

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043407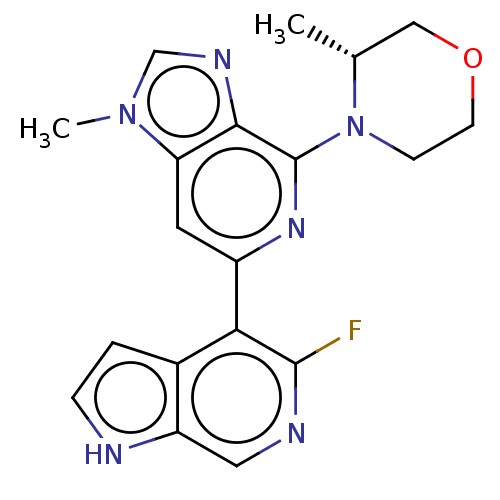 (CHEMBL3355476) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ATR using ATP substrate measured after 3 hrs | ACS Med Chem Lett 6: 42-6 (2015) Article DOI: 10.1021/ml500352s BindingDB Entry DOI: 10.7270/Q2XG9SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043409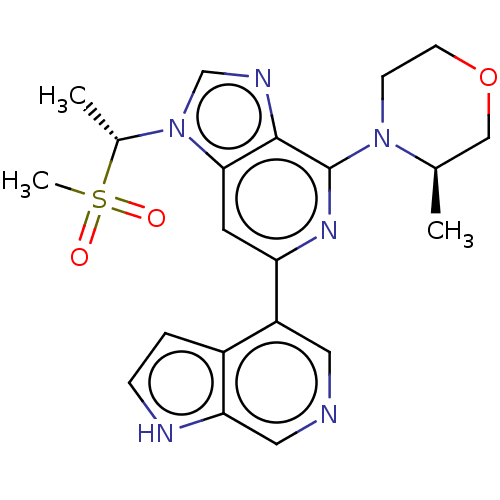 (CHEMBL3355474) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ATR using ATP substrate measured after 3 hrs | ACS Med Chem Lett 6: 42-6 (2015) Article DOI: 10.1021/ml500352s BindingDB Entry DOI: 10.7270/Q2XG9SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043385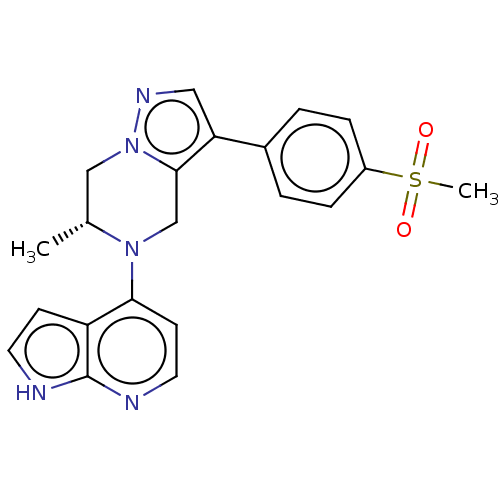 (CHEMBL3355072) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay | ACS Med Chem Lett 6: 37-41 (2015) Article DOI: 10.1021/ml500353p BindingDB Entry DOI: 10.7270/Q228097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043387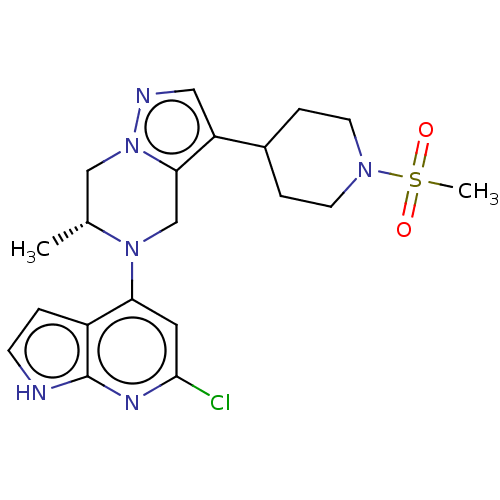 (CHEMBL3355074) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay | ACS Med Chem Lett 6: 37-41 (2015) Article DOI: 10.1021/ml500353p BindingDB Entry DOI: 10.7270/Q228097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043408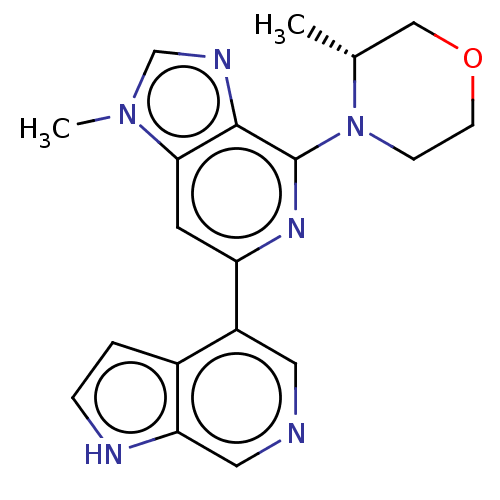 (CHEMBL3355475) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ATR using ATP substrate measured after 3 hrs | ACS Med Chem Lett 6: 42-6 (2015) Article DOI: 10.1021/ml500352s BindingDB Entry DOI: 10.7270/Q2XG9SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043382 (CHEMBL3355069) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay | ACS Med Chem Lett 6: 37-41 (2015) Article DOI: 10.1021/ml500353p BindingDB Entry DOI: 10.7270/Q228097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043411 (CHEMBL3355480) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ATR using ATP substrate measured after 3 hrs | ACS Med Chem Lett 6: 42-6 (2015) Article DOI: 10.1021/ml500352s BindingDB Entry DOI: 