 Found 44 hits of Enzyme Inhibition Constant Data
Found 44 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089328
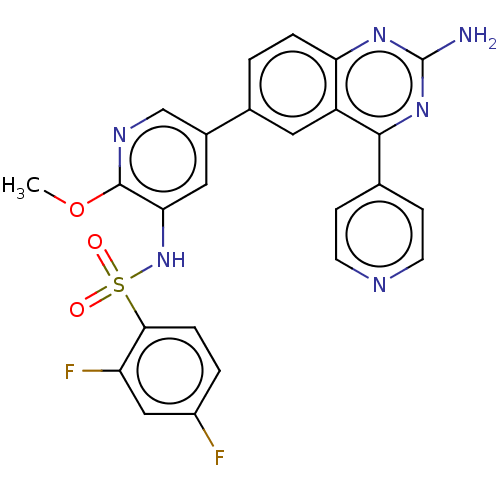
(CHEMBL3577914)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nc(N)nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H18F2N6O3S/c1-36-24-21(33-37(34,35)22-5-3-17(26)12-19(22)27)11-16(13-30-24)15-2-4-20-18(10-15)23(32-25(28)31-20)14-6-8-29-9-7-14/h2-13,33H,1H3,(H2,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0700 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089318
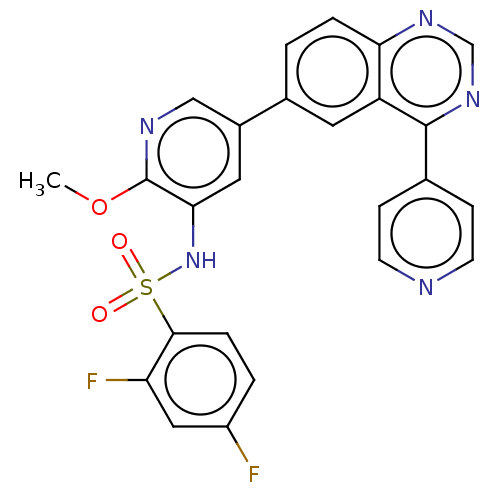
(CHEMBL3577911)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncnc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-22(32-36(33,34)23-5-3-18(26)12-20(23)27)11-17(13-29-25)16-2-4-21-19(10-16)24(31-14-30-21)15-6-8-28-9-7-15/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.0950 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089321

(CHEMBL3577908)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccncc3)c2c1 Show InChI InChI=1S/C26H18F2N4O3S/c1-35-26-24(32-36(33,34)25-5-3-19(27)14-22(25)28)13-18(15-31-26)17-2-4-23-21(12-17)20(8-11-30-23)16-6-9-29-10-7-16/h2-15,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.100 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089305

(CHEMBL3577927)Show SMILES Clc1ccc(s1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C20H11Cl2N5O2S3/c21-16-1-2-17(31-16)32(28,29)27-14-7-12(9-24-19(14)22)15-8-13-18(11-3-5-23-6-4-11)25-10-26-20(13)30-15/h1-10,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.150 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089318

