

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296936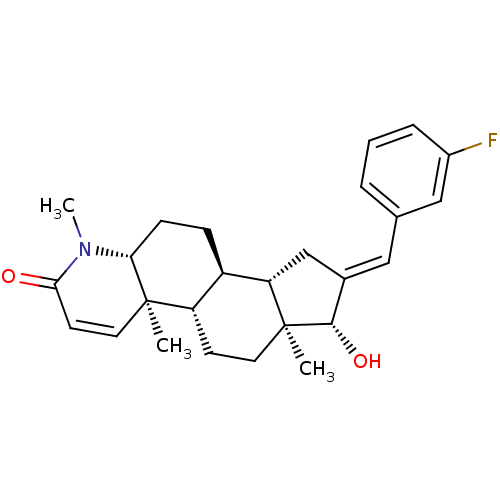 (16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 29 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296937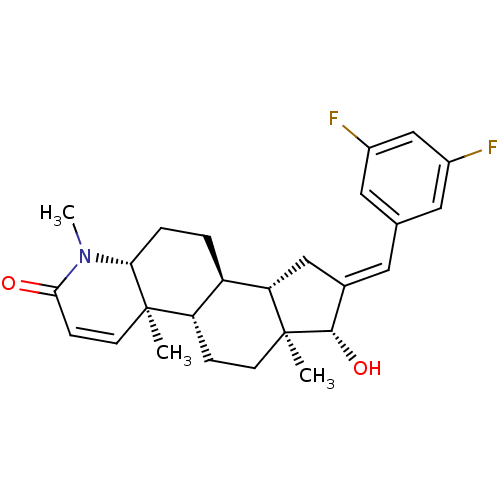 (16-[(3,5-Difluorophenyl)methylidene]-17beta-hydrox...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 46 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296938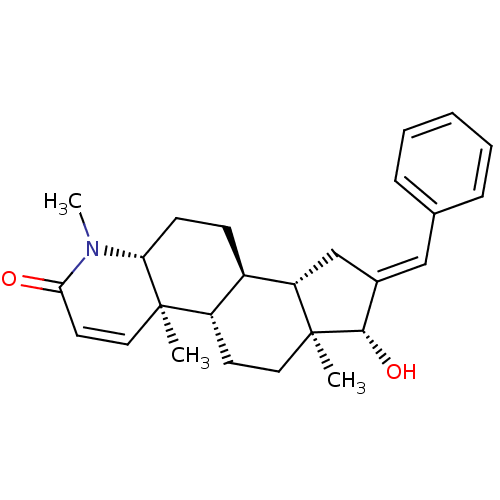 (16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 225 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296935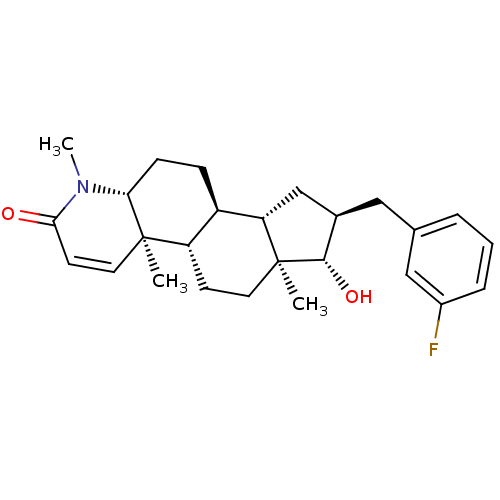 (16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | 240 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296941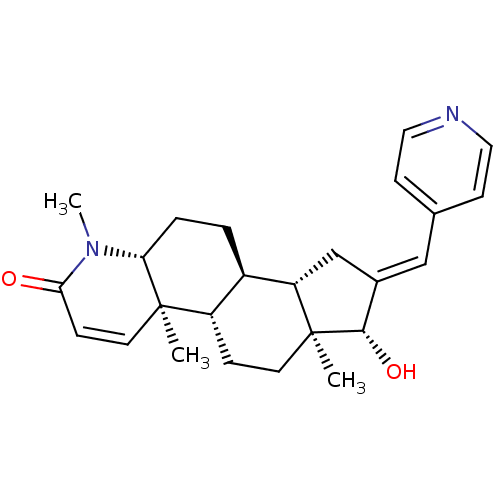 (16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 320 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296942 (16-(Pyridin-3-ylmethylidene)-17beta-hydroxy-4-meth...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296944 (16-[(2-cyclopropylpyrimidin-5-yl)methylidene]-17be...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 4.65E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296945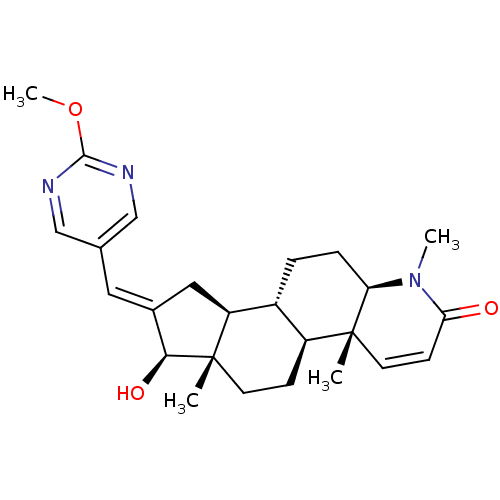 (16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-h...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 7.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296939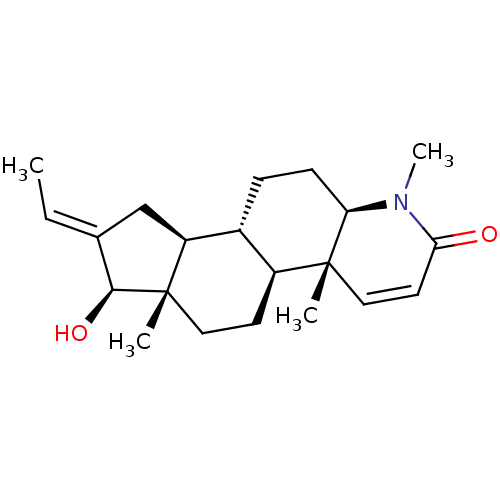 (16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-a...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 8.05E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296943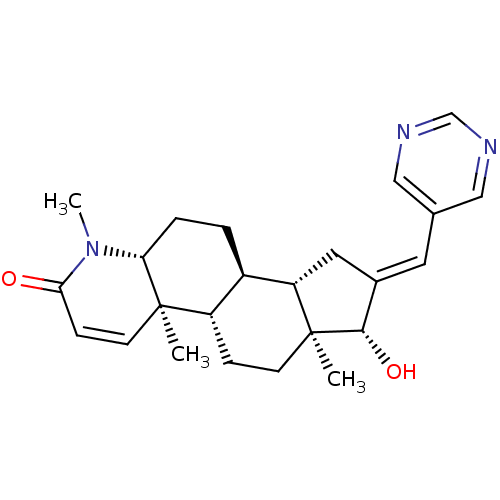 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 9.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296946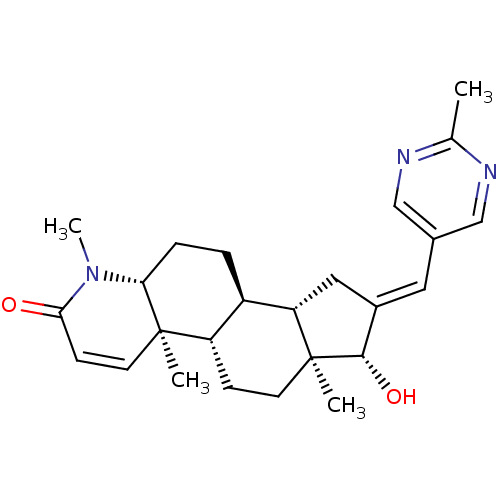 (16-[(2-Methylpyrimidin-5-yl)methylidene]-17beta-hy...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.50E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296933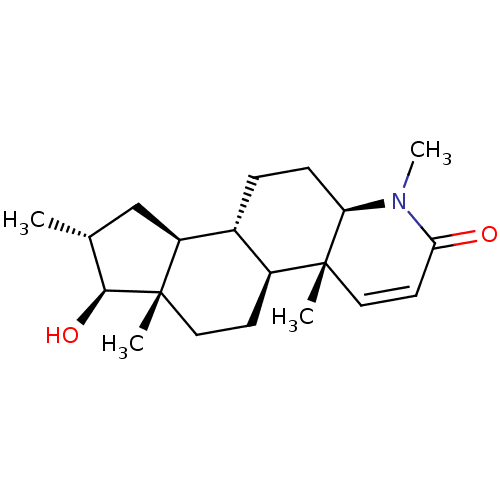 (16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5al...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296947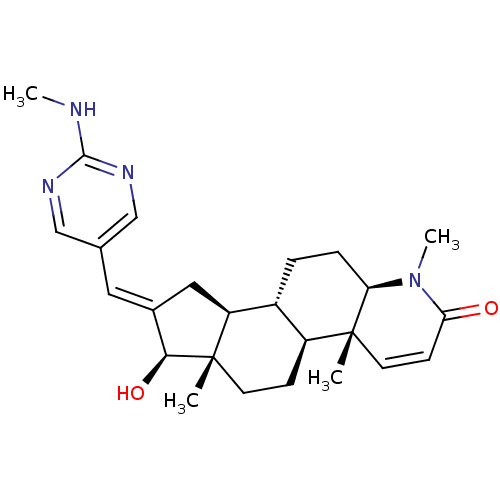 (16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 2.25E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296934 (16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 3.90E+4 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Potassium voltage-gated channel subfamily H member 2 (Homo sapiens (Human)) | BDBM50296932 ((4aR,4bS,6aS,7S,9aS,9bR,11aR)-7-hydroxy-1,4a,6a-tr...) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | 1.30E+5 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Binding affinity to human ERG potassium channel | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296932 ((4aR,4bS,6aS,7S,9aS,9bR,11aR)-7-hydroxy-1,4a,6a-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296945 (16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296935 (16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296934 (16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 24 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296936 (16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 27 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296933 (16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5al...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296947 (16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 31 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296939 (16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296937 (16-[(3,5-Difluorophenyl)methylidene]-17beta-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 41 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296946 (16-[(2-Methylpyrimidin-5-yl)methylidene]-17beta-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296944 (16-[(2-cyclopropylpyrimidin-5-yl)methylidene]-17be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 51 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 59 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296942 (16-(Pyridin-3-ylmethylidene)-17beta-hydroxy-4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296938 (16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 116 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296941 (16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 129 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296940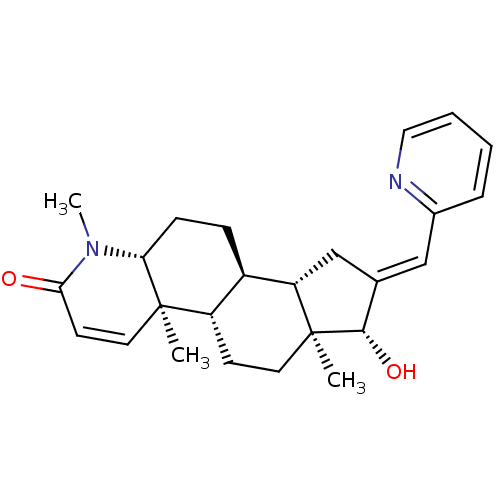 (16-(Pyridin-2-ylmethylidene)-17beta-hydroxy-4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 292 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296948 (16-{[(Pyridin-2-yl)pyrimidin-5-yl]methylidene}-17b...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 715 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Displacement of [3H]methyltrienolone from androgen receptor in human MDA-MB-453 cells | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Mineralocorticoid receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB MMDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of mineralocorticoid receptor | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Glucocorticoid receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of glucocorticoid receptor | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Estrogen receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB UniProtKB/SwissProt antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of estrogen receptor | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Progesterone receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Inhibition of progesterone receptor | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296932 ((4aR,4bS,6aS,7S,9aS,9bR,11aR)-7-hydroxy-1,4a,6a-tr...