

 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data
| Target/Host (Institution) | Ligand | Target/Host Links | Ligand Links | