 Found 29 hits of Enzyme Inhibition Constant Data
Found 29 hits of Enzyme Inhibition Constant Data Target/Host
(Institution) | Ligand | Target/Host
Links | Ligand
Links | Trg + Lig
Links | Ki
nM | ΔG°
kJ/mole | IC50
nM | Kd
nM | EC50/IC50
nM | koff
s-1 | kon
M-1s-1 | pH | Temp
°C |
|---|
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249567
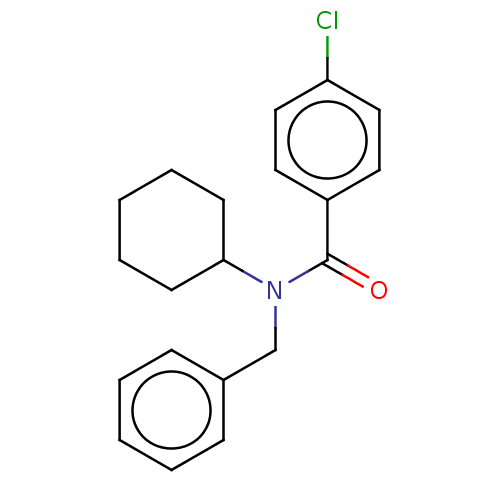
(CHEMBL4075936)Show InChI InChI=1S/C20H22ClNO/c21-18-13-11-17(12-14-18)20(23)22(19-9-5-2-6-10-19)15-16-7-3-1-4-8-16/h1,3-4,7-8,11-14,19H,2,5-6,9-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 25 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Displacement of [I125]amyloid beta (1 to 40) from human RAGE domain V expressed in CHO cells |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249567

(CHEMBL4075936)Show InChI InChI=1S/C20H22ClNO/c21-18-13-11-17(12-14-18)20(23)22(19-9-5-2-6-10-19)15-16-7-3-1-4-8-16/h1,3-4,7-8,11-14,19H,2,5-6,9-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 148 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of HMGB1 binding to human RAGE domain V expressed in CHO cells |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249567

(CHEMBL4075936)Show InChI InChI=1S/C20H22ClNO/c21-18-13-11-17(12-14-18)20(23)22(19-9-5-2-6-10-19)15-16-7-3-1-4-8-16/h1,3-4,7-8,11-14,19H,2,5-6,9-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| 230 | n/a | n/a | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of S110B binding to human RAGE domain V expressed in CHO cells |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249568
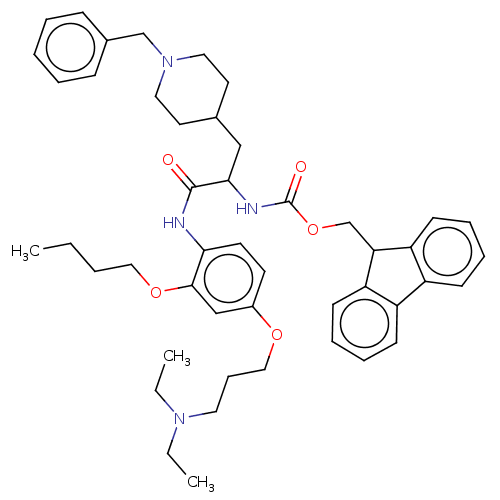
(CHEMBL4068269)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)C(CC1CCN(Cc2ccccc2)CC1)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C47H60N4O5/c1-4-7-29-55-45-32-37(54-30-15-26-50(5-2)6-3)22-23-43(45)48-46(52)44(31-35-24-27-51(28-25-35)33-36-16-9-8-10-17-36)49-47(53)56-34-42-40-20-13-11-18-38(40)39-19-12-14-21-41(39)42/h8-14,16-23,32,35,42,44H,4-7,15,24-31,33-34H2,1-3H3,(H,48,52)(H,49,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of S110B binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249569
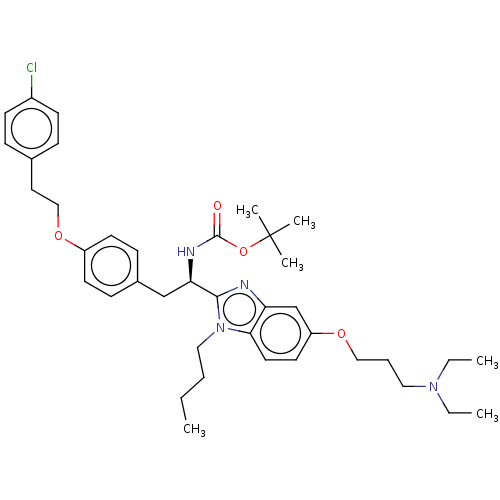
(CHEMBL4063487)Show SMILES CCCCn1c(nc2cc(OCCCN(CC)CC)ccc12)[C@@H](Cc1ccc(OCCc2ccc(Cl)cc2)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H53ClN4O4/c1-7-10-24-44-36-21-20-33(46-25-11-23-43(8-2)9-3)28-34(36)41-37(44)35(42-38(45)48-39(4,5)6)27-30-14-18-32(19-15-30)47-26-22-29-12-16-31(40)17-13-29/h12-21,28,35H,7-11,22-27H2,1-6H3,(H,42,45)/t35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of S110B binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249569

(CHEMBL4063487)Show SMILES CCCCn1c(nc2cc(OCCCN(CC)CC)ccc12)[C@@H](Cc1ccc(OCCc2ccc(Cl)cc2)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H53ClN4O4/c1-7-10-24-44-36-21-20-33(46-25-11-23-43(8-2)9-3)28-34(36)41-37(44)35(42-38(45)48-39(4,5)6)27-30-14-18-32(19-15-30)47-26-22-29-12-16-31(40)17-13-29/h12-21,28,35H,7-11,22-27H2,1-6H3,(H,42,45)/t35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249568

(CHEMBL4068269)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)C(CC1CCN(Cc2ccccc2)CC1)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C47H60N4O5/c1-4-7-29-55-45-32-37(54-30-15-26-50(5-2)6-3)22-23-43(45)48-46(52)44(31-35-24-27-51(28-25-35)33-36-16-9-8-10-17-36)49-47(53)56-34-42-40-20-13-11-18-38(40)39-19-12-14-21-41(39)42/h8-14,16-23,32,35,42,44H,4-7,15,24-31,33-34H2,1-3H3,(H,48,52)(H,49,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249569

(CHEMBL4063487)Show SMILES CCCCn1c(nc2cc(OCCCN(CC)CC)ccc12)[C@@H](Cc1ccc(OCCc2ccc(Cl)cc2)cc1)NC(=O)OC(C)(C)C |r| Show InChI InChI=1S/C39H53ClN4O4/c1-7-10-24-44-36-21-20-33(46-25-11-23-43(8-2)9-3)28-34(36)41-37(44)35(42-38(45)48-39(4,5)6)27-30-14-18-32(19-15-30)47-26-22-29-12-16-31(40)17-13-29/h12-21,28,35H,7-11,22-27H2,1-6H3,(H,42,45)/t35-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249568