10.7270/Q2XG9SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043384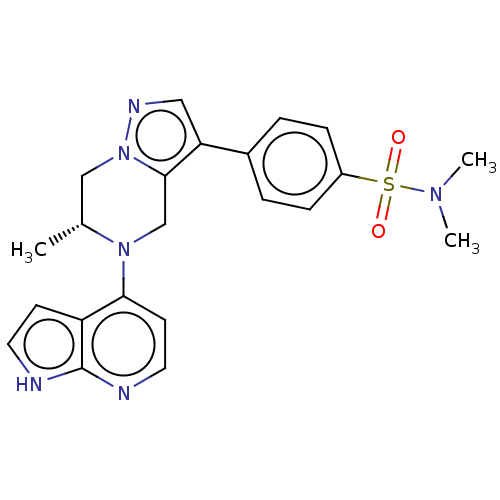 (CHEMBL3355071) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 0.5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay | ACS Med Chem Lett 6: 37-41 (2015) Article DOI: 10.1021/ml500353p BindingDB Entry DOI: 10.7270/Q228097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043383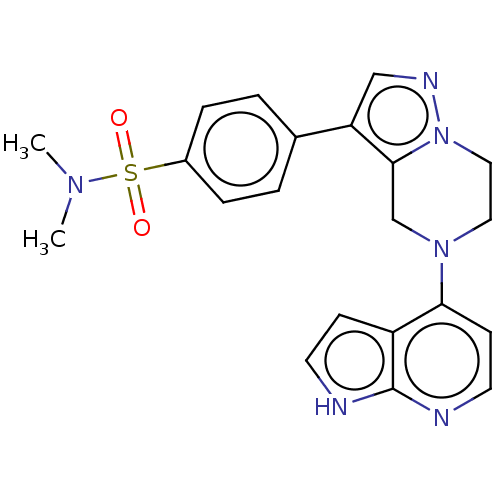 (CHEMBL3355070) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay | ACS Med Chem Lett 6: 37-41 (2015) Article DOI: 10.1021/ml500353p BindingDB Entry DOI: 10.7270/Q228097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50468005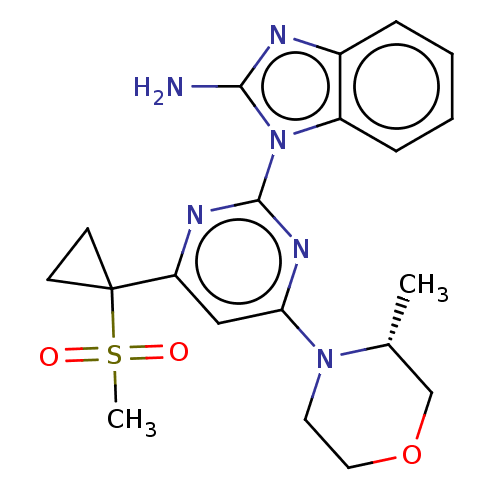 (CHEMBL4288033) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043410 (CHEMBL3355473) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ATR using ATP substrate measured after 3 hrs | ACS Med Chem Lett 6: 42-6 (2015) Article DOI: 10.1021/ml500352s BindingDB Entry DOI: 10.7270/Q2XG9SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043386 (CHEMBL3355073) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay | ACS Med Chem Lett 6: 37-41 (2015) Article DOI: 10.1021/ml500353p BindingDB Entry DOI: 10.7270/Q228097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616182 (US20230271968, Compound A22) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616171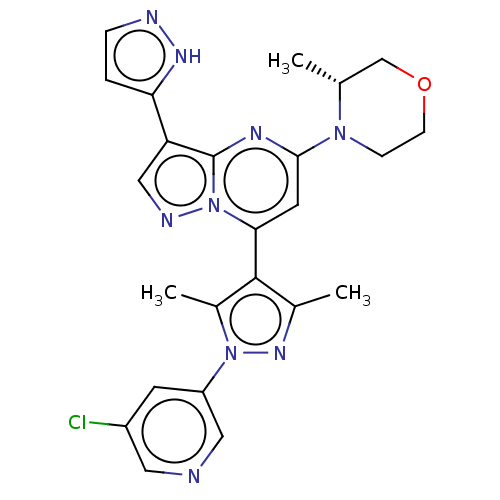 (US20230271968, Compound A12) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043389 (CHEMBL3352844) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of ATR (unknown origin) using Avi-tagged protein substrate by alphascreen assay | ACS Med Chem Lett 6: 37-41 (2015) Article DOI: 10.1021/ml500353p BindingDB Entry DOI: 10.7270/Q228097V | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50468002 (CHEMBL4291436) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50468003 (CHEMBL4281240) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616172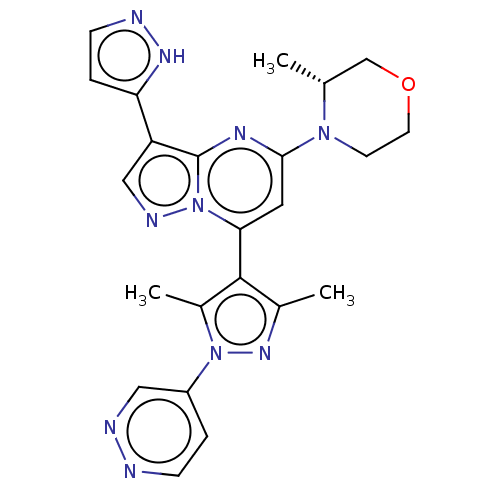 (US20230271968, Compound A13) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616184 (US20230271968, Compound A24) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM459773 (3,3-dimethyl-2-{2-[(3R)-3-methylmorpholin-4-yl]-8-...