 Found 35 hits of Enzyme Inhibition Constant Data
Found 35 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin E-cyclin-dependent kinase 2 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin E-cyclin-dependent kinase 2 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PDB
PubMed
| n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase A |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM2672
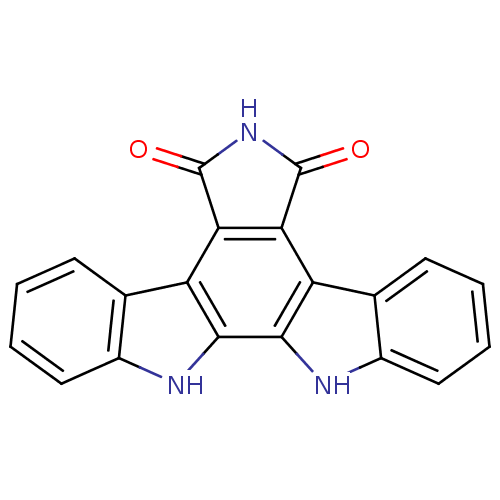
(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H11N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,21-22H,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 25 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin E-cyclin-dependent kinase 2 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM14187

(3,13-diazahexacyclo[14.8.0.0^{2,10}.0^{4,9}.0^{11,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3ccccc3c21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 45 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM2579

((2S,3R,4R,6R)-3-methoxy-2-methyl-4-(methylamino)-2...)Show SMILES CN[C@@H]1C[C@H]2O[C@@](C)([C@@H]1OC)n1c3ccccc3c3c4CNC(=O)c4c4c5ccccc5n2c4c13 |r| Show InChI InChI=1S/C28H26N4O3/c1-28-26(34-3)17(29-2)12-20(35-28)31-18-10-6-4-8-14(18)22-23-16(13-30-27(23)33)21-15-9-5-7-11-19(15)32(28)25(21)24(22)31/h4-11,17,20,26,29H,12-13H2,1-3H3,(H,30,33)/t17-,20-,26-,28+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin B-cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM2672