(CHEMBL4068269)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)C(CC1CCN(Cc2ccccc2)CC1)NC(=O)OCC1c2ccccc2-c2ccccc12 Show InChI InChI=1S/C47H60N4O5/c1-4-7-29-55-45-32-37(54-30-15-26-50(5-2)6-3)22-23-43(45)48-46(52)44(31-35-24-27-51(28-25-35)33-36-16-9-8-10-17-36)49-47(53)56-34-42-40-20-13-11-18-38(40)39-19-12-14-21-41(39)42/h8-14,16-23,32,35,42,44H,4-7,15,24-31,33-34H2,1-3H3,(H,48,52)(H,49,53) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | <500 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249578
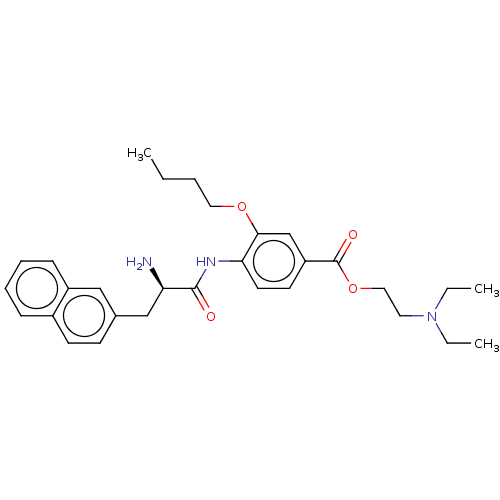
(CHEMBL4095634)Show SMILES CCCCOc1cc(ccc1NC(=O)[C@H](N)Cc1ccc2ccccc2c1)C(=O)OCCN(CC)CC |r| Show InChI InChI=1S/C30H39N3O4/c1-4-7-17-36-28-21-25(30(35)37-18-16-33(5-2)6-3)14-15-27(28)32-29(34)26(31)20-22-12-13-23-10-8-9-11-24(23)19-22/h8-15,19,21,26H,4-7,16-18,20,31H2,1-3H3,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.75E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of S110B binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50005634
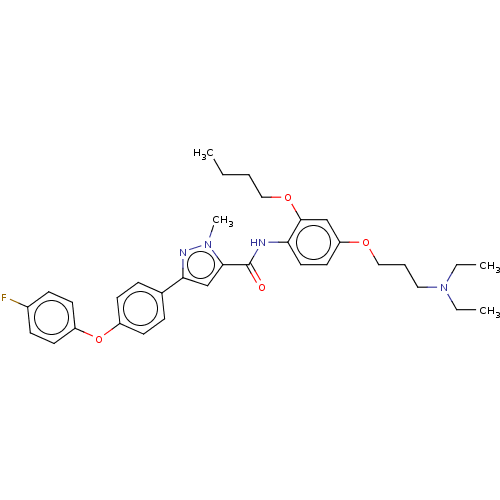
(CHEMBL3235375)Show SMILES CCCCOc1cc(OCCCN(CC)CC)ccc1NC(=O)c1cc(nn1C)-c1ccc(Oc2ccc(F)cc2)cc1 Show InChI InChI=1S/C34H41FN4O4/c1-5-8-21-42-33-23-29(41-22-9-20-39(6-2)7-3)18-19-30(33)36-34(40)32-24-31(37-38(32)4)25-10-14-27(15-11-25)43-28-16-12-26(35)13-17-28/h10-19,23-24H,5-9,20-22H2,1-4H3,(H,36,40) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | 1.90E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to biotin-labeled human RAGE domain V after 60 mins by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249578

(CHEMBL4095634)Show SMILES CCCCOc1cc(ccc1NC(=O)[C@H](N)Cc1ccc2ccccc2c1)C(=O)OCCN(CC)CC |r| Show InChI InChI=1S/C30H39N3O4/c1-4-7-17-36-28-21-25(30(35)37-18-16-33(5-2)6-3)14-15-27(28)32-29(34)26(31)20-22-12-13-23-10-8-9-11-24(23)19-22/h8-15,19,21,26H,4-7,16-18,20,31H2,1-3H3,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 2.29E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249578

