 Found 23 hits of Enzyme Inhibition Constant Data
Found 23 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115402
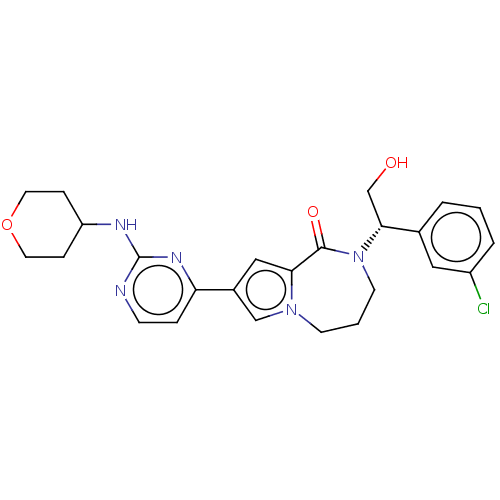
(CHEMBL3608588)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H28ClN5O3/c26-19-4-1-3-17(13-19)23(16-32)31-10-2-9-30-15-18(14-22(30)24(31)33)21-5-8-27-25(29-21)28-20-6-11-34-12-7-20/h1,3-5,8,13-15,20,23,32H,2,6-7,9-12,16H2,(H,27,28,29)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0100 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115401
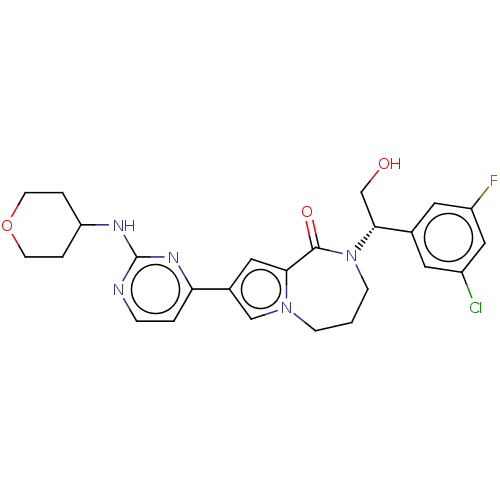
(CHEMBL3608589)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cc(F)cc(Cl)c1 |r| Show InChI InChI=1S/C25H27ClFN5O3/c26-18-10-16(11-19(27)13-18)23(15-33)32-7-1-6-31-14-17(12-22(31)24(32)34)21-2-5-28-25(30-21)29-20-3-8-35-9-4-20/h2,5,10-14,20,23,33H,1,3-4,6-9,15H2,(H,28,29,30)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 0.0190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115398
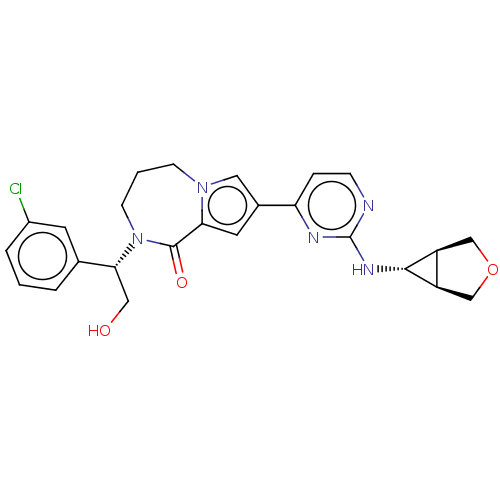
(CHEMBL3608586)Show SMILES [H][C@@]12COC[C@]1([H])[C@H]2Nc1nccc(n1)-c1cc2C(=O)N(CCCn2c1)[C@H](CO)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26ClN5O3/c26-17-4-1-3-15(9-17)22(12-32)31-8-2-7-30-11-16(10-21(30)24(31)33)20-5-6-27-25(28-20)29-23-18-13-34-14-19(18)23/h1,3-6,9-11,18-19,22-23,32H,2,7-8,12-14H2,(H,27,28,29)/t18-,19+,22-,23+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50094465
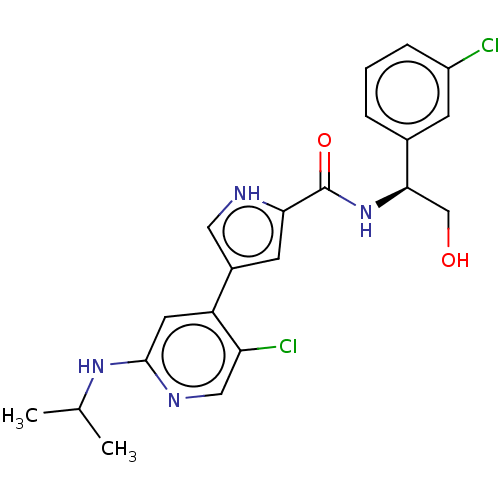
(CHEMBL3590106 | US10525036, Example BVD-523 | US10...)Show SMILES CC(C)Nc1cc(-c2c[nH]c(c2)C(=O)N[C@H](CO)c2cccc(Cl)c2)c(Cl)cn1 |r| Show InChI InChI=1S/C19H21NO3/c21-18(23-17-11-13-20-14-12-17)19(22,15-7-3-1-4-8-15)16-9-5-2-6-10-16/h1-10,17,20,22H,11-14H2 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| Purchase