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H11N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,21-22H,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 140 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50134579
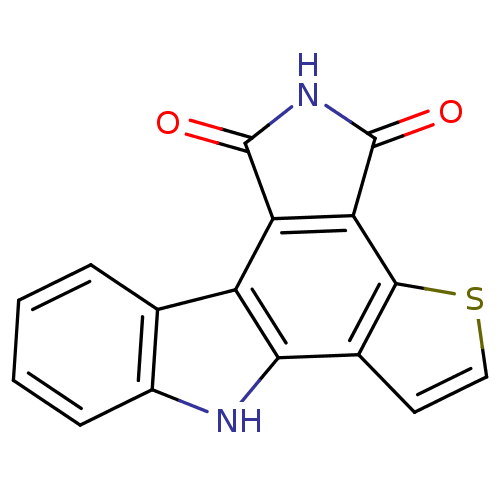
(11H-3-Thia-5,11-diaza-benzo[a]trindene-4,6-dione |...)Show InChI InChI=1S/C16H8N2O2S/c19-15-11-10-7-3-1-2-4-9(7)17-13(10)8-5-6-21-14(8)12(11)16(20)18-15/h1-6,17H,(H,18,19,20) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| PubMed
| n/a | n/a | 150 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50134578
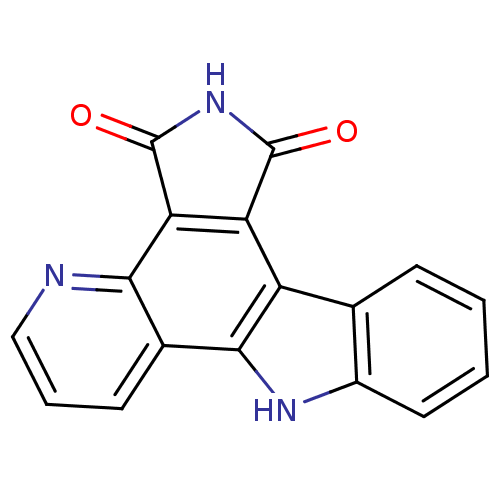
(1,2,3,8-tetrahydropyrido[3,2-a]pyrrolo[4,3-c]carba...)Show InChI InChI=1S/C17H9N3O2/c21-16-12-11-8-4-1-2-6-10(8)19-15(11)9-5-3-7-18-14(9)13(12)17(22)20-16/h1-7,19H,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 180 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Calcium/calmodulin-dependent protein kinase type II subunit alpha/beta/delta/gamma
(Homo sapiens (Human)) | BDBM2581
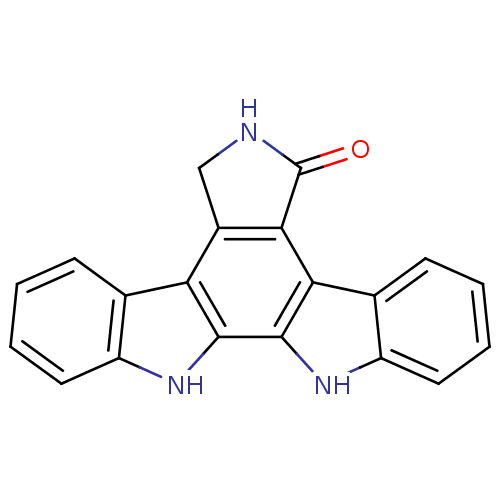
(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 184 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM14188
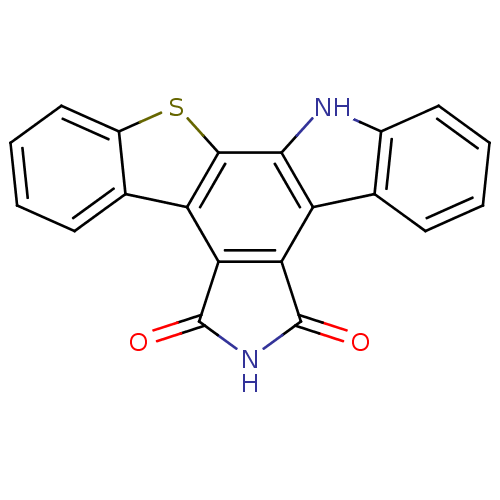
(3-thia-13,23-diazahexacyclo[14.7.0.0^{2,10}.0^{4,9...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1sc3ccccc3c21 Show InChI InChI=1S/C20H10N2O2S/c23-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(24)22-19)10-6-2-4-8-12(10)25-18/h1-8,21H,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 220 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50134576
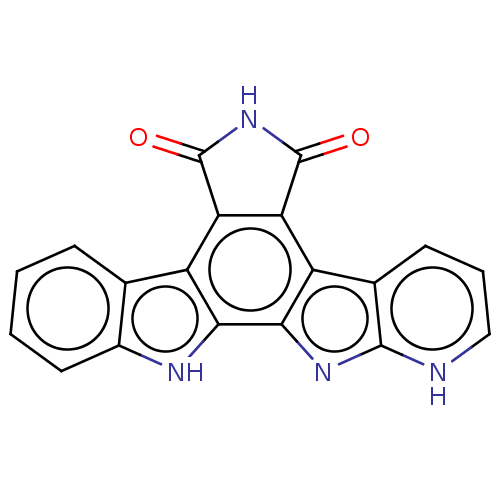
(6,7,12,13-tetrahydro-5H-pyrido[3',2':4,5]pyrrolo[2...)Show SMILES O=C1NC(=O)c2c1c1c3ccc[nH]c3nc1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C19H8N4O2/c24-18-13-11-8-4-1-2-6-10(8)21-15(11)16-12(14(13)19(25)23-18)9-5-3-7-20-17(9)22-16/h1-7H,(H,23,24,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 250 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50134580

(1,2,3,8-tetrahydro-benzo[a]pyrrolo[3,4-c]carbazole...)Show InChI InChI=1S/C18H10N2O2/c21-17-14-9-5-1-2-6-10(9)16-13(15(14)18(22)20-17)11-7-3-4-8-12(11)19-16/h1-8,19H,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 260 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin E-cyclin-dependent kinase 2 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM14187

