

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM36456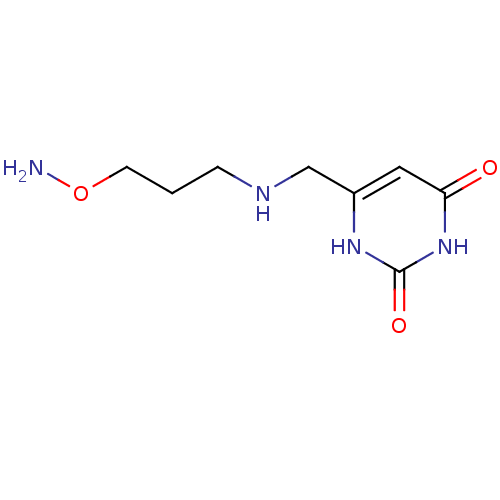 (6-[3-(Aminooxypropylamino)methyl]uracil, dihydroch...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | Article PubMed | 7.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
Johns Hopkins University | Assay Description Inhibition of human uracil DNA glycosylase using high-throughput fluorescent molecular beacon DNA substrate. | Nat Chem Biol 5: 407-13 (2009) Article DOI: 10.1038/nchembio.163 BindingDB Entry DOI: 10.7270/Q2W37TP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM36457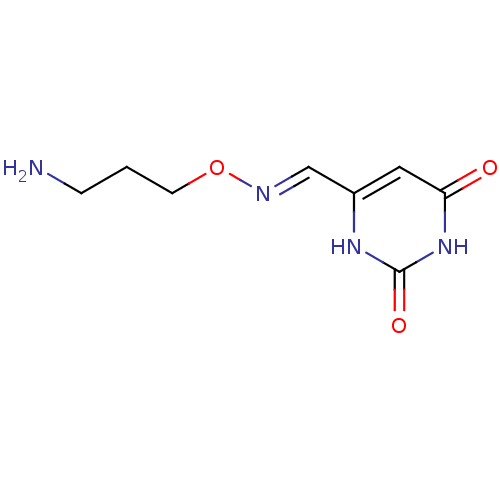 (3-[(3-Aminopropoxyimino)methyl]uracil, hydrochlori...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | Article PubMed | 7.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a | 8.0 | n/a |
Johns Hopkins University | Assay Description Inhibition of human uracil DNA glycosylase using high-throughput fluorescent molecular beacon DNA substrate. | Nat Chem Biol 5: 407-13 (2009) Article DOI: 10.1038/nchembio.163 BindingDB Entry DOI: 10.7270/Q2W37TP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (docked) | ||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM36452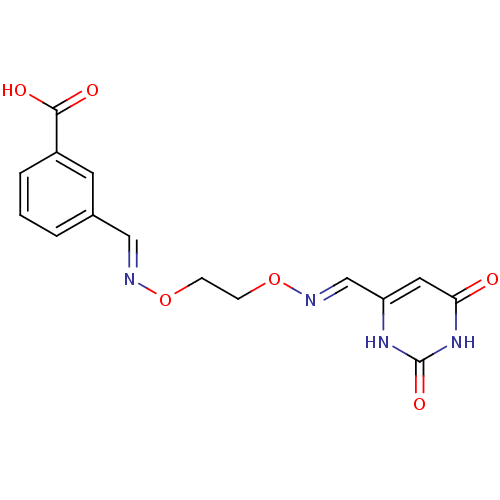 (6-[[(3-Carboxy-benzylidene)-aminooxy]ethoxyimino]m...