(CHEMBL3577911)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2ncnc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-22(32-36(33,34)23-5-3-18(26)12-20(23)27)11-17(13-29-25)16-2-4-21-19(10-16)24(31-14-30-21)15-6-8-28-9-7-15/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 0.200 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089303
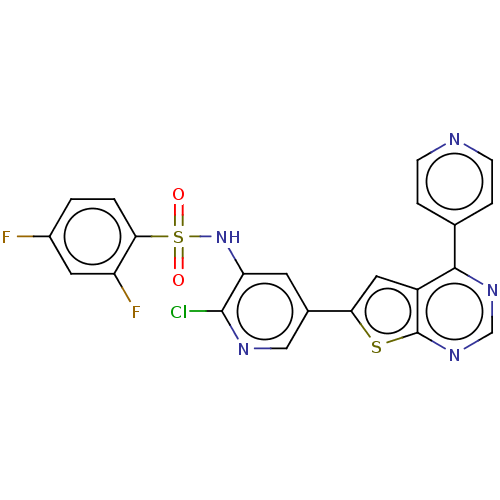
(CHEMBL3577925)Show SMILES Fc1ccc(c(F)c1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H12ClF2N5O2S2/c23-21-17(30-34(31,32)19-2-1-14(24)8-16(19)25)7-13(10-27-21)18-9-15-20(12-3-5-26-6-4-12)28-11-29-22(15)33-18/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.230 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089329
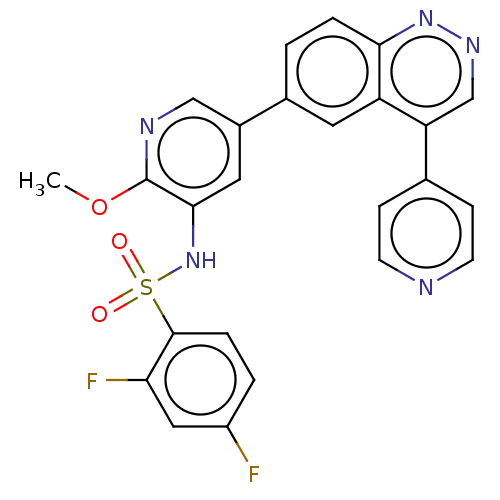
(CHEMBL3577913)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nncc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)16-2-4-22-19(10-16)20(14-30-31-22)15-6-8-28-9-7-15/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.270 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089328

(CHEMBL3577914)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nc(N)nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H18F2N6O3S/c1-36-24-21(33-37(34,35)22-5-3-17(26)12-19(22)27)11-16(13-30-24)15-2-4-20-18(10-15)23(32-25(28)31-20)14-6-8-29-9-7-14/h2-13,33H,1H3,(H2,28,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.290 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089304
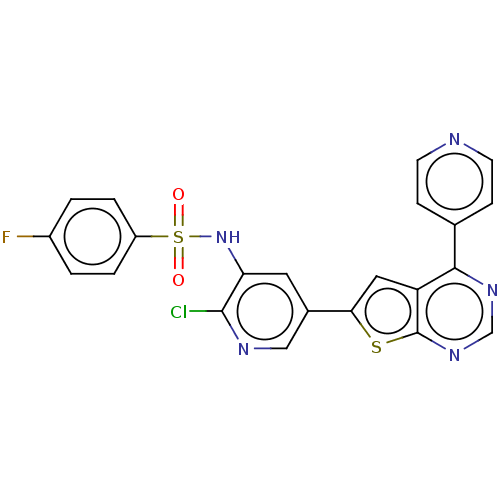
(CHEMBL3577926)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H13ClFN5O2S2/c23-21-18(29-33(30,31)16-3-1-15(24)2-4-16)9-14(11-26-21)19-10-17-20(13-5-7-25-8-6-13)27-12-28-22(17)32-19/h1-12,29H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089321

(CHEMBL3577908)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nccc(-c3ccncc3)c2c1 Show InChI InChI=1S/C26H18F2N4O3S/c1-35-26-24(32-36(33,34)25-5-3-19(27)14-22(25)28)13-18(15-31-26)17-2-4-23-21(12-17)20(8-11-30-23)16-6-9-29-10-7-16/h2-15,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.75 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089319