CHEMBL
MCE
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.0300 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115397
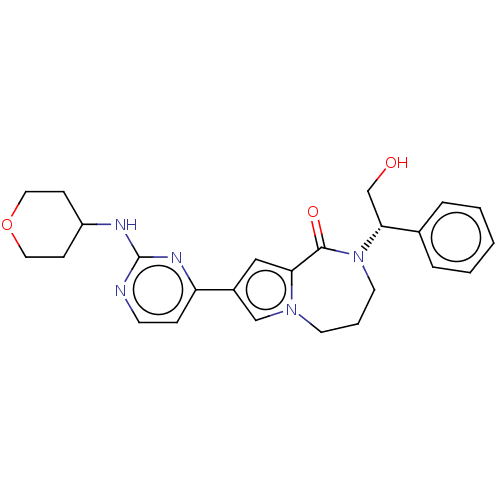
(CHEMBL3608462)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3/c31-17-23(18-5-2-1-3-6-18)30-12-4-11-29-16-19(15-22(29)24(30)32)21-7-10-26-25(28-21)27-20-8-13-33-14-9-20/h1-3,5-7,10,15-16,20,23,31H,4,8-9,11-14,17H2,(H,26,27,28)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0330 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115399
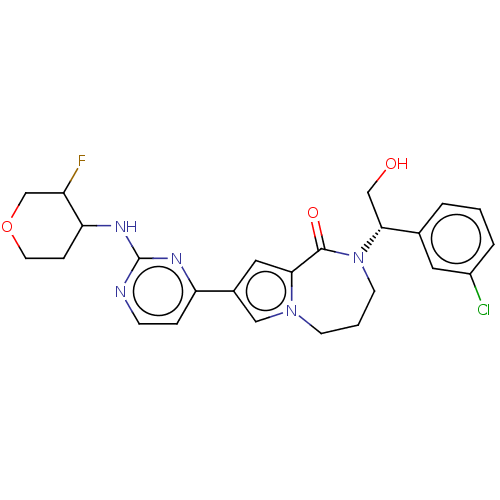
(CHEMBL3608464)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2F)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H27ClFN5O3/c26-18-4-1-3-16(11-18)23(14-33)32-9-2-8-31-13-17(12-22(31)24(32)34)20-5-7-28-25(29-20)30-21-6-10-35-15-19(21)27/h1,3-5,7,11-13,19,21,23,33H,2,6,8-10,14-15H2,(H,28,29,30)/t19?,21?,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0350 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115396
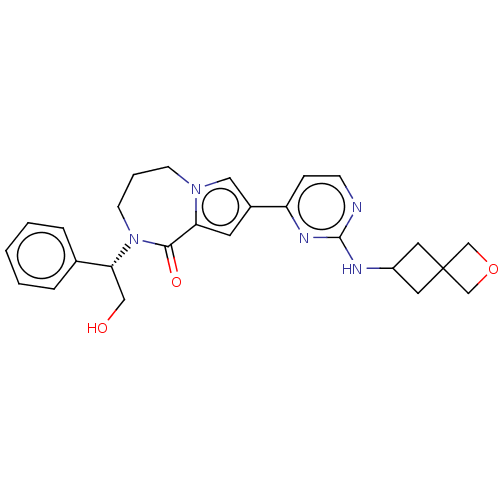
(CHEMBL3608463)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(NC2CC3(COC3)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C26H29N5O3/c32-15-23(18-5-2-1-3-6-18)31-10-4-9-30-14-19(11-22(30)24(31)33)21-7-8-27-25(29-21)28-20-12-26(13-20)16-34-17-26/h1-3,5-8,11,14,20,23,32H,4,9-10,12-13,15-17H2,(H,27,28,29)/t23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0400 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115400