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of 5'-TAMRA-labeled tracer 3',6'-bis(dimethylamino)-N-(4-{[2(1H-indol-4-yl)-6-(morpholin-4-yl)-pyrimidin-4-yl]amino}butyl)-3-oxo-3H-spiro[... | J Med Chem 63: 7293-7325 (2020) Article DOI: 10.1021/acs.jmedchem.0c00369 BindingDB Entry DOI: 10.7270/Q29C71ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50468009 (CHEMBL4286105) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616166 (US20230271968, Compound A9) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | MCE PC cid PC sid UniChem | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM103469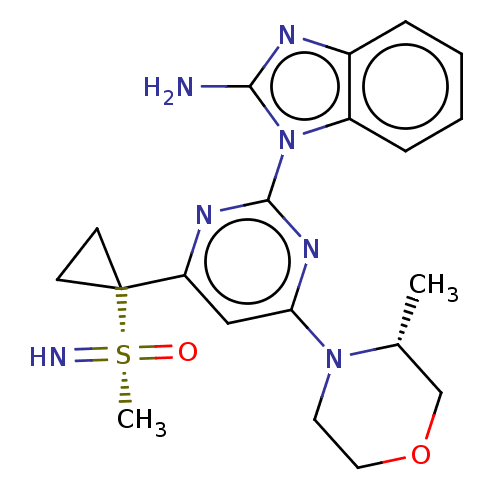 (US8552004, 2.07) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.58 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description ATM and ATR have distinct and overlapping responses to DNA damage. They must participate together and responses must be co-ordinated. Both pathways... | US Patent US8552004 (2013) BindingDB Entry DOI: 10.7270/Q20P0XN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM103481 (US8552004, 2.08) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 2.76 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca AB US Patent | Assay Description ATM and ATR have distinct and overlapping responses to DNA damage. They must participate together and responses must be co-ordinated. Both pathways... | US Patent US8552004 (2013) BindingDB Entry DOI: 10.7270/Q20P0XN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427326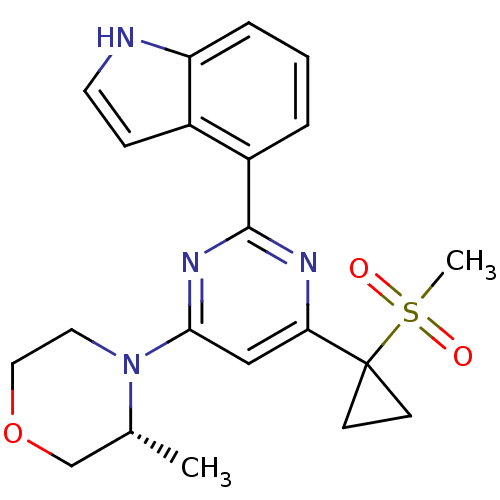 (CHEMBL2325697) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of 5'-TAMRA-labeled tracer 3',6'-bis(dimethylamino)-N-(4-{[2(1H-indol-4-yl)-6-(morpholin-4-yl)-pyrimidin-4-yl]amino}butyl)-3-oxo-3H-spiro[... | J