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | DrugBank MMDB PC cid PC sid PDB UniChem | DrugBank MMDB PDB Article PubMed | n/a | n/a | 4.00E+4 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Johns Hopkins University | Assay Description Inhibition of human uracil DNA glycosylase using high-throughput fluorescent molecular beacon DNA substrate. | Nat Chem Biol 5: 407-13 (2009) Article DOI: 10.1038/nchembio.163 BindingDB Entry DOI: 10.7270/Q2W37TP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM36455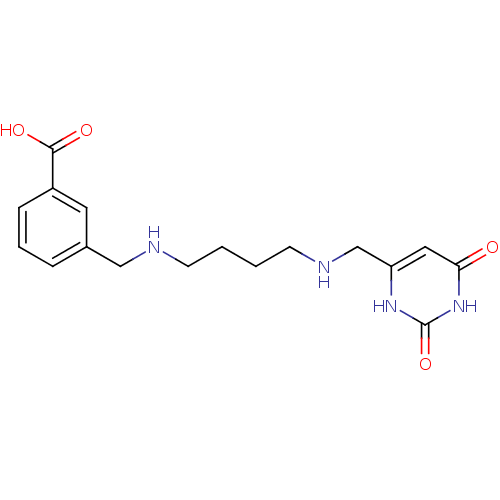 (6-[4-[(3-Carboxybenzylamino)butylamino]methyl]urac...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 3.15E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Johns Hopkins University | Assay Description Inhibition of human uracil DNA glycosylase using high-throughput fluorescent molecular beacon DNA substrate. | Nat Chem Biol 5: 407-13 (2009) Article DOI: 10.1038/nchembio.163 BindingDB Entry DOI: 10.7270/Q2W37TP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM36454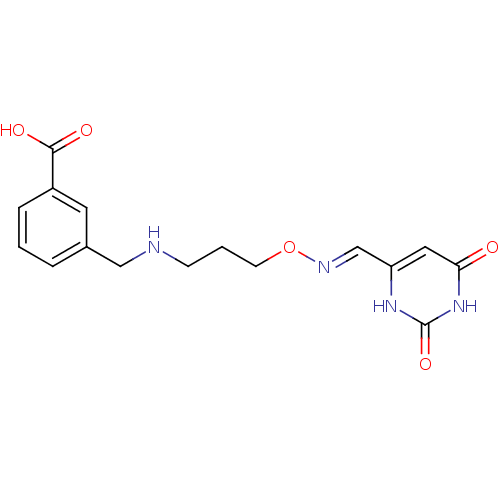 (6-[[3-(3-Carboxybenzyl)aminopropylimino]methyl]ura...