(CHEMBL3608587)Show SMILES OC[C@@H](N1C[C@H](F)Cn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H27ClFN5O3/c26-18-3-1-2-16(10-18)23(15-33)32-14-19(27)13-31-12-17(11-22(31)24(32)34)21-4-7-28-25(30-21)29-20-5-8-35-9-6-20/h1-4,7,10-12,19-20,23,33H,5-6,8-9,13-15H2,(H,28,29,30)/t19-,23-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.0500 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115403

(CHEMBL3608591)Show SMILES OC[C@@H](N1CCn2cc(cc2C1=O)-c1ccnc(NC2CCOCC2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C24H26ClN5O3/c25-18-3-1-2-16(12-18)22(15-31)30-9-8-29-14-17(13-21(29)23(30)32)20-4-7-26-24(28-20)27-19-5-10-33-11-6-19/h1-4,7,12-14,19,22,31H,5-6,8-11,15H2,(H,26,27,28)/t22-/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.160 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115393

(CHEMBL3608458)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)[C@@H](F)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c26-19-12-18(13-23(19)33)28-25-27-8-7-20(29-25)17-11-21-24(34)31(10-4-9-30(21)14-17)22(15-32)16-5-2-1-3-6-16/h1-3,5-8,11,14,18-19,22-23,32-33H,4,9-10,12-13,15H2,(H,27,28,29)/t18-,19-,22+,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115394

(CHEMBL3608461)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@H]2CC[C@@H](O)C2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H28ClN5O3/c26-18-4-1-3-16(11-18)23(15-32)31-10-2-9-30-14-17(12-22(30)24(31)34)21-7-8-27-25(29-21)28-19-5-6-20(33)13-19/h1,3-4,7-8,11-12,14,19-20,23,32-33H,2,5-6,9-10,13,15H2,(H,27,28,29)/t19-,20+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 0.190 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115405
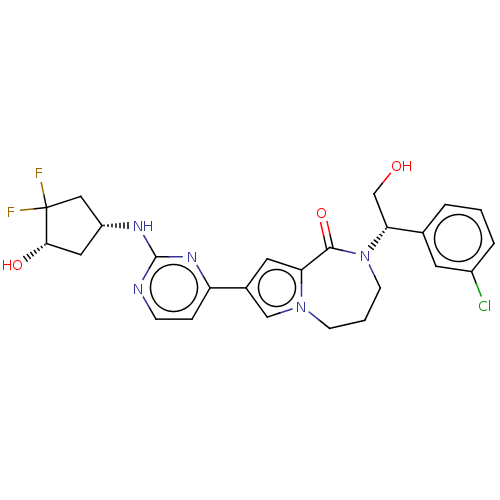
(CHEMBL3608459)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)C(F)(F)C2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26ClF2N5O3/c26-17-4-1-3-15(9-17)21(14-34)33-8-2-7-32-13-16(10-20(32)23(33)36)19-5-6-29-24(31-19)30-18-11-22(35)25(27,28)12-18/h1,3-6,9-10,13,18,21-22,34-35H,2,7-8,11-12,14H2,(H,29,30,31)/t18-,21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 0.260 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115395

(CHEMBL3608460)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@H]2CC[C@@H](O)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3/c31-16-23(17-5-2-1-3-6-17)30-12-4-11-29-15-18(13-22(29)24(30)33)21-9-10-26-25(28-21)27-19-7-8-20(32)14-19/h1-3,5-6,9-10,13,15,19-20,23,31-32H,4,7-8,11-12,14,16H2,(H,26,27,28)/t19-,20+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 2.30 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115394

(CHEMBL3608461)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@H]2CC[C@@H](O)C2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H28ClN5O3/c26-18-4-1-3-16(11-18)23(15-32)31-10-2-9-30-14-17(12-22(30)24(31)34)21-7-8-27-25(29-21)28-19-5-6-20(33)13-19/h1,3-4,7-8,11-12,14,19-20,23,32-33H,2,5-6,9-10,13,15H2,(H,27,28,29)/t19-,20+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
PDB
UniChem
Similars
| PDB
Article
PubMed
| n/a | n/a | 52 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 in human A375 cells assessed as phosphorylated FRA level |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | 
3D Structure (crystal) |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115405

(CHEMBL3608459)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)C(F)(F)C2)n1)c1cccc(Cl)c1 |r| Show InChI InChI=1S/C25H26ClF2N5O3/c26-17-4-1-3-15(9-17)21(14-34)33-8-2-7-32-13-16(10-20(32)23(33)36)19-5-6-29-24(31-19)30-18-11-22(35)25(27,28)12-18/h1,3-6,9-10,13,18,21-22,34-35H,2,7-8,11-12,14H2,(H,29,30,31)/t18-,21-,22+/m1/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 61 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 in human A375 cells assessed as phosphorylated FRA level |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115393