(CHEMBL4095634)Show SMILES CCCCOc1cc(ccc1NC(=O)[C@H](N)Cc1ccc2ccccc2c1)C(=O)OCCN(CC)CC |r| Show InChI InChI=1S/C30H39N3O4/c1-4-7-17-36-28-21-25(30(35)37-18-16-33(5-2)6-3)14-15-27(28)32-29(34)26(31)20-22-12-13-23-10-8-9-11-24(23)19-22/h8-15,19,21,26H,4-7,16-18,20,31H2,1-3H3,(H,32,34)/t26-/m1/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 3.40E+3 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50397836

(CHEMBL2179073)Show SMILES CCN(CC)CCOc1cccc(Nc2nc(cc(n2)-c2ccc(Cl)cc2)-c2ccc(Cl)cc2)c1 Show InChI InChI=1S/C28H28Cl2N4O/c1-3-34(4-2)16-17-35-25-7-5-6-24(18-25)31-28-32-26(20-8-12-22(29)13-9-20)19-27(33-28)21-10-14-23(30)15-11-21/h5-15,18-19H,3-4,16-17H2,1-2H3,(H,31,32,33) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| CHEMBL
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | 1.65E+4 | n/a | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to biotin-labeled human RAGE domain V after 60 mins by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249579
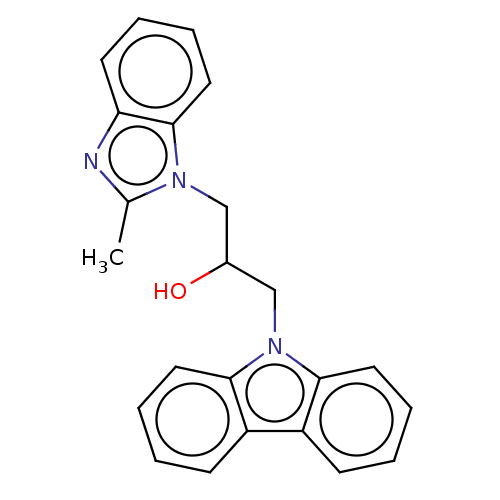
(CHEMBL1477694 | US10729695, Compound CB-3)Show InChI InChI=1S/C23H21N3O/c1-16-24-20-10-4-7-13-23(20)25(16)14-17(27)15-26-21-11-5-2-8-18(21)19-9-3-6-12-22(19)26/h2-13,17,27H,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249582
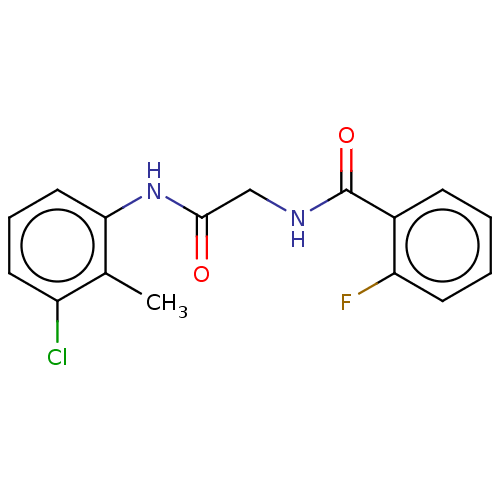
(CHEMBL1547178 | US10729695, Compound CB-13)Show InChI InChI=1S/C16H14ClFN2O2/c1-10-12(17)6-4-8-14(10)20-15(21)9-19-16(22)11-5-2-3-7-13(11)18/h2-8H,9H2,1H3,(H,19,22)(H,20,21) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 32 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249566
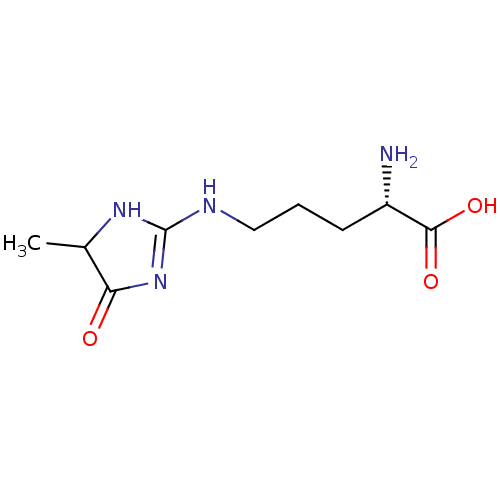
(CHEMBL4085254)Show InChI InChI=1S/C9H16N4O3/c1-5-7(14)13-9(12-5)11-4-2-3-6(10)8(15)16/h5-6H,2-4,10H2,1H3,(H,15,16)(H2,11,12,13,14)/t5?,6-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 30 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Binding affinity to human C-terminal His-tagged RAGE domain V (24 to 125 residues) expressed in Escherichia coli BL21(DE3) by tryptophan fluorescence... |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249574