(CHEMBL3577910)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ccnc2cn1)-c1ccncc1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-22(32-36(33,34)24-3-2-17(26)11-20(24)27)10-16(13-31-25)21-12-19-18(15-4-7-28-8-5-15)6-9-29-23(19)14-30-21/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089301
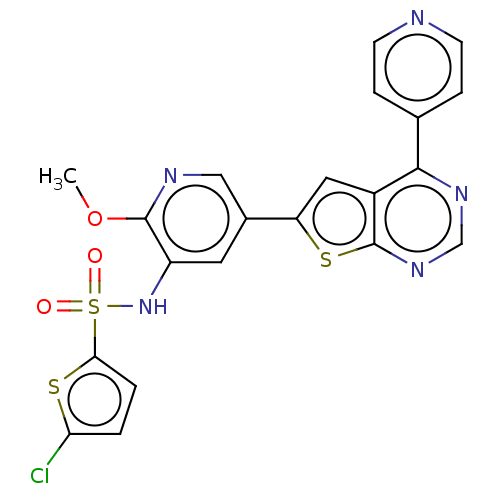
(CHEMBL3577923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(Cl)s1)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C21H14ClN5O3S3/c1-30-20-15(27-33(28,29)18-3-2-17(22)32-18)8-13(10-24-20)16-9-14-19(12-4-6-23-7-5-12)25-11-26-21(14)31-16/h2-11,27H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.30 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089302
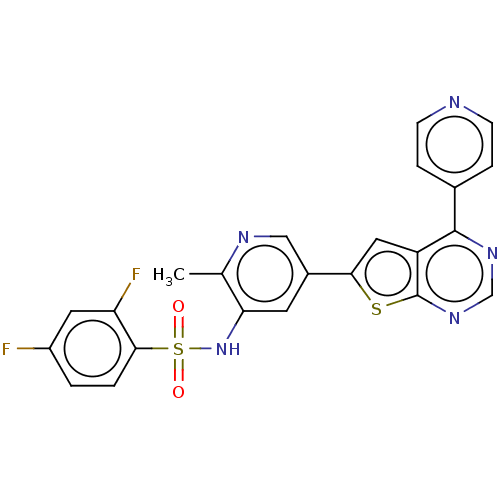
(CHEMBL3577924)Show SMILES Cc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C23H15F2N5O2S2/c1-13-19(30-34(31,32)21-3-2-16(24)9-18(21)25)8-15(11-27-13)20-10-17-22(14-4-6-26-7-5-14)28-12-29-23(17)33-20/h2-12,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.70 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089299
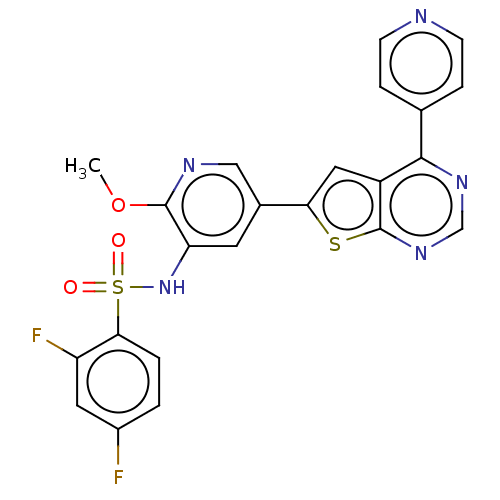
(CHEMBL3577921)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C23H15F2N5O3S2/c1-33-22-18(30-35(31,32)20-3-2-15(24)9-17(20)25)8-14(11-27-22)19-10-16-21(13-4-6-26-7-5-13)28-12-29-23(16)34-19/h2-12,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.10 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089319

(CHEMBL3577910)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ccnc2cn1)-c1ccncc1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-22(32-36(33,34)24-3-2-17(26)11-20(24)27)10-16(13-31-25)21-12-19-18(15-4-7-28-8-5-15)6-9-29-23(19)14-30-21/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50089303

(CHEMBL3577925)Show SMILES Fc1ccc(c(F)c1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H12ClF2N5O2S2/c23-21-17(30-34(31,32)19-2-1-14(24)8-16(19)25)7-13(10-27-21)18-9-15-20(12-3-5-26-6-4-12)28-11-29-22(15)33-18/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.10 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089300
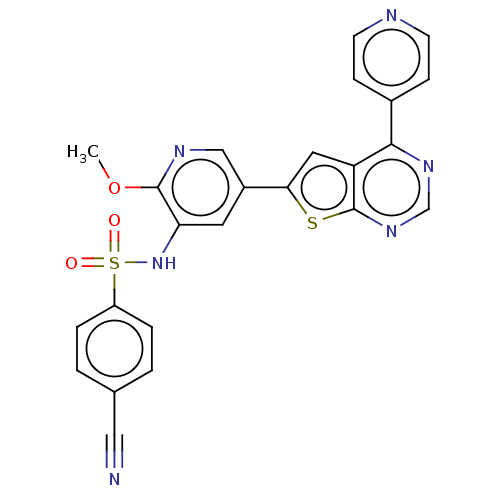
(CHEMBL3577922)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(cc1)C#N)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C24H16N6O3S2/c1-33-23-20(30-35(31,32)18-4-2-15(12-25)3-5-18)10-17(13-27-23)21-11-19-22(16-6-8-26-9-7-16)28-14-29-24(19)34-21/h2-11,13-14,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50089303