Med Chem 63: 7293-7325 (2020) Article DOI: 10.1021/acs.jmedchem.0c00369 BindingDB Entry DOI: 10.7270/Q29C71ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427322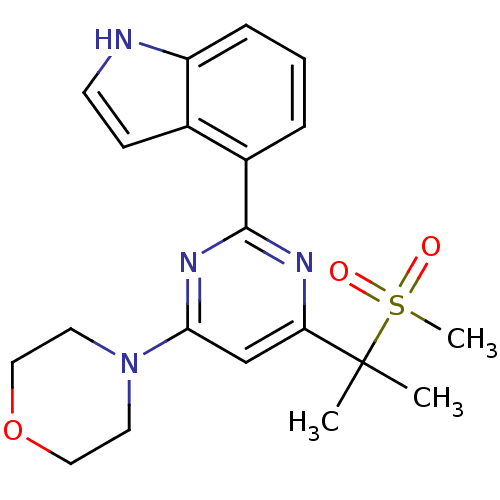 (CHEMBL2325703) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50468017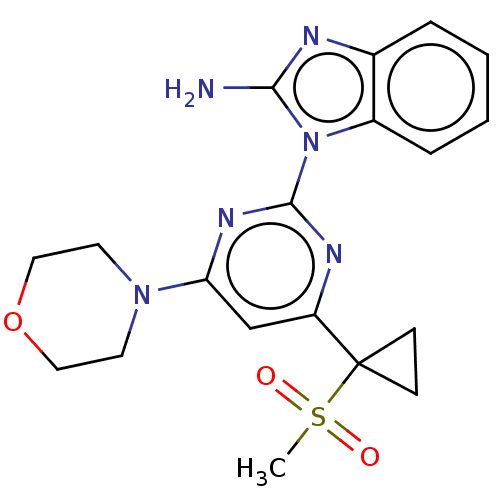 (CHEMBL4291783) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616177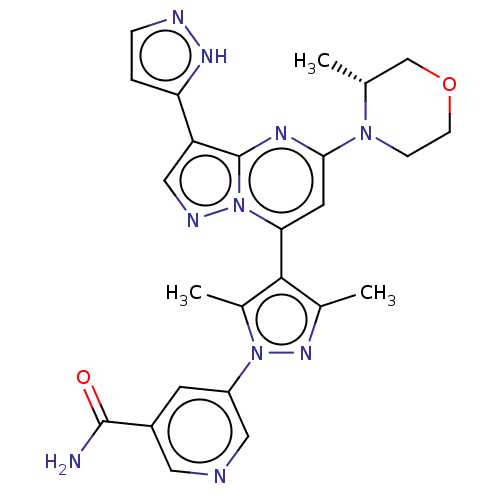 (US20230271968, Compound A17) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616167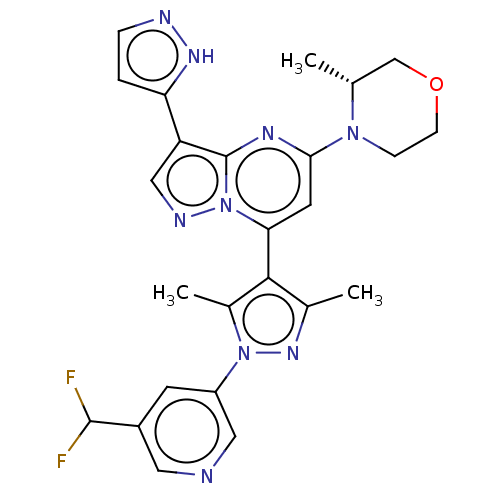 (US20230271968, Compound A10) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616173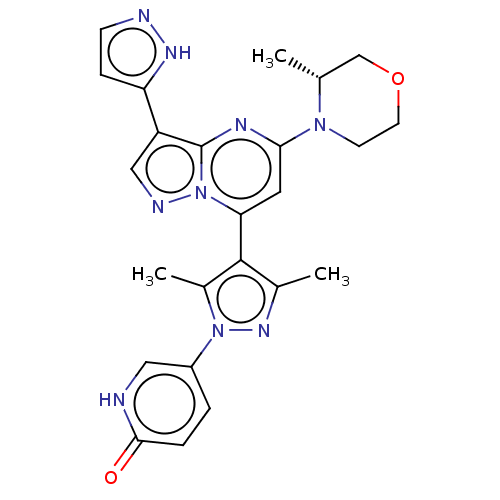 (US20230271968, Compound A14) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM60432 (BDBM50468001 | US11236089, Compound AZD-6738 | US8...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 3.75 | n/a | n/a | n/a | n/a | 7.4 | 37 |
AstraZeneca AB US Patent | Assay Description ATR for use in the in vitro enzyme assay was obtained from HeLa nuclear extract (CIL Biotech, Mons, Belgium) by immunoprecipitation with rabbit polyc... | US Patent US8552004 (2013) BindingDB Entry DOI: 10.7270/Q20P0XN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427320 (CHEMBL2325705) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616179 (US20230271968, Compound A19) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM60432 (BDBM50468001 | US11236089, Compound AZD-6738 | US8...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase MCE PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM616183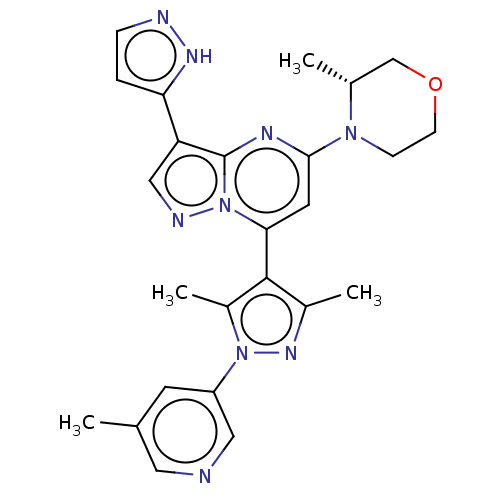 (US20230271968, Compound A23) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a | |
