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
| PDB
Article
PubMed
| n/a | n/a | 1.44E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103557
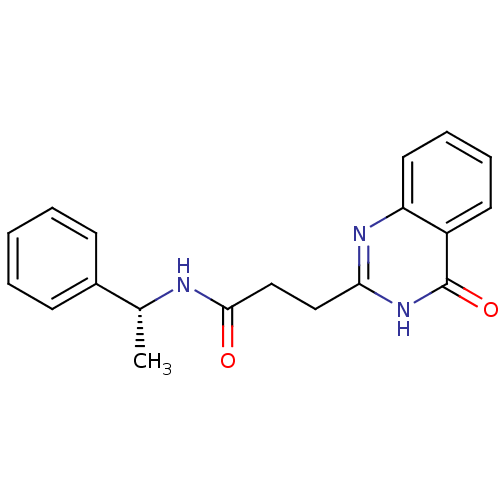
(ME0327)Show SMILES C[C@@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C19H19N3O2/c1-13(14-7-3-2-4-8-14)20-18(23)12-11-17-21-16-10-6-5-9-15(16)19(24)22-17/h2-10,13H,11-12H2,1H3,(H,20,23)(H,21,22,24)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.52E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103557

(ME0327)Show SMILES C[C@@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccc1 |r| Show InChI InChI=1S/C19H19N3O2/c1-13(14-7-3-2-4-8-14)20-18(23)12-11-17-21-16-10-6-5-9-15(16)19(24)22-17/h2-10,13H,11-12H2,1H3,(H,20,23)(H,21,22,24)/t13-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.53E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
| Assay Description
Experiments todetermine IC50 values were conducted with compound concentrationsin the range between 10 nM and 450 μM with a DMSO concentrationof... |
ACS Chem Biol 8: 1698-703 (2013)
Article DOI: 10.1021/cb4002014
BindingDB Entry DOI: 10.7270/Q2668BS1 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50104288
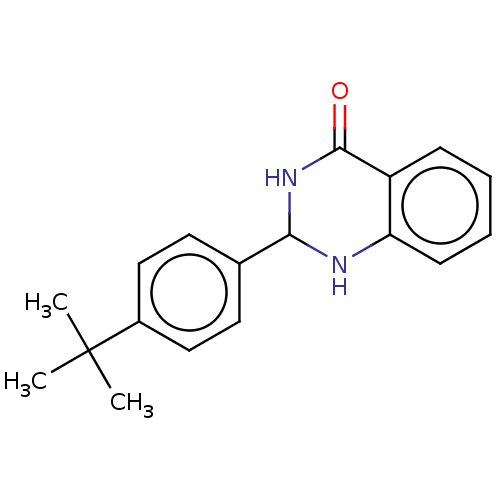
(CHEMBL3594137)Show InChI InChI=1S/C18H20N2O/c1-18(2,3)13-10-8-12(9-11-13)16-19-15-7-5-4-6-14(15)17(21)20-16/h4-11,16,19H,1-3H3,(H,20,21) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 3.40E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of full-length human ARTD3 using 500 nM NAD substrate after 1 hrs incubation |
Bioorg Med Chem 23: 4139-49 (2015)
Article DOI: 10.1016/j.bmc.2015.06.063
BindingDB Entry DOI: 10.7270/Q2W66NJ2 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50104287
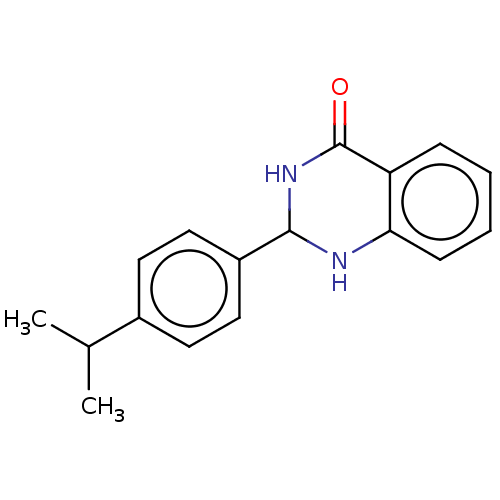
(CHEMBL3594136)Show InChI InChI=1S/C17H18N2O/c1-11(2)12-7-9-13(10-8-12)16-18-15-6-4-3-5-14(15)17(20)19-16/h3-11,16,18H,1-2H3,(H,19,20) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 5.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of full-length human ARTD3 using 500 nM NAD substrate after 1 hrs incubation |
Bioorg Med Chem 23: 4139-49 (2015)
Article DOI: 10.1016/j.bmc.2015.06.063
BindingDB Entry DOI: 10.7270/Q2W66NJ2 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50104285
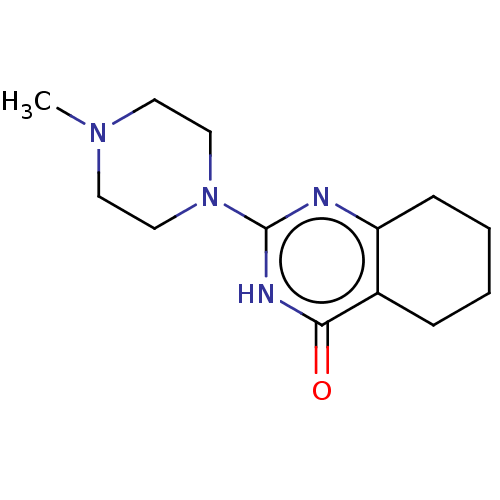
(CHEMBL3593716)Show InChI InChI=1S/C13H20N4O/c1-16-6-8-17(9-7-16)13-14-11-5-3-2-4-10(11)12(18)15-13/h2-9H2,1H3,(H,14,15,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | 9.10E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of full-length human ARTD3 using 500 nM NAD substrate after 1 hrs incubation |
Bioorg Med Chem 23: 4139-49 (2015)
Article DOI: 10.1016/j.bmc.2015.06.063
BindingDB Entry DOI: 10.7270/Q2W66NJ2 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50136755
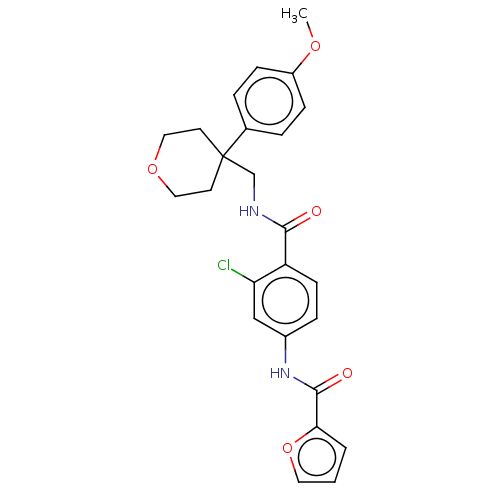
(CHEMBL3752386)Show SMILES COc1ccc(cc1)C1(CNC(=O)c2ccc(NC(=O)c3ccco3)cc2Cl)CCOCC1 Show InChI InChI=1S/C25H25ClN2O5/c1-31-19-7-4-17(5-8-19)25(10-13-32-14-11-25)16-27-23(29)20-9-6-18(15-21(20)26)28-24(30)22-3-2-12-33-22/h2-9,12,15H,10-11,13-14,16H2,1H3,(H,27,29)(H,28,30) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of human ARTD3 by fluorescence analysis |
Bioorg Med Chem Lett 26: 328-33 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.018
BindingDB Entry DOI: 10.7270/Q20003ZD |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103555
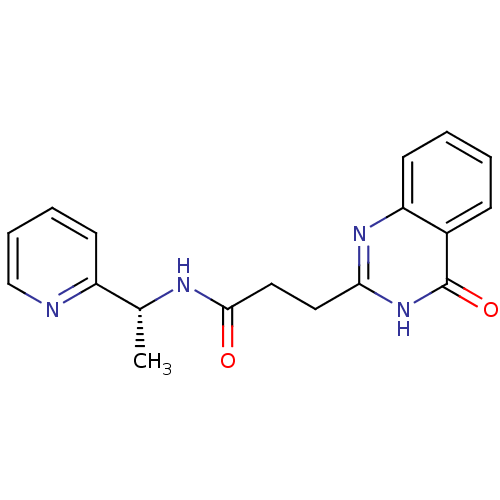
(ME0354)Show SMILES C[C@@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccn1 |r| Show InChI InChI=1S/C18H18N4O2/c1-12(14-7-4-5-11-19-14)20-17(23)10-9-16-21-15-8-3-2-6-13(15)18(24)22-16/h2-8,11-12H,9-10H2,1H3,(H,20,23)(H,21,22,24)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
| Assay Description
Experiments todetermine IC50 values were conducted with compound concentrationsin the range between 10 nM and 450 μM with a DMSO concentrationof... |
ACS Chem Biol 8: 1698-703 (2013)
Article DOI: 10.1021/cb4002014
BindingDB Entry DOI: 10.7270/Q2668BS1 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM103555

(ME0354)Show SMILES C[C@@H](NC(=O)CCc1nc2ccccc2c(=O)[nH]1)c1ccccn1 |r| Show InChI InChI=1S/C18H18N4O2/c1-12(14-7-4-5-11-19-14)20-17(23)10-9-16-21-15-8-3-2-6-13(15)18(24)22-16/h2-8,11-12H,9-10H2,1H3,(H,20,23)(H,21,22,24)/t12-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MMDB
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444036
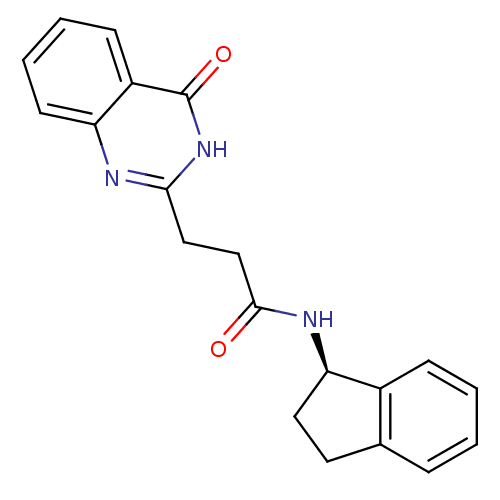