(CHEMBL3577925)Show SMILES Fc1ccc(c(F)c1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H12ClF2N5O2S2/c23-21-17(30-34(31,32)19-2-1-14(24)8-16(19)25)7-13(10-27-21)18-9-15-20(12-3-5-26-6-4-12)28-11-29-22(15)33-18/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.60 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 6.20 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50089303

(CHEMBL3577925)Show SMILES Fc1ccc(c(F)c1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H12ClF2N5O2S2/c23-21-17(30-34(31,32)19-2-1-14(24)8-16(19)25)7-13(10-27-21)18-9-15-20(12-3-5-26-6-4-12)28-11-29-22(15)33-18/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 10 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM92862

(US9284315, BEZ-235 | mTOR Inhibitor, BEZ235)Show SMILES Cn1c2cnc3ccc(cc3c2n(-c2ccc(cc2)C(C)(C)C#N)c1=O)-c1cnc2ccccc2c1 Show InChI InChI=1S/C30H23N5O/c1-30(2,18-31)22-9-11-23(12-10-22)35-28-24-15-19(21-14-20-6-4-5-7-25(20)32-16-21)8-13-26(24)33-17-27(28)34(3)29(35)36/h4-17H,1-3H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 12 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit delta isoform
(Homo sapiens (Human)) | BDBM50089299

(CHEMBL3577921)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C23H15F2N5O3S2/c1-33-22-18(30-35(31,32)20-3-2-15(24)9-17(20)25)8-14(11-27-22)19-10-16-21(13-4-6-26-7-5-13)28-12-29-23(16)34-19/h2-12,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 13 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kdelta (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089322
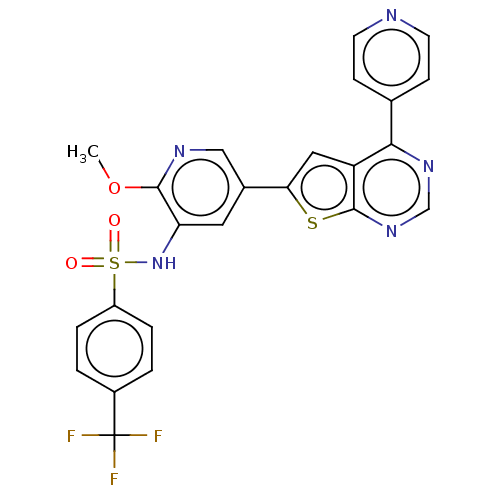
(CHEMBL3577920)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(cc1)C(F)(F)F)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C24H16F3N5O3S2/c1-35-22-19(32-37(33,34)17-4-2-16(3-5-17)24(25,26)27)10-15(12-29-22)20-11-18-21(14-6-8-28-9-7-14)30-13-31-23(18)36-20/h2-13,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 17 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50447619
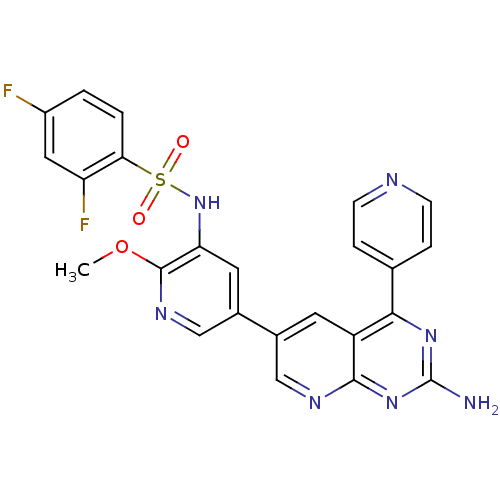
(CHEMBL3112719)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cnc2nc(N)nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C24H17F2N7O3S/c1-36-23-19(33-37(34,35)20-3-2-16(25)10-18(20)26)9-15(12-30-23)14-8-17-21(13-4-6-28-7-5-13)31-24(27)32-22(17)29-11-14/h2-12,33H,1H3,(H2,27,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 18 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit gamma isoform
(Homo sapiens (Human)) | BDBM50089299