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid PDB UniChem | PDB Article PubMed | n/a | n/a | 1.00E+5 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Johns Hopkins University | Assay Description Inhibition of human uracil DNA glycosylase using high-throughput fluorescent molecular beacon DNA substrate. | Nat Chem Biol 5: 407-13 (2009) Article DOI: 10.1038/nchembio.163 BindingDB Entry DOI: 10.7270/Q2W37TP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM36453 (6-[3-[(3-Carboxy-benzylidene)aminooxypropylamino]-...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | MMDB PC cid PC sid PDB UniChem | MMDB PDB Article PubMed | n/a | n/a | 1.30E+3 | n/a | n/a | n/a | n/a | 8.0 | n/a |
Johns Hopkins University | Assay Description Inhibition of human uracil DNA glycosylase using high-throughput fluorescent molecular beacon DNA substrate. | Nat Chem Biol 5: 407-13 (2009) Article DOI: 10.1038/nchembio.163 BindingDB Entry DOI: 10.7270/Q2W37TP8 | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078338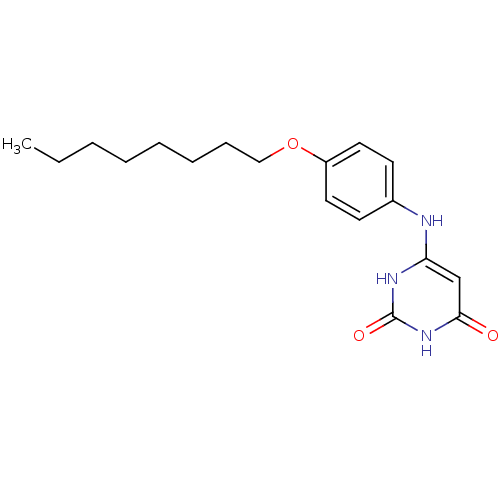 (6-(4-Octyloxy-phenylamino)-1H-pyrimidine-2,4-dione...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 Uracil-DNA glycosylase | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078339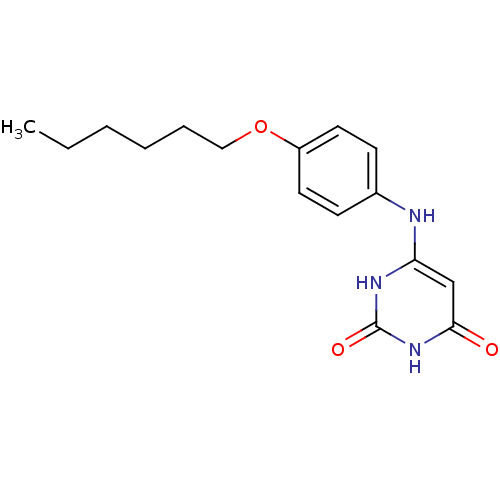 (6-(4-Hexyloxy-phenylamino)-1H-pyrimidine-2,4-dione...