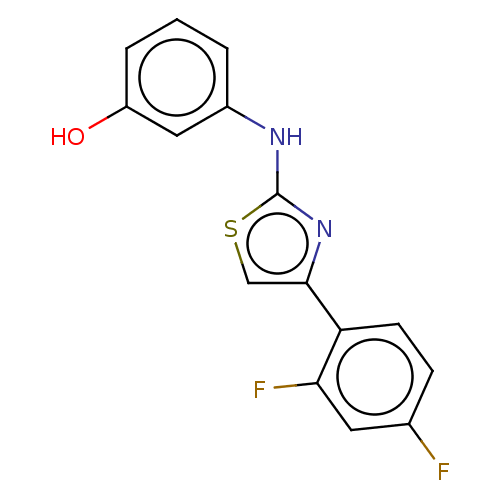
(CHEMBL4077165 | US10729695, Compound CB-5)Show InChI InChI=1S/C15H10F2N2OS/c16-9-4-5-12(13(17)6-9)14-8-21-15(19-14)18-10-2-1-3-11(20)7-10/h1-8,20H,(H,18,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 1 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249570
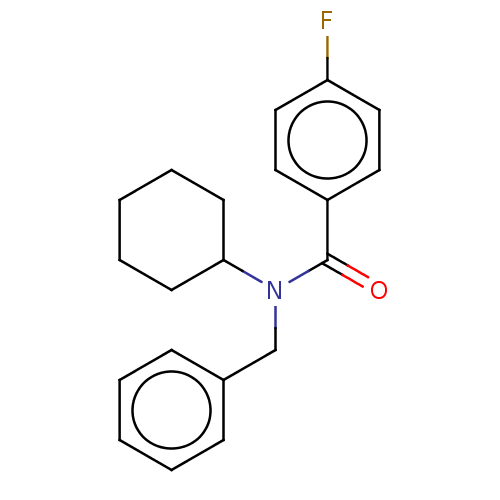
(CHEMBL4066660)Show InChI InChI=1S/C20H22FNO/c21-18-13-11-17(12-14-18)20(23)22(19-9-5-2-6-10-19)15-16-7-3-1-4-8-16/h1,3-4,7-8,11-14,19H,2,5-6,9-10,15H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 15 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Binding affinity to human RAGE domain V by autoradiography |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249571
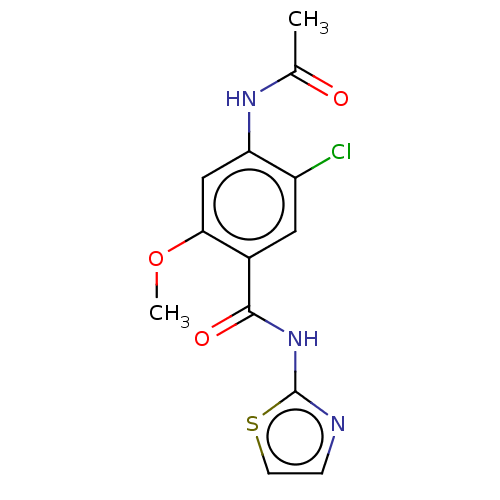
(CHEMBL4101927 | US10729695, Compound CB-6)Show InChI InChI=1S/C13H12ClN3O3S/c1-7(18)16-10-6-11(20-2)8(5-9(10)14)12(19)17-13-15-3-4-21-13/h3-6H,1-2H3,(H,16,18)(H,15,17,19) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
| Article
PubMed
| n/a | n/a | n/a | 0.300 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249575
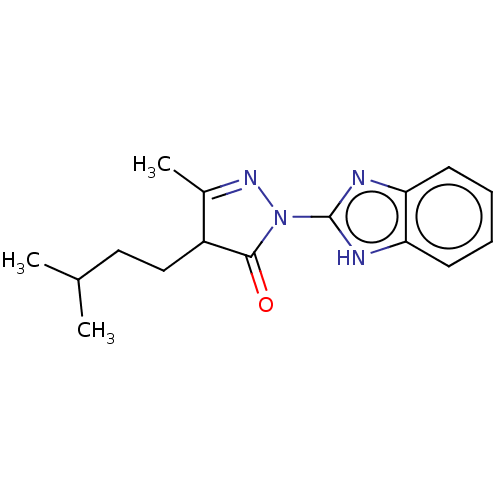
(CHEMBL4100986 | US10729695, Compound CB-10)Show SMILES CC(C)CCC1C(C)=NN(C1=O)c1nc2ccccc2[nH]1 |c:7| Show InChI InChI=1S/C16H20N4O/c1-10(2)8-9-12-11(3)19-20(15(12)21)16-17-13-6-4-5-7-14(13)18-16/h4-7,10,12H,8-9H2,1-3H3,(H,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 3 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249580
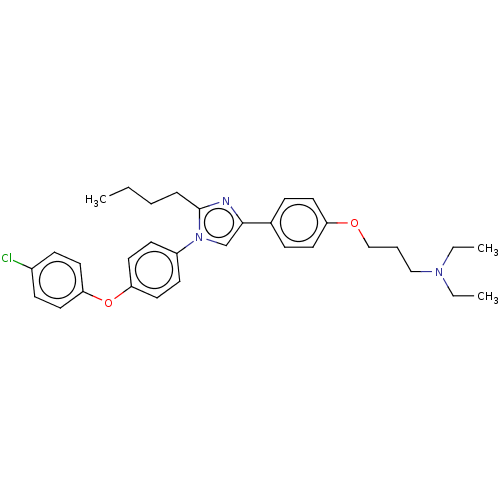