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296937 (16-[(3,5-Difluorophenyl)methylidene]-17beta-hydrox...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296943 (16-(Pyrimidin-5-ylmethylidene)-17beta-hydroxy-4-me...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296935 (16alpha-(3-Fluorobenzyl)-17beta-hydroxy-4-methyl-4...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | n/a | n/a | 14 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296938 (16-(Phenylmethylidene)-17beta-hydroxy-4-methyl-4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296941 (16-(Pyridin-4-ylmethylidene)-17beta-hydroxy-4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 138 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296934 (16alpha-(Methoxymethyl)-17beta-hydroxy-4-methyl-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 612 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296933 (16alpha-(Methyl)-17beta-hydroxy-4-methyl-4-aza-5al...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 119 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM18161 ((1S,2S,7S,10R,11S,14S,15S)-14-hydroxy-2,15-dimethy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | Purchase CHEMBL KEGG MMDB PC cid PC sid PDB UniChem Patents Similars | PDB Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair |  3D Structure (crystal) | ||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296942 (16-(Pyridin-3-ylmethylidene)-17beta-hydroxy-4-meth...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 62 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296946 (16-[(2-Methylpyrimidin-5-yl)methylidene]-17beta-hy...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 8 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296939 (16-(Methylmethylidene)-17beta-hydroxy-4-methyl-4-a...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 17 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296945 (16-[(2-Methoxypyrimidin-5-yl)methylidene]-17beta-h...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296947 (16-{[(2-Methylamino)pyrimidin-5-yl]methylidene}-17...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 5 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296936 (16-[(3-Fluorophenyl)methylidene]-17beta-hydroxy-4-...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 12 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Androgen receptor (Homo sapiens (Human)) | BDBM50296944 (16-[(2-cyclopropylpyrimidin-5-yl)methylidene]-17be...) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | n/a | n/a | 26 | n/a | n/a | n/a | n/a |
Merck Research Laboratories Curated by ChEMBL | Assay Description Agonist activity at androgen receptor in human MDA-MB-453 cells transfected with MMTV-LUC assessed as induction of MMTV-LTR/promoter linked LUC gene ... | J Med Chem 52: 4578-81 (2009) Article DOI: 10.1021/jm900880r BindingDB Entry DOI: 10.7270/Q2K35TPQ | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||