TBA | Citation and Details BindingDB Entry DOI: 10.7270/Q2SQ94HS | ||||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM268208 (US10772893, Example 241 | US11529356, Example 241 ...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.06 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine of binding activity of the test compounds, full-length human ATR protein was expressed and purified together with ATRIP as described abo... | US Patent US9549932 (2017) BindingDB Entry DOI: 10.7270/Q27S7QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM268080 (2-[(3R)-3-methylmorpholin-4-yl]-4-(3-methyl-1,2-ox...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.74 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine of binding activity of the test compounds, full-length human ATR protein was expressed and purified together with ATRIP as described abo... | US Patent US9549932 (2017) BindingDB Entry DOI: 10.7270/Q27S7QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM268050 (2-[(3R)-3-methylmorpholin-4-yl]-4-[4-(methylsulfon...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 4.90 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine of binding activity of the test compounds, full-length human ATR protein was expressed and purified together with ATRIP as described abo... | US Patent US9549932 (2017) BindingDB Entry DOI: 10.7270/Q27S7QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427326 (CHEMBL2325697) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427314 (CHEMBL2325711) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR-mediated CHK1 phosphorylation at serine 345 in human HT29 cells after 1 hr in presence of 4-nitroquinoline 1-oxide | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50043403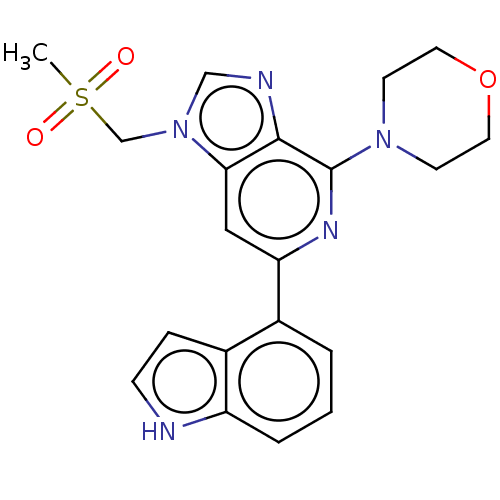 (CHEMBL3355472) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for Biomedical Research Curated by ChEMBL | Assay Description Inhibition of human ATR using ATP substrate measured after 3 hrs | ACS Med Chem Lett 6: 42-6 (2015) Article DOI: 10.1021/ml500352s BindingDB Entry DOI: 10.7270/Q2XG9SRM | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50427326 (CHEMBL2325697) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MCE