(CHEMBL3577921)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C23H15F2N5O3S2/c1-33-22-18(30-35(31,32)20-3-2-15(24)9-17(20)25)8-14(11-27-22)19-10-16-21(13-4-6-26-7-5-13)28-12-29-23(16)34-19/h2-12,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 21 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kgamma (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089329

(CHEMBL3577913)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1ccc2nncc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-23(32-36(33,34)24-5-3-18(26)12-21(24)27)11-17(13-29-25)16-2-4-22-19(10-16)20(14-30-31-22)15-6-8-28-9-7-15/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 22 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
(Homo sapiens (Human)) | BDBM50089299

(CHEMBL3577921)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C23H15F2N5O3S2/c1-33-22-18(30-35(31,32)20-3-2-15(24)9-17(20)25)8-14(11-27-22)19-10-16-21(13-4-6-26-7-5-13)28-12-29-23(16)34-19/h2-12,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 23 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kbeta (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089305

(CHEMBL3577927)Show SMILES Clc1ccc(s1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C20H11Cl2N5O2S3/c21-16-1-2-17(31-16)32(28,29)27-14-7-12(9-24-19(14)22)15-8-13-18(11-3-5-23-6-4-11)25-10-26-20(13)30-15/h1-10,27H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 26 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089320
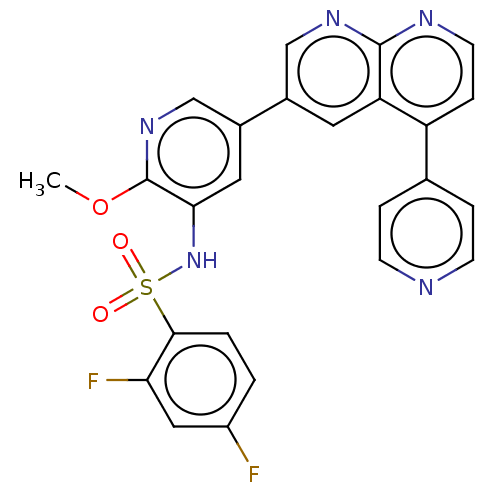
(CHEMBL3577909)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cnc2nccc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-22(32-36(33,34)23-3-2-18(26)12-21(23)27)11-17(14-31-25)16-10-20-19(15-4-7-28-8-5-15)6-9-29-24(20)30-13-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 35 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50447619

(CHEMBL3112719)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cnc2nc(N)nc(-c3ccncc3)c2c1 Show InChI InChI=1S/C24H17F2N7O3S/c1-36-23-19(33-37(34,35)20-3-2-16(25)10-18(20)26)9-15(12-30-23)14-8-17-21(13-4-6-28-7-5-13)31-24(27)32-22(17)29-11-14/h2-12,33H,1H3,(H2,27,29,31,32) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 36 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089324

(CHEMBL3577918)Show SMILES COc1ncc(cc1NS(C)(=O)=O)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C18H15N5O3S2/c1-26-17-14(23-28(2,24)25)7-12(9-20-17)15-8-13-16(11-3-5-19-6-4-11)21-10-22-18(13)27-15/h3-10,23H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089304

(CHEMBL3577926)Show SMILES Fc1ccc(cc1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H13ClFN5O2S2/c23-21-18(29-33(30,31)16-3-1-15(24)2-4-16)9-14(11-26-21)19-10-17-20(13-5-7-25-8-6-13)27-12-28-22(17)32-19/h1-12,29H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 44 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089317
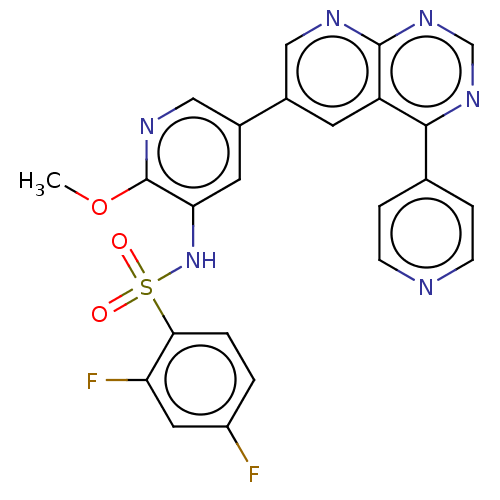
(CHEMBL3577912)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cnc2ncnc(-c3ccncc3)c2c1 Show InChI InChI=1S/C24H16F2N6O3S/c1-35-24-20(32-36(33,34)21-3-2-17(25)10-19(21)26)9-16(12-29-24)15-8-18-22(14-4-6-27-7-5-14)30-13-31-23(18)28-11-15/h2-13,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089303