(3,13-diazahexacyclo[14.8.0.0^{2,10}.0^{4,9}.0^{11,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3ccccc3c21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 275 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50134577
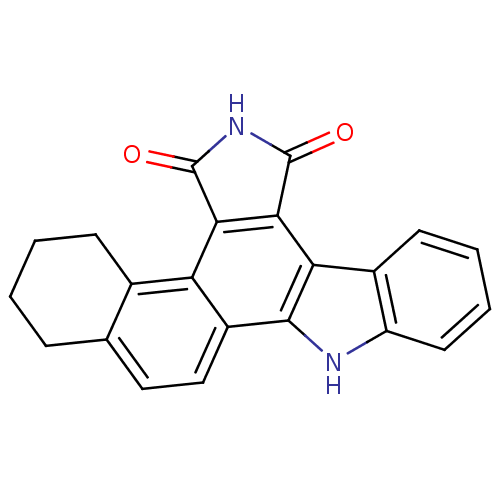
(1,2,3,4,5,6,7,12-octahydronaphtho[2,1-a]pyrrolo[4,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3CCCCc3c21 Show InChI InChI=1S/C22H16N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h3-4,7-10,23H,1-2,5-6H2,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 410 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50134575

(7,8,9,14-tetrahydronaphtho[1,2-a]pyrrolo[4,3-c]car...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1c2ccc2ccccc12 Show InChI InChI=1S/C22H12N2O2/c25-21-18-14-10-9-11-5-1-2-6-12(11)16(14)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 430 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3
(Homo sapiens (Human)) | BDBM14187

(3,13-diazahexacyclo[14.8.0.0^{2,10}.0^{4,9}.0^{11,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3ccccc3c21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 590 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin B-cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50216165
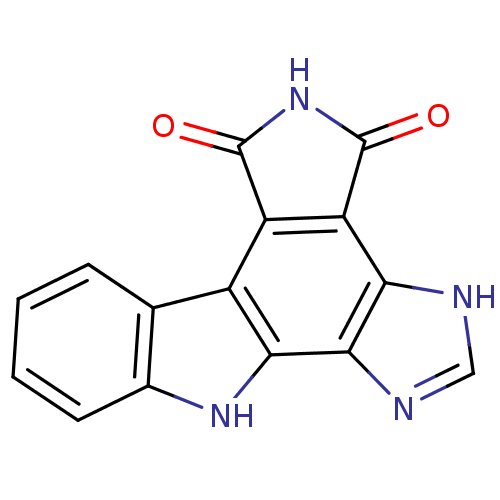
(1,11-dihydro-1,3,5,11-tetraaza-benzo[a]trindene-4,...)Show InChI InChI=1S/C15H8N4O2/c20-14-9-8-6-3-1-2-4-7(6)18-12(8)13-11(16-5-17-13)10(9)15(21)19-14/h1-5,18H,(H,16,17)(H,19,20,21) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 740 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM2581

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 830 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM2672

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H11N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,21-22H,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 900 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin B-cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 2/G1/S-specific cyclin-E1/G1/S-specific cyclin-E2
(Homo sapiens (Human)) | BDBM2581

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 1/G2/mitotic-specific cyclin-B1/G2/mitotic-specific cyclin-B2/G2/mitotic-specific cyclin-B3
(Homo sapiens (Human)) | BDBM2672

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H11N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,21-22H,(H,23,24,25) | PDB
UniProtKB/SwissProt
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 1.13E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50134582

(1,2,3,8-tetrahydropyrido[3,4-a]pyrrolo[4,3-c]carba...)Show InChI InChI=1S/C17H9N3O2/c21-16-13-8-5-6-18-7-10(8)15-12(14(13)17(22)20-16)9-3-1-2-4-11(9)19-15/h1-7,19H,(H,20,21,22) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 1.81E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin E-cyclin-dependent kinase 2 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM2672

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H11N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,21-22H,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM2581

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NCc2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O/c24-20-17-12(9-21-20)15-10-5-1-3-7-13(10)22-18(15)19-16(17)11-6-2-4-8-14(11)23-19/h1-8,22-23H,9H2,(H,21,24) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
PC cid
PC sid
PDB
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >2.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50054426
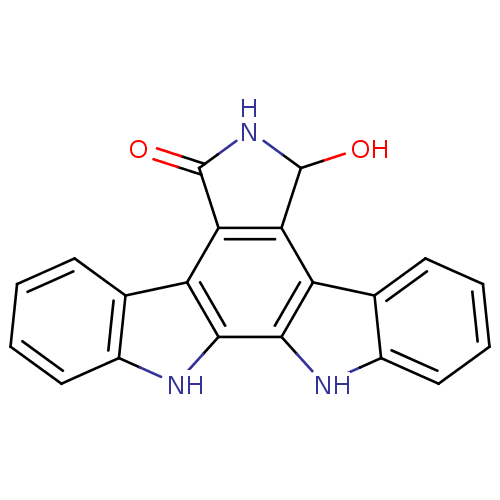
(7-hydroxy-6,7,12,13-tetrahydro-5H-indolo[2,3-a]pyr...)Show SMILES OC1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H13N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,19,21-22,24H,(H,23,25) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | 3.83E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Cyclin B-cyclin-dependent kinase 1 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Glycogen synthase kinase-3 beta
(Homo sapiens (Human)) | BDBM14187