(Azeliragon | PF-04494700 | TTP448 | US11524942, Co...)Show SMILES CCCCc1nc(cn1-c1ccc(Oc2ccc(Cl)cc2)cc1)-c1ccc(OCCCN(CC)CC)cc1 Show InChI InChI=1S/C32H38ClN3O2/c1-4-7-9-32-34-31(25-10-16-28(17-11-25)37-23-8-22-35(5-2)6-3)24-36(32)27-14-20-30(21-15-27)38-29-18-12-26(33)13-19-29/h10-21,24H,4-9,22-23H2,1-3H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 500 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta (1 to 42) binding to RAGE domain V (unknown origin) by fluorescence polarization assay |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249577

(CHEMBL4072531 | US10729695, Compound CB-4)Show InChI InChI=1S/C18H17N3O/c22-15(11-20-10-9-14-5-1-3-7-17(14)20)12-21-13-19-16-6-2-4-8-18(16)21/h1-10,13,15,22H,11-12H2 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249581
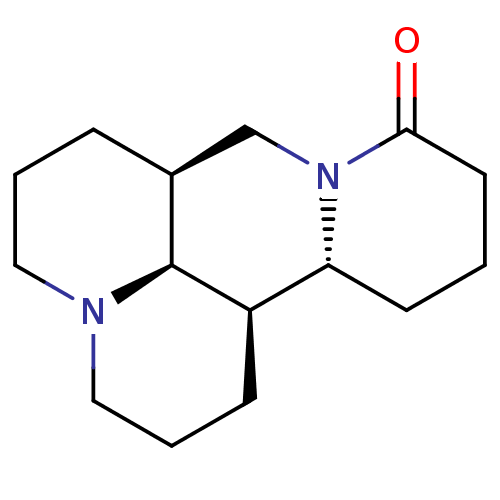
(CHEBI:6700 | Matrine)Show SMILES [H][C@]12CCCN3CCC[C@]([H])([C@@]4([H])CCCC(=O)N4C1)[C@]23[H] Show InChI InChI=1S/C15H24N2O/c18-14-7-1-6-13-12-5-3-9-16-8-2-4-11(15(12)16)10-17(13)14/h11-13,15H,1-10H2/t11-,12+,13+,15-/m0/s1 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
MCE
KEGG
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 2.40E+7 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of amyloid beta binding to human RAGE domain V (Met1 to Ala 344 residues) expressed in HEK293 cells after 10 mins by MST assay |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Rattus norvegicus) | BDBM26115
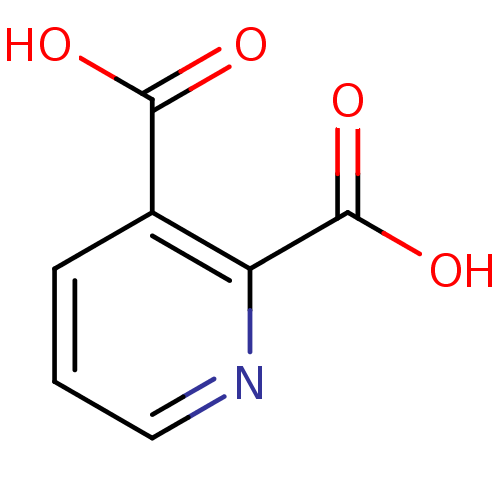
(CHEMBL286204 | Quinolinate | Quinolinic acid | pyr...)Show InChI InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-8-5(4)7(11)12/h1-3H,(H,9,10)(H,11,12) | PDB
UniProtKB/SwissProt
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Binding affinity to Wistar rat His-tagged RAGE domain VC1 expressed in Escherichia coli by fluorescence titration method |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249573
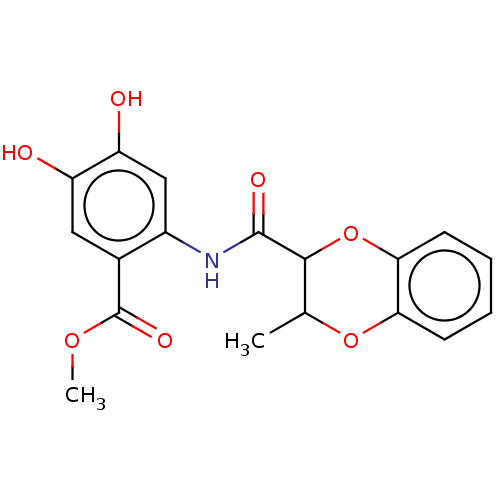
(CHEMBL4063929)Show InChI InChI=1S/C18H17NO7/c1-9-16(26-15-6-4-3-5-14(15)25-9)17(22)19-11-8-13(21)12(20)7-10(11)18(23)24-2/h3-9,16,20-21H,1-2H3,(H,19,22) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| PC cid