(CHEMBL3608458)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)[C@@H](F)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c26-19-12-18(13-23(19)33)28-25-27-8-7-20(29-25)17-11-21-24(34)31(10-4-9-30(21)14-17)22(15-32)16-5-2-1-3-6-16/h1-3,5-8,11,14,18-19,22-23,32-33H,4,9-10,12-13,15H2,(H,27,28,29)/t18-,19-,22+,23-/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 69 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 in human A375 cells assessed as phosphorylated FRA level |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115404

(CHEMBL3608590)Show SMILES CC(C)Nc1nccc(n1)-c1cc2C(=O)N(Cc3ccccc3)CCn2c1 Show InChI InChI=1S/C21H23N5O/c1-15(2)23-21-22-9-8-18(24-21)17-12-19-20(27)26(11-10-25(19)14-17)13-16-6-4-3-5-7-16/h3-9,12,14-15H,10-11,13H2,1-2H3,(H,22,23,24) | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 76 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Mitogen-activated protein kinase 1
(Homo sapiens (Human)) | BDBM50115395

(CHEMBL3608460)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@H]2CC[C@@H](O)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H29N5O3/c31-16-23(17-5-2-1-3-6-17)30-12-4-11-29-15-18(13-22(29)24(30)33)21-9-10-26-25(28-21)27-19-7-8-20(32)14-19/h1-3,5-6,9-10,13,15,19-20,23,31-32H,4,7-8,11-12,14,16H2,(H,26,27,28)/t19-,20+,23+/m0/s1 | PDB
MMDB
NCI pathway
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | 278 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of ERK2 in human A375 cells assessed as phosphorylated FRA level |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50115393

(CHEMBL3608458)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)[C@@H](F)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c26-19-12-18(13-23(19)33)28-25-27-8-7-20(29-25)17-11-21-24(34)31(10-4-9-30(21)14-17)22(15-32)16-5-2-1-3-6-16/h1-3,5-8,11,14,18-19,22-23,32-33H,4,9-10,12-13,15H2,(H,27,28,29)/t18-,19-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.88E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using testosterone as substrate |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2D6
(Homo sapiens (Human)) | BDBM50115393

(CHEMBL3608458)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)[C@@H](F)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c26-19-12-18(13-23(19)33)28-25-27-8-7-20(29-25)17-11-21-24(34)31(10-4-9-30(21)14-17)22(15-32)16-5-2-1-3-6-16/h1-3,5-8,11,14,18-19,22-23,32-33H,4,9-10,12-13,15H2,(H,27,28,29)/t18-,19-,22+,23-/m0/s1 | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2D6 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 3A4
(Homo sapiens (Human)) | BDBM50115393

(CHEMBL3608458)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)[C@@H](F)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c26-19-12-18(13-23(19)33)28-25-27-8-7-20(29-25)17-11-21-24(34)31(10-4-9-30(21)14-17)22(15-32)16-5-2-1-3-6-16/h1-3,5-8,11,14,18-19,22-23,32-33H,4,9-10,12-13,15H2,(H,27,28,29)/t18-,19-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP3A4 (unknown origin) using midazolam as substrate |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 1A2
(Homo sapiens (Human)) | BDBM50115393

(CHEMBL3608458)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)[C@@H](F)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c26-19-12-18(13-23(19)33)28-25-27-8-7-20(29-25)17-11-21-24(34)31(10-4-9-30(21)14-17)22(15-32)16-5-2-1-3-6-16/h1-3,5-8,11,14,18-19,22-23,32-33H,4,9-10,12-13,15H2,(H,27,28,29)/t18-,19-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP1A2 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |
Cytochrome P450 2C9
(Homo sapiens (Human)) | BDBM50115393

(CHEMBL3608458)Show SMILES OC[C@@H](N1CCCn2cc(cc2C1=O)-c1ccnc(N[C@@H]2C[C@H](O)[C@@H](F)C2)n1)c1ccccc1 |r| Show InChI InChI=1S/C25H28FN5O3/c26-19-12-18(13-23(19)33)28-25-27-8-7-20(29-25)17-11-21-24(34)31(10-4-9-30(21)14-17)22(15-32)16-5-2-1-3-6-16/h1-3,5-8,11,14,18-19,22-23,32-33H,4,9-10,12-13,15H2,(H,27,28,29)/t18-,19-,22+,23-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
DrugBank
antibodypedia
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | >4.00E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
Novartis Institutes for BioMedical Research
Curated by ChEMBL
| Assay Description
Inhibition of CYP2C9 (unknown origin) |
Bioorg Med Chem Lett 25: 3788-92 (2015)
Article DOI: 10.1016/j.bmcl.2015.07.091
BindingDB Entry DOI: 10.7270/Q2G44S3Q |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data