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078340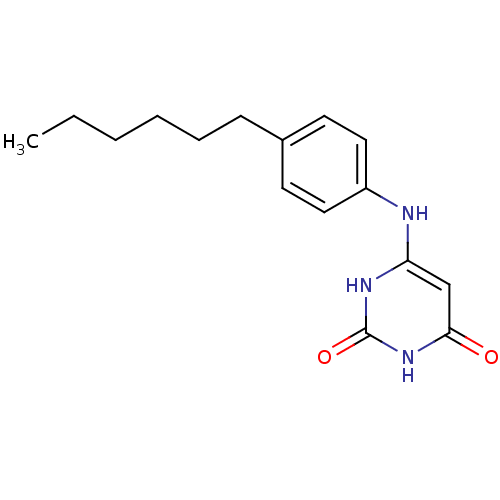 (6-(4-Hexyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50028325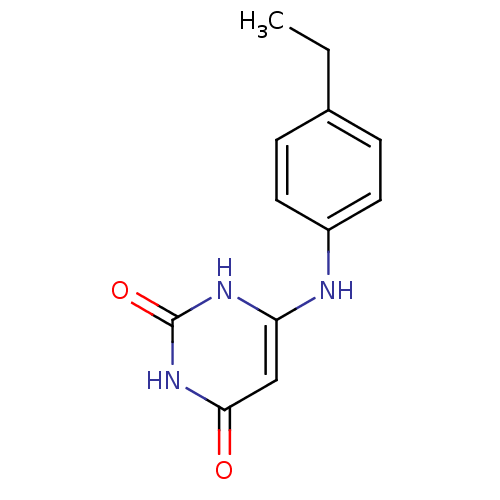 (6-(4-Ethyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078341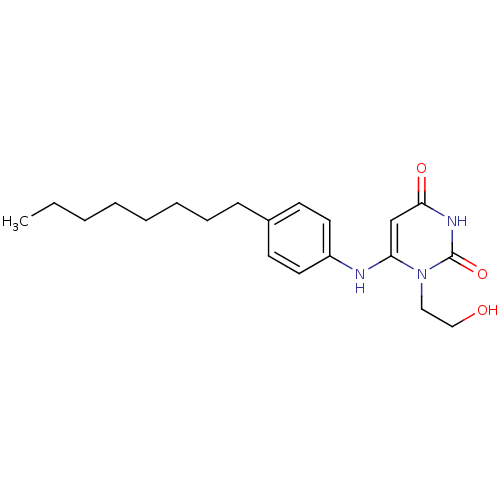 (1-(2-Hydroxy-ethyl)-6-(4-octyl-phenylamino)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 Uracil-DNA glycosylase | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078342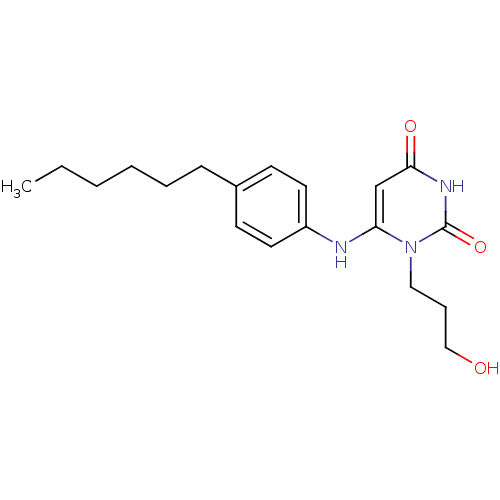 (6-(4-Hexyl-phenylamino)-1-(3-hydroxy-propyl)-1H-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 9.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078344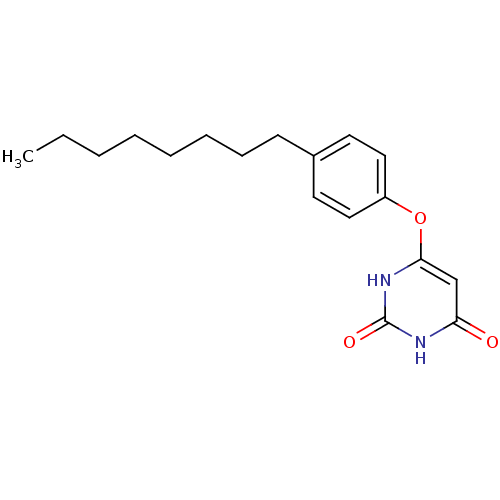 (6-(4-Octyl-phenoxy)-1H-pyrimidine-2,4-dione | CHEM...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 Uracil-DNA glycosylase | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078337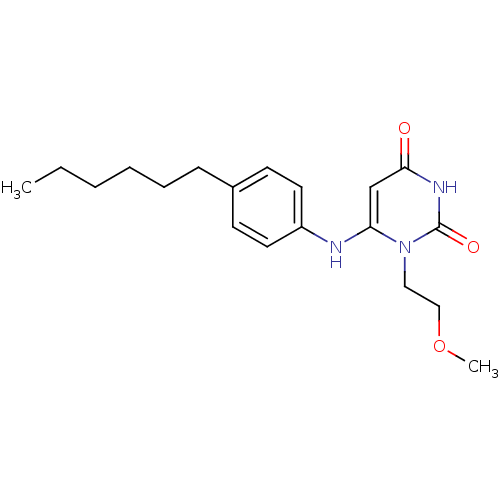 (6-(4-Hexyl-phenylamino)-1-(2-methoxy-ethyl)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078336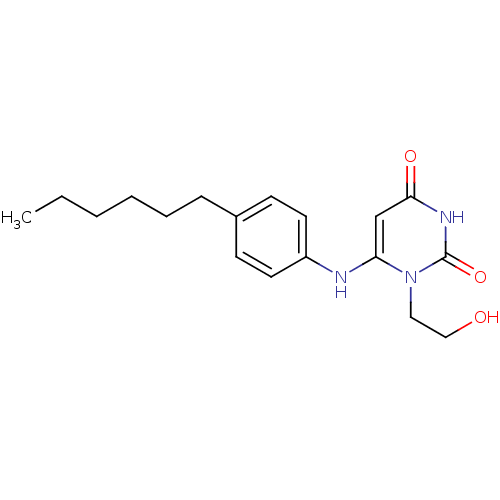 (6-(4-Hexyl-phenylamino)-1-(2-hydroxy-ethyl)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078343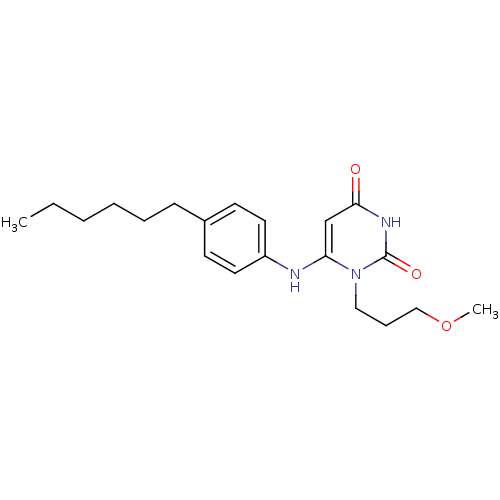 (6-(4-Hexyl-phenylamino)-1-(3-methoxy-propyl)-1H-py...