

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Collagenase 3 (Homo sapiens (Human)) | BDBM50356946 (CHEMBL1916054) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356961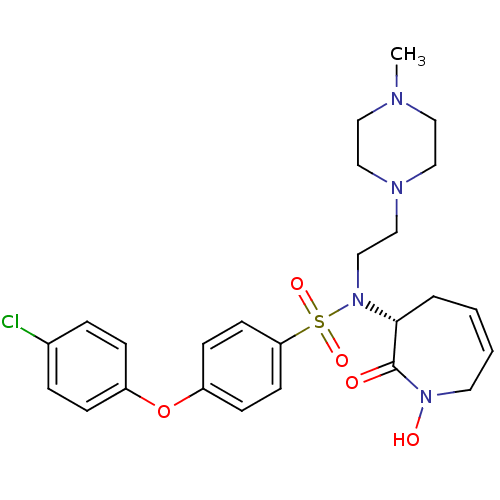 (CHEMBL1916212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356960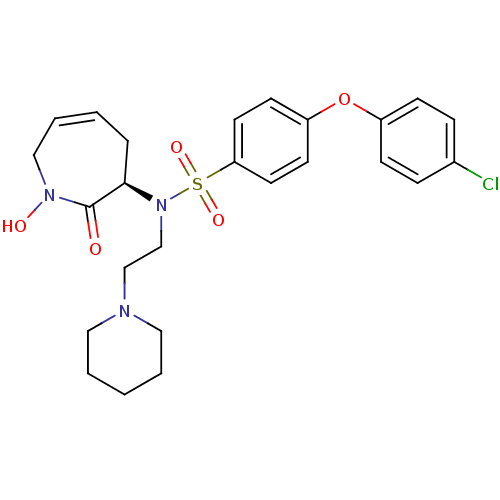 (CHEMBL1916211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.210 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356959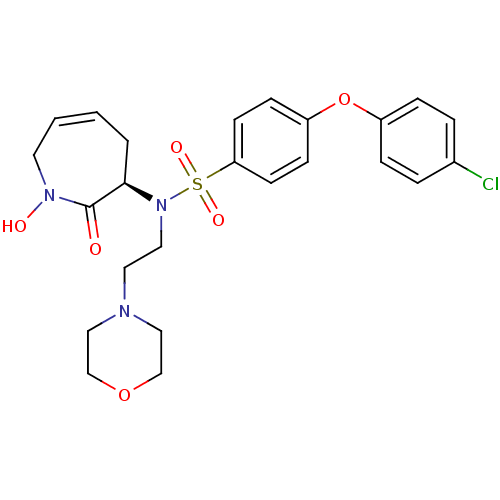 (CHEMBL1916210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.220 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356959 (CHEMBL1916210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.390 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50356942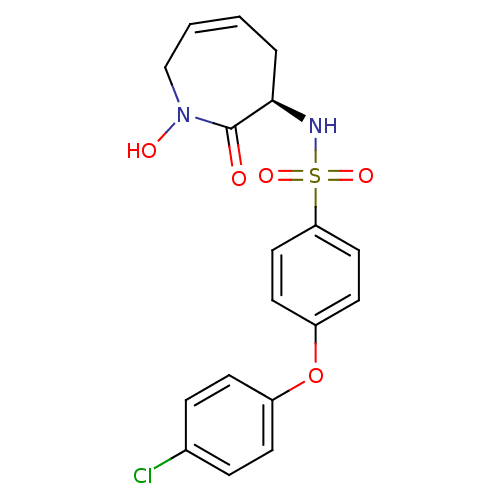 (CHEMBL1916050) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.400 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356946 (CHEMBL1916054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.460 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356961 (CHEMBL1916212) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.490 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356946 (CHEMBL1916054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.530 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356960 (CHEMBL1916211) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.550 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356942 (CHEMBL1916050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.570 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356942 (CHEMBL1916050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.580 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50356959 (CHEMBL1916210) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 0.700 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356964 (CHEMBL1916215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 0.800 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356950 (CHEMBL1916201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356949 (CHEMBL1916057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356945 (CHEMBL1916053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356951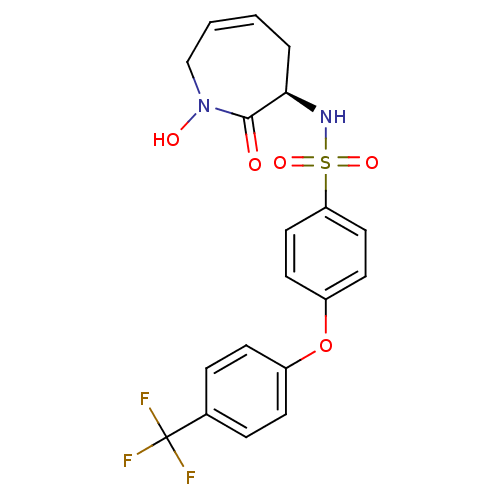 (CHEMBL1916202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356950 (CHEMBL1916201) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356949 (CHEMBL1916057) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356951 (CHEMBL1916202) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356945 (CHEMBL1916053) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356964 (CHEMBL1916215) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 2.40 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Collagenase 3 (Homo sapiens (Human)) | BDBM50356956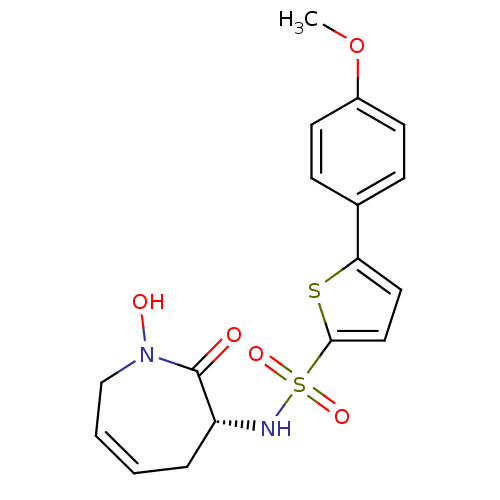 (CHEMBL1916207) | PDB MMDB NCI pathway Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP13 catalytic domain (amino acids 103 to 268) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preinc... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356953 (CHEMBL1916204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356943 (CHEMBL1916051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 3.70 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356956 (CHEMBL1916207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 3.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356954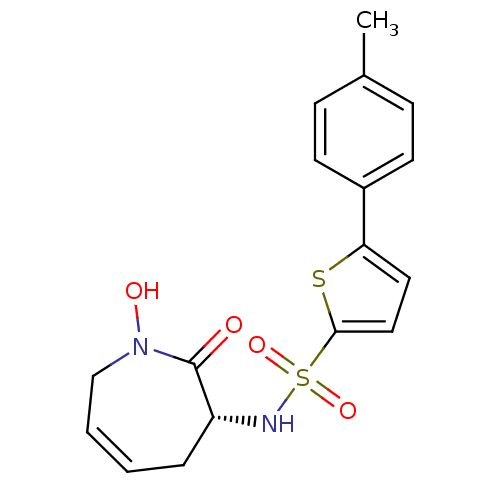 (CHEMBL1916205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356943 (CHEMBL1916051) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 5.90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356963 (CHEMBL1916214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 7 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356954 (CHEMBL1916205) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 9 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356963 (CHEMBL1916214) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356956 (CHEMBL1916207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356955 (CHEMBL1916206) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 11 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356953 (CHEMBL1916204) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356952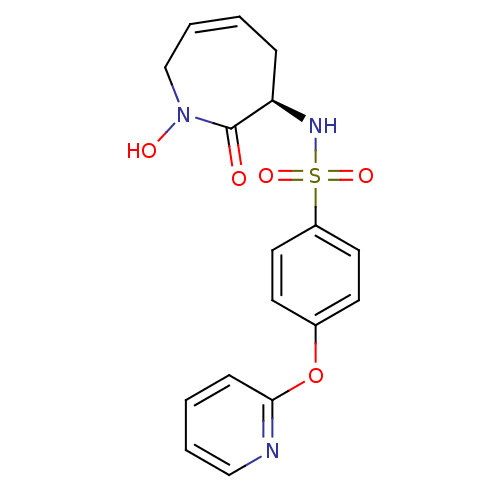 (CHEMBL1916203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356962 (CHEMBL1916213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356955 (CHEMBL1916206) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 16 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356962 (CHEMBL1916213) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356952 (CHEMBL1916203) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50356946 (CHEMBL1916054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 39 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP3 catalytic domain (amino acids 100 to 265) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356948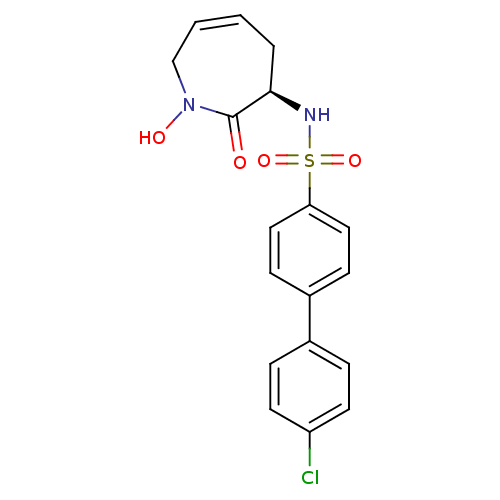 (CHEMBL1916056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 85 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50356942 (CHEMBL1916050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 86 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP3 catalytic domain (amino acids 100 to 265) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50356959 (CHEMBL1916210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 90 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP3 catalytic domain (amino acids 100 to 265) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Stromelysin-1 (Homo sapiens (Human)) | BDBM50356956 (CHEMBL1916207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 493 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP3 catalytic domain (amino acids 100 to 265) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356944 (CHEMBL1916052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356958 (CHEMBL1916209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50356959 (CHEMBL1916210) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP1 catalytic domain (amino acids 100 to 262) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50356942 (CHEMBL1916050) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP1 catalytic domain (amino acids 100 to 262) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356958 (CHEMBL1916209) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356957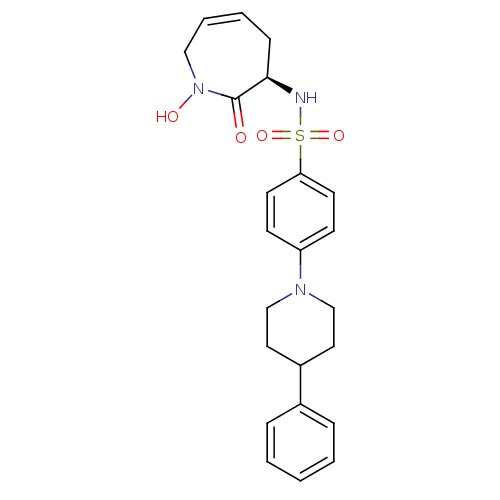 (CHEMBL1916208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50356956 (CHEMBL1916207) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP1 catalytic domain (amino acids 100 to 262) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356944 (CHEMBL1916052) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Interstitial collagenase (Homo sapiens (Human)) | BDBM50356946 (CHEMBL1916054) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP1 catalytic domain (amino acids 100 to 262) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356957 (CHEMBL1916208) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | >1.00E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356941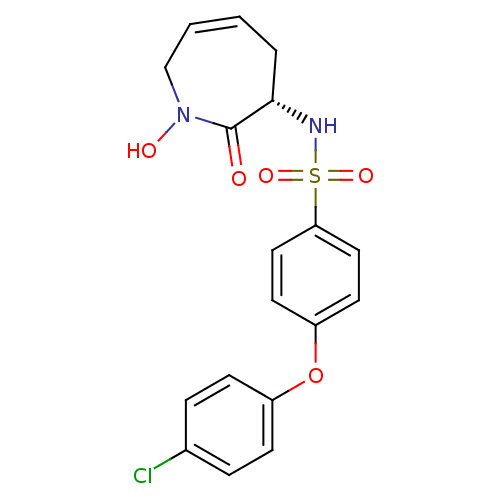 (CHEMBL1916049) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.12E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356948 (CHEMBL1916056) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | Article PubMed | n/a | n/a | 1.51E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356941 (CHEMBL1916049) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Similars | Article PubMed | n/a | n/a | 1.60E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| 72 kDa type IV collagenase (Homo sapiens (Human)) | BDBM50356947 (CHEMBL1916055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 2.32E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of full length human MMP2 (amino acids 1 to 660) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincubated f... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Matrix metalloproteinase-9 (Homo sapiens (Human)) | BDBM50356947 (CHEMBL1916055) | PDB MMDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD DrugBank antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem | Article PubMed | n/a | n/a | 5.09E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
Johnson& Johnson Pharmaceutical Research& Development Curated by ChEMBL | Assay Description Inhibition of human MMP9 catalytic domain (amino acids 107 to 446) using acetyl-Cys(Eu)-Pro-Leu-Gly-Leu-Lys-(QSY7)-Ala-Arg-amide as substrate preincu... | Bioorg Med Chem Lett 21: 6485-90 (2011) Article DOI: 10.1016/j.bmcl.2011.08.068 BindingDB Entry DOI: 10.7270/Q2BP0353 | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||