(CHEMBL3577925)Show SMILES Fc1ccc(c(F)c1)S(=O)(=O)Nc1cc(cnc1Cl)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C22H12ClF2N5O2S2/c23-21-17(30-34(31,32)19-2-1-14(24)8-16(19)25)7-13(10-27-21)18-9-15-20(12-3-5-26-6-4-12)28-11-29-22(15)33-18/h1-11,30H | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 54 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089317

(CHEMBL3577912)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cnc2ncnc(-c3ccncc3)c2c1 Show InChI InChI=1S/C24H16F2N6O3S/c1-35-24-20(32-36(33,34)21-3-2-17(25)10-19(21)26)9-16(12-29-24)15-8-18-22(14-4-6-27-7-5-14)30-13-31-23(18)28-11-15/h2-13,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 62 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089320

(CHEMBL3577909)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cnc2nccc(-c3ccncc3)c2c1 Show InChI InChI=1S/C25H17F2N5O3S/c1-35-25-22(32-36(33,34)23-3-2-18(26)12-21(23)27)11-17(14-31-25)16-10-20-19(15-4-7-28-8-5-15)6-9-29-24(20)30-13-16/h2-14,32H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 67 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089301

(CHEMBL3577923)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(Cl)s1)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C21H14ClN5O3S3/c1-30-20-15(27-33(28,29)18-3-2-17(22)32-18)8-13(10-24-20)16-9-14-19(12-4-6-23-7-5-12)25-11-26-21(14)31-16/h2-11,27H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 68 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089323
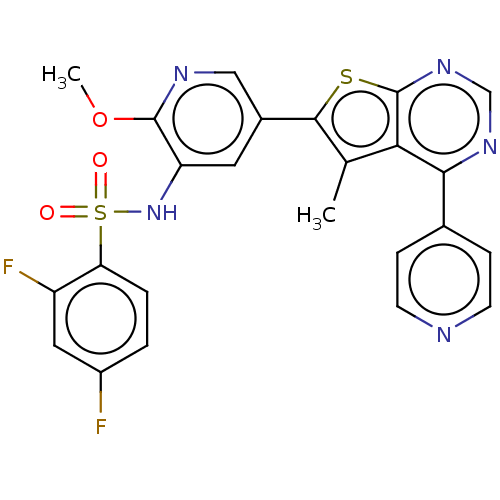
(CHEMBL3577919)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1sc2ncnc(-c3ccncc3)c2c1C Show InChI InChI=1S/C24H17F2N5O3S2/c1-13-20-21(14-5-7-27-8-6-14)29-12-30-24(20)35-22(13)15-9-18(23(34-2)28-11-15)31-36(32,33)19-4-3-16(25)10-17(19)26/h3-12,31H,1-2H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 72 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089299

(CHEMBL3577921)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C23H15F2N5O3S2/c1-33-22-18(30-35(31,32)20-3-2-15(24)9-17(20)25)8-14(11-27-22)19-10-16-21(13-4-6-26-7-5-13)28-12-29-23(16)34-19/h2-12,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 218 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089302

(CHEMBL3577924)Show SMILES Cc1ncc(cc1NS(=O)(=O)c1ccc(F)cc1F)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C23H15F2N5O2S2/c1-13-19(30-34(31,32)21-3-2-16(24)9-18(21)25)8-15(11-27-13)20-10-17-22(14-4-6-26-7-5-14)28-12-29-23(17)33-20/h2-12,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 345 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Serine/threonine-protein kinase mTOR
(Homo sapiens (Human)) | BDBM50089300