PC sid
UniChem
Patents
Similars
| Article
PubMed
| n/a | n/a | n/a | 2 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249576
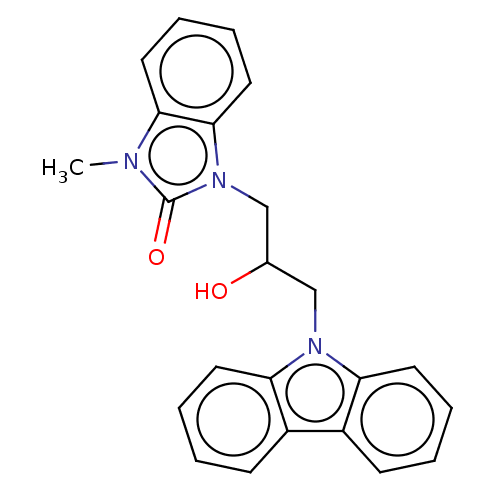
(CHEMBL4075539 | US10729695, Compound CD-6)Show SMILES Cn1c2ccccc2n(CC(O)Cn2c3ccccc3c3ccccc23)c1=O Show InChI InChI=1S/C23H21N3O2/c1-24-21-12-6-7-13-22(21)26(23(24)28)15-16(27)14-25-19-10-4-2-8-17(19)18-9-3-5-11-20(18)25/h2-13,16,27H,14-15H2,1H3 | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Similars
| Article
PubMed
| n/a | n/a | n/a | 4 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM50249572
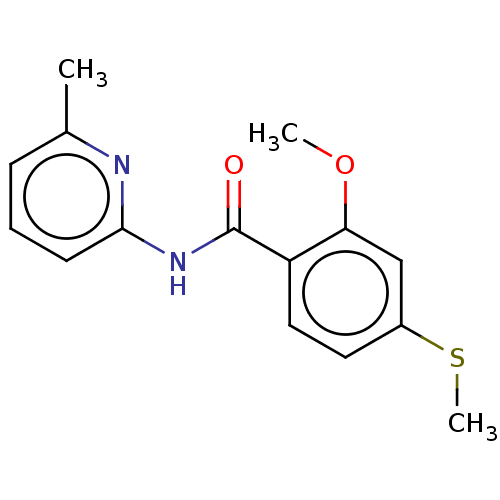
(CHEMBL1463447 | US10729695, Compound CB-1)Show InChI InChI=1S/C15H16N2O2S/c1-10-5-4-6-14(16-10)17-15(18)12-8-7-11(20-3)9-13(12)19-2/h4-9H,1-3H3,(H,16,17,18) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
PC cid
PC sid
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 6 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Inhibition of CML binding to RAGE (unknown origin) by ELISA |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |
Advanced glycosylation end product-specific receptor
(Homo sapiens (Human)) | BDBM26115

(CHEMBL286204 | Quinolinate | Quinolinic acid | pyr...)Show InChI InChI=1S/C7H5NO4/c9-6(10)4-2-1-3-8-5(4)7(11)12/h1-3H,(H,9,10)(H,11,12) | PDB
MMDB
Reactome pathway
KEGG
UniProtKB/SwissProt
B.MOAD
GoogleScholar
AffyNet 
| Purchase
CHEMBL
DrugBank
MCE
KEGG
MMDB
PC cid
PC sid
PDB
UniChem
Patents
| Article
PubMed
| n/a | n/a | n/a | 43 | n/a | n/a | n/a | n/a | n/a |
King's College London
Curated by ChEMBL
| Assay Description
Binding affinity to human His-tagged RAGE domain VC1 expressed in Escherichia coli by fluorescence titration method |
J Med Chem 60: 7213-7232 (2017)
Article DOI: 10.1021/acs.jmedchem.7b00058
BindingDB Entry DOI: 10.7270/Q2KD21BP |
More data for this
Ligand-Target Pair | |


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data