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078334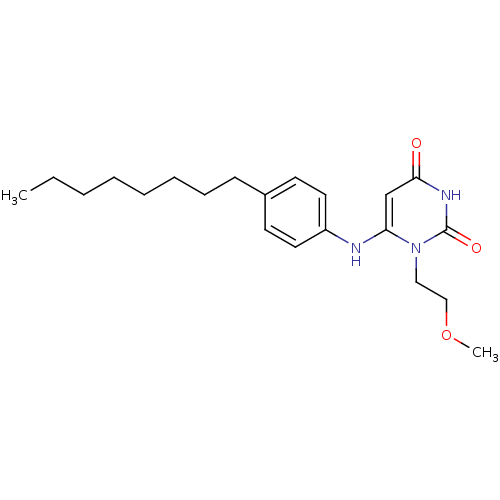 (1-(2-Methoxy-ethyl)-6-(4-octyl-phenylamino)-1H-pyr...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 Uracil-DNA glycosylase | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078333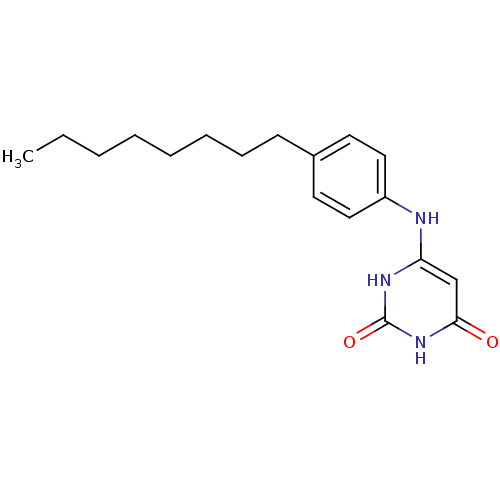 (6-(4-Octyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 8.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 Uracil-DNA glycosylase | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50028333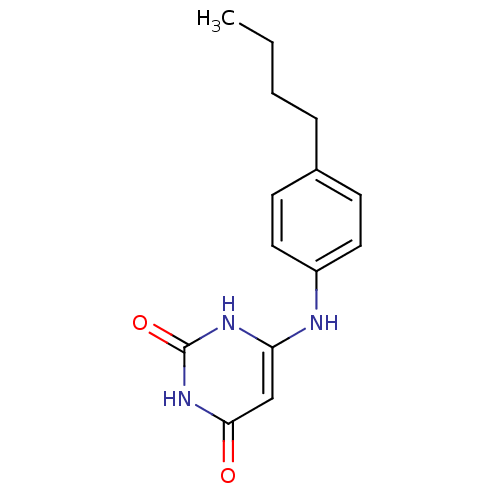 (6-(4-Butyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.50E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 UDG (Herpes Simplex virus type-I Uracil DNA glycosylase) | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Uracil-DNA glycosylase (Homo sapiens (Human)) | BDBM50078335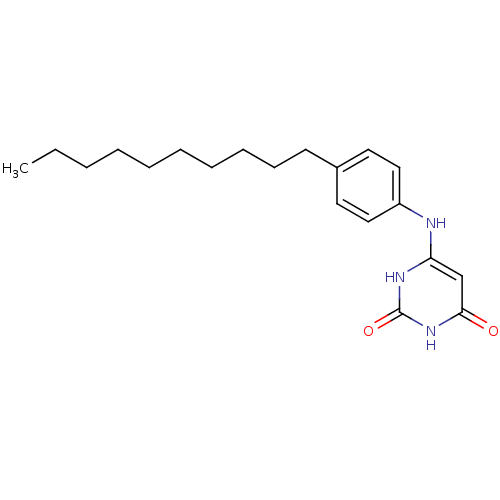 (6-(4-Decyl-phenylamino)-1H-pyrimidine-2,4-dione | ...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Massachusetts Medical Center Curated by ChEMBL | Assay Description Inhibitory potency of the compound against HSV-1 Uracil-DNA glycosylase | J Med Chem 42: 2344-50 (1999) Article DOI: 10.1021/jm980718d BindingDB Entry DOI: 10.7270/Q21J98ZQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||