(CHEMBL3092540)Show SMILES O=C(CCc1nc2ccccc2c(=O)[nH]1)N[C@@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C20H19N3O2/c24-19(22-17-10-9-13-5-1-2-6-14(13)17)12-11-18-21-16-8-4-3-7-15(16)20(25)23-18/h1-8,17H,9-12H2,(H,22,24)(H,21,23,25)/t17-/m1/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50136772

(CHEMBL3752492)Show SMILES COc1ccc(cc1)C(=O)Nc1ccc(cc1)C(=O)N(c1ccncn1)c1ccccc1Cl Show InChI InChI=1S/C25H19ClN4O3/c1-33-20-12-8-17(9-13-20)24(31)29-19-10-6-18(7-11-19)25(32)30(23-14-15-27-16-28-23)22-5-3-2-4-21(22)26/h2-16H,1H3,(H,29,31) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
Curated by ChEMBL
| Assay Description
Inhibition of human ARTD3 by fluorescence analysis |
Bioorg Med Chem Lett 26: 328-33 (2016)
Article DOI: 10.1016/j.bmcl.2015.12.018
BindingDB Entry DOI: 10.7270/Q20003ZD |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM199181
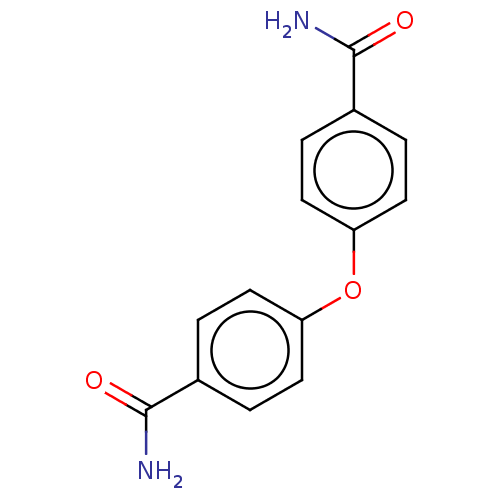
(4-[(4-Carbamoylcyclohexyl)oxy]cyclohexane-1-carbox...)Show InChI InChI=1S/C14H12N2O3/c15-13(17)9-1-5-11(6-2-9)19-12-7-3-10(4-8-12)14(16)18/h1-8H,(H2,15,17)(H2,16,18) | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
PDB
UniChem
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Oulu
| Assay Description
This assay measures the substrate consumption by quantifying the remaining NAD+ through its chemical conversion to a stable fluorescent condensation ... |
Cell Chem Biol 23: 1251-1260 (2016)
Article DOI: 10.1016/j.chembiol.2016.08.012
BindingDB Entry DOI: 10.7270/Q2B56HJ3 |
More data for this
Ligand-Target Pair | |
Protein mono-ADP-ribosyltransferase PARP3 [R100H]
(Homo sapiens (Human)) | BDBM50444035
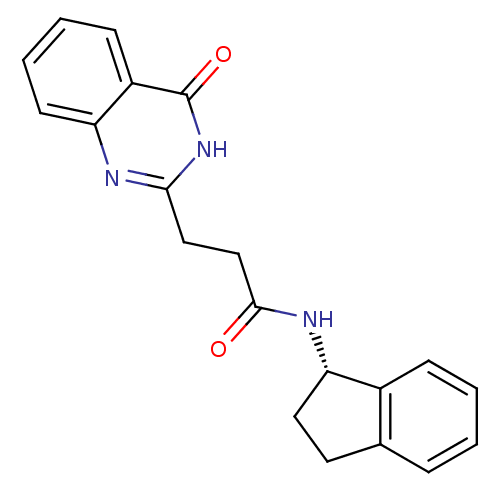
(CHEMBL3092541)Show SMILES O=C(CCc1nc2ccccc2c(=O)[nH]1)N[C@H]1CCc2ccccc12 |r| Show InChI InChI=1S/C20H19N3O2/c24-19(22-17-10-9-13-5-1-2-6-14(13)17)12-11-18-21-16-8-4-3-7-15(16)20(25)23-18/h1-8,17H,9-12H2,(H,22,24)(H,21,23,25)/t17-/m0/s1 | PDB
MMDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
GoogleScholar
AffyNet 
| Purchase
CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Ume£ University
Curated by ChEMBL
| Assay Description
Inhibition of full length ATRD3 (unknown origin) |
J Med Chem 56: 9556-68 (2014)
Article DOI: 10.1021/jm401394u
BindingDB Entry DOI: 10.7270/Q2Q52R32 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data