PC cid PC sid PDB UniChem Similars | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extracts using glutathione S-transferase-p53N66 and ATP as substrate incubated for 10 mins prior to ATP ... | J Med Chem 56: 2125-38 (2013) Article DOI: 10.1021/jm301859s BindingDB Entry DOI: 10.7270/Q2VH5Q5C | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50468004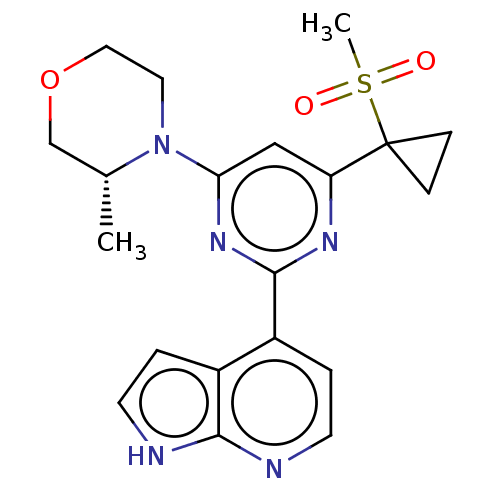 (CHEMBL4286472) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM50468021 (CHEMBL4278556) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
AstraZeneca Curated by ChEMBL | Assay Description Inhibition of ATR in human HeLa cell nuclear extract using GST-fused p53N66 as substrate preincubated for 10 mins followed by ATP addition and measur... | J Med Chem 61: 9889-9907 (2018) Article DOI: 10.1021/acs.jmedchem.8b01187 BindingDB Entry DOI: 10.7270/Q2S46VPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM459573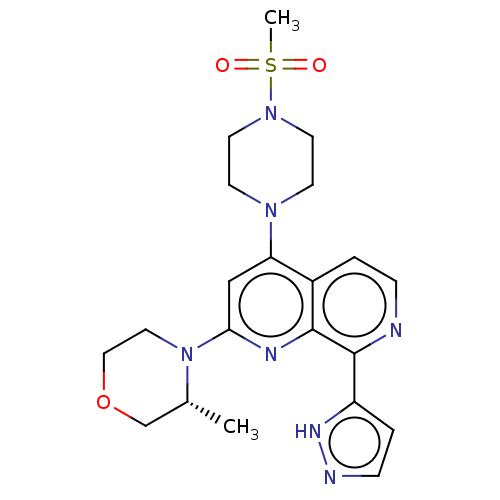 (US10772893, Example 82 | US11529356, Example 82) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5 | n/a | n/a | n/a | n/a | n/a | n/a |
Bayer AG Curated by ChEMBL | Assay Description Inhibition of 5'-TAMRA-labeled tracer 3',6'-bis(dimethylamino)-N-(4-{[2(1H-indol-4-yl)-6-(morpholin-4-yl)-pyrimidin-4-yl]amino}butyl)-3-oxo-3H-spiro[... | J Med Chem 63: 7293-7325 (2020) Article DOI: 10.1021/acs.jmedchem.0c00369 BindingDB Entry DOI: 10.7270/Q29C71ZT | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM268038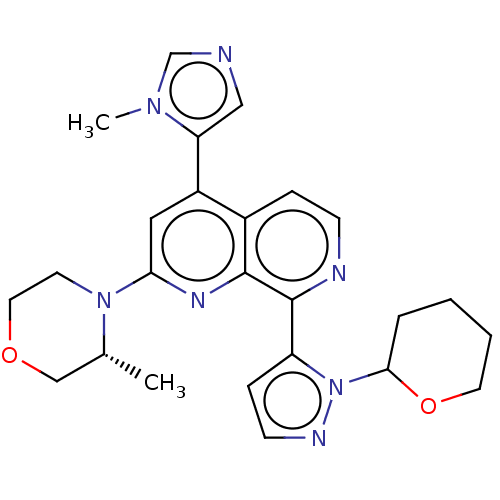 (4-(1-methyl-1H-imidazol-5-yl)-2-[(3R)-3-methylmorp...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.17 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine of binding activity of the test compounds, full-length human ATR protein was expressed and purified together with ATRIP as described abo... | US Patent US9549932 (2017) BindingDB Entry DOI: 10.7270/Q27S7QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM268037 (4-(1-ethyl-1H-pyrazol-5-yl)-2-[(3R)-3-methylmorpho...