(3,13-diazahexacyclo[14.8.0.0^{2,10}.0^{4,9}.0^{11,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3ccccc3c21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 4.38E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Glycogen synthase kinase-3 beta |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Protein kinase C beta type
(Homo sapiens (Human)) | BDBM14187

(3,13-diazahexacyclo[14.8.0.0^{2,10}.0^{4,9}.0^{11,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3ccccc3c21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 9.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C beta 2 |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Cyclin-dependent kinase 4/G1/S-specific cyclin-D1
(Homo sapiens (Human)) | BDBM50134581
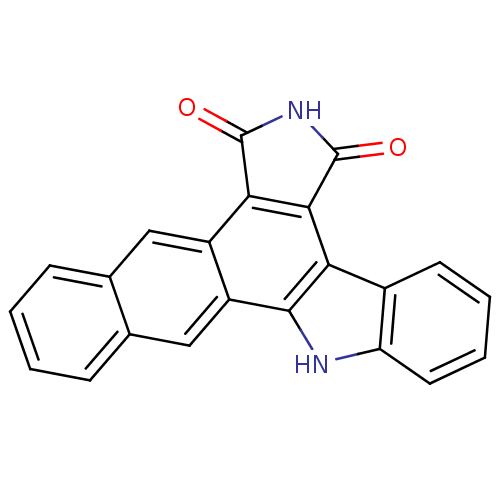
(5,6,7,14-tetrahydronaphtho[3,2-a]pyrrolo[4,3-c]car...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1cc3ccccc3cc21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-14-9-11-5-1-2-6-12(11)10-15(14)20-17(19(18)22(26)24-21)13-7-3-4-8-16(13)23-20/h1-10,23H,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >1.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
cAMP-dependent protein kinase catalytic subunit alpha/beta/gamma
(Homo sapiens (Human)) | BDBM14187

(3,13-diazahexacyclo[14.8.0.0^{2,10}.0^{4,9}.0^{11,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3ccccc3c21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | >2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Protein kinase C alpha type
(Homo sapiens (Human)) | BDBM14187

(3,13-diazahexacyclo[14.8.0.0^{2,10}.0^{4,9}.0^{11,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3ccccc3c21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.28E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C alpha |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
Protein kinase C gamma type
(Homo sapiens (Human)) | BDBM14187

(3,13-diazahexacyclo[14.8.0.0^{2,10}.0^{4,9}.0^{11,...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1ccc3ccccc3c21 Show InChI InChI=1S/C22H12N2O2/c25-21-18-16-12-6-2-1-5-11(12)9-10-14(16)20-17(19(18)22(26)24-21)13-7-3-4-8-15(13)23-20/h1-10,23H,(H,24,25,26) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| PubMed
| n/a | n/a | 5.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibition of Protein kinase C gamma |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |
O94806/P05129/P05771/P17252/P24723/P41743/Q02156/Q04759/Q05513/Q05655/Q15139
(Homo sapiens (Human)) | BDBM2672

(3,13,23-triazahexacyclo[14.7.0.0^{2,10}.0^{4,9}.0^...)Show SMILES O=C1NC(=O)c2c1c1c3ccccc3[nH]c1c1[nH]c3ccccc3c21 Show InChI InChI=1S/C20H11N3O2/c24-19-15-13-9-5-1-3-7-11(9)21-17(13)18-14(16(15)20(25)23-19)10-6-2-4-8-12(10)22-18/h1-8,21-22H,(H,23,24,25) | PDB
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
UniChem
Patents
Similars
| PubMed
| n/a | n/a | >1.00E+5 | n/a | n/a | n/a | n/a | n/a | n/a |
Lilly Spain S.A.
Curated by ChEMBL
| Assay Description
Inhibitory activity against Cyclin D1-cyclin-dependent kinase 4 by measuring the phosphorylation of RbING |
Bioorg Med Chem Lett 13: 3835-9 (2003)
BindingDB Entry DOI: 10.7270/Q2Z89BTC |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data