

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121753 (1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | 17 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against human thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122770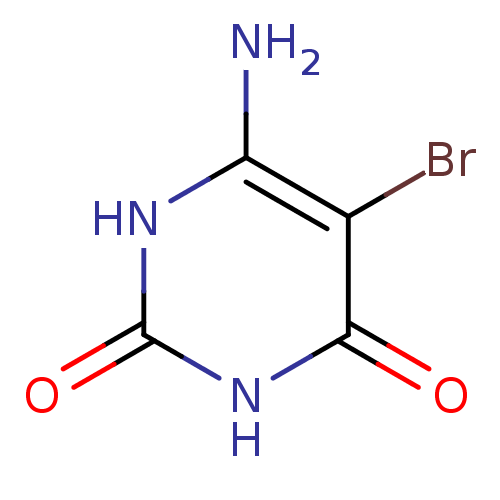 (6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 2.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50134398 (1-methyl-2,5-dioxo-4-pentylimidazolidine-4-carbald...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 1.25E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50134398 (1-methyl-2,5-dioxo-4-pentylimidazolidine-4-carbald...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | 2.50E+7 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Mus musculus) | BDBM50134397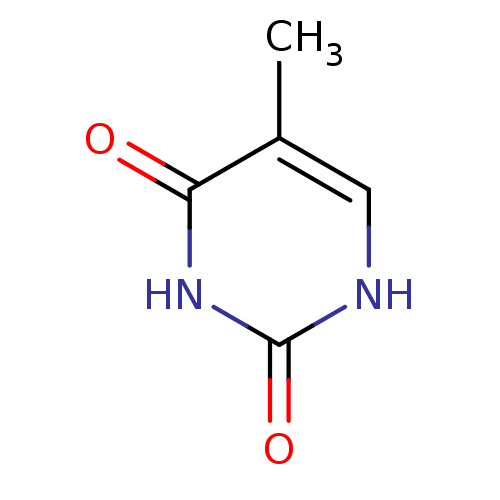 (5-Methyl-1H-pyrimidine-2,4-dione | CHEMBL993 | THY...) | Reactome pathway KEGG UniProtKB/SwissProt GoogleScholar AffyNet | Purchase CHEMBL DrugBank MCE KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | 3.47E+8 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against mouse liver thymidine phosphorylase at 0.15 mM thymidine substrate | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122764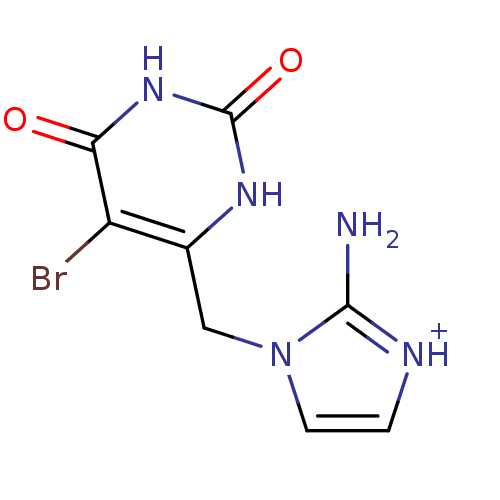 (2-Amino-1-(5-bromo-2,6-dioxo-1,2,3,6-tetrahydro-py...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | PubMed | n/a | n/a | 19 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50121753 (1-(5-Chloro-2,6-dioxo-1,2,3,6-tetrahydro-pyrimidin...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | PubMed | n/a | n/a | 20 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50134399 (1,7-Dihydro-pyrrolo[2,3-d]pyrimidine-2,4-dione | 7...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem Patents | PubMed | n/a | n/a | 6.30E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50122770 (6-Amino-5-bromo-1H-pyrimidine-2,4-dione | 6-Amino-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL MMDB PC cid PC sid PDB UniChem Patents Similars | PubMed | n/a | n/a | 6.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against human thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50134398 (1-methyl-2,5-dioxo-4-pentylimidazolidine-4-carbald...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 1.96E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against human thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50134398 (1-methyl-2,5-dioxo-4-pentylimidazolidine-4-carbald...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 7.70E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against human thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Thymidine phosphorylase (Homo sapiens (Human)) | BDBM50134396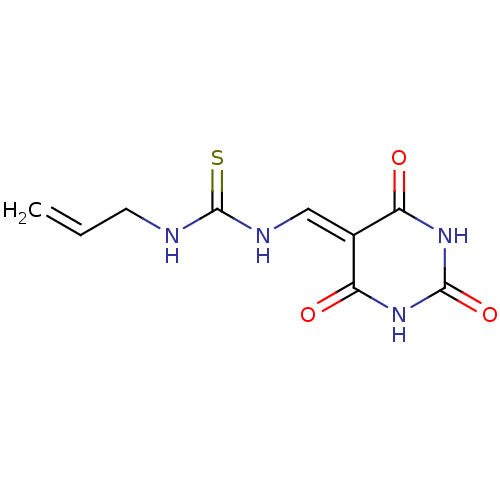 (1-Allyl-3-(2,4,6-trioxo-tetrahydro-pyrimidin-5-yli...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL PC cid PC sid UniChem | PubMed | n/a | n/a | 9.48E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
University of Manchester Curated by ChEMBL | Assay Description Inhibitory activity against Escherichia coli thymidine phosphorylase | Bioorg Med Chem Lett 13: 3705-9 (2003) BindingDB Entry DOI: 10.7270/Q2SQ90ZZ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||