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.34 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine of binding activity of the test compounds, full-length human ATR protein was expressed and purified together with ATRIP as described abo... | US Patent US9549932 (2017) BindingDB Entry DOI: 10.7270/Q27S7QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM268051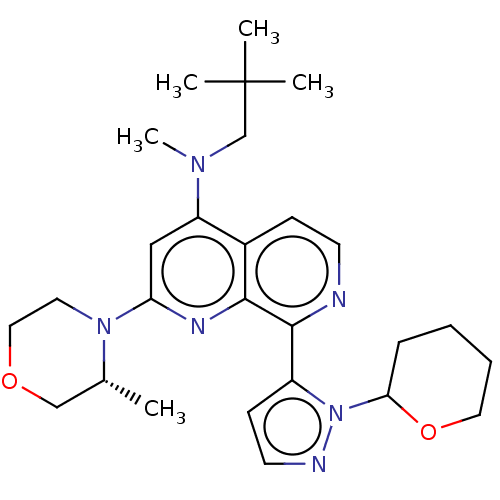 (N-(2,2-dimethylpropyl)-N-methyl-2-[(3R)-3-methylmo...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.38 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine of binding activity of the test compounds, full-length human ATR protein was expressed and purified together with ATRIP as described abo... | US Patent US9549932 (2017) BindingDB Entry DOI: 10.7270/Q27S7QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM103467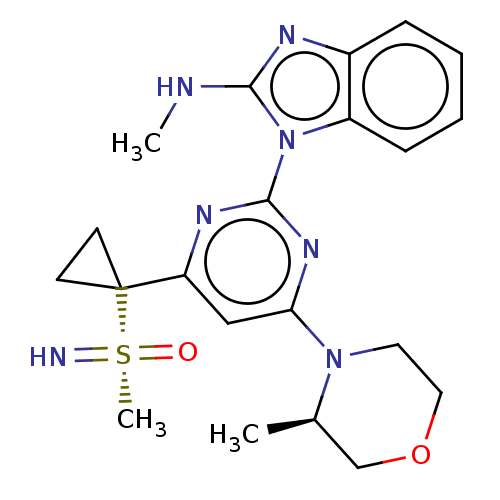 (US8552004, 2.03) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | 5.61 | n/a | n/a | n/a | n/a | 7.4 | 37 |
AstraZeneca AB US Patent | Assay Description ATR for use in the in vitro enzyme assay was obtained from HeLa nuclear extract (CIL Biotech, Mons, Belgium) by immunoprecipitation with rabbit polyc... | US Patent US8552004 (2013) BindingDB Entry DOI: 10.7270/Q20P0XN6 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Serine/threonine-protein kinase ATR (Homo sapiens (Human)) | BDBM268039 (2-{2-[(3R)-3-methylmorpholin-4-yl]-8-(1H-pyrazol-5...) | PDB Reactome pathway UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | 5.65 | n/a | n/a | n/a | n/a | 7.0 | n/a |
Bayer Pharma Aktiengesellschaft US Patent | Assay Description To determine of binding activity of the test compounds, full-length human ATR protein was expressed and purified together with ATRIP as described abo... | US Patent US9549932 (2017) BindingDB Entry DOI: 10.7270/Q27S7QSC | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 5545 total ) | Next | Last >> |