(CHEMBL3577922)Show SMILES COc1ncc(cc1NS(=O)(=O)c1ccc(cc1)C#N)-c1cc2c(ncnc2s1)-c1ccncc1 Show InChI InChI=1S/C24H16N6O3S2/c1-33-23-20(30-35(31,32)18-4-2-15(12-25)3-5-18)10-17(13-27-23)21-11-19-22(16-6-8-26-9-7-16)28-14-29-24(19)34-21/h2-11,13-14,30H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 363 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of mTOR (unknown origin) using GFP-4E-BP1 as substrate after 1 hr by TR-FRET assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089327
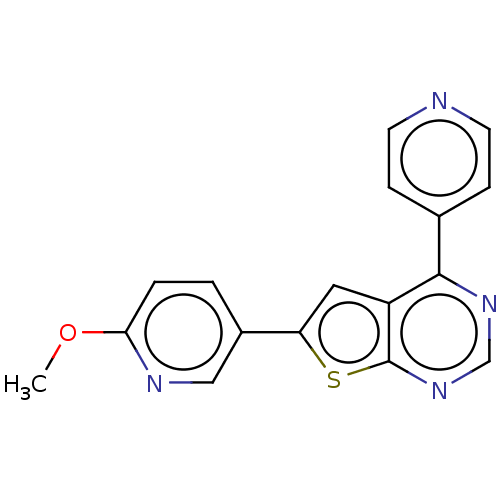
(CHEMBL3577915)Show InChI InChI=1S/C17H12N4OS/c1-22-15-3-2-12(9-19-15)14-8-13-16(11-4-6-18-7-5-11)20-10-21-17(13)23-14/h2-10H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.23E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089325
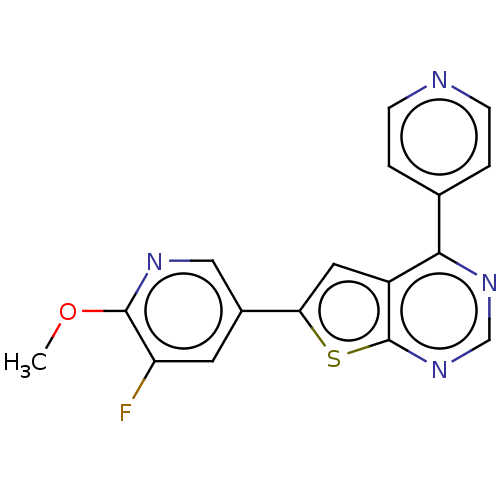
(CHEMBL3577917)Show InChI InChI=1S/C17H11FN4OS/c1-23-16-13(18)6-11(8-20-16)14-7-12-15(10-2-4-19-5-3-10)21-9-22-17(12)24-14/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >2.50E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
(Homo sapiens (Human)) | BDBM50089326
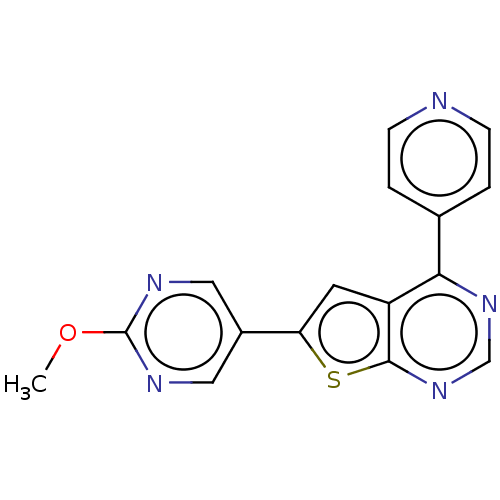
(CHEMBL3577916)Show InChI InChI=1S/C16H11N5OS/c1-22-16-18-7-11(8-19-16)13-6-12-14(10-2-4-17-5-3-10)20-9-21-15(12)23-13/h2-9H,1H3 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.49E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
PKUCare Pharmaceutical R&D Center
Curated by ChEMBL
| Assay Description
Inhibition of PI3Kalpha (unknown origin) using PIP2/PS as substrate after 1 hr by luciferase-based luminescence assay |
ACS Med Chem Lett 6: 434-8 (2015)
Article DOI: 10.1021/ml5005014
BindingDB Entry DOI: 10.7270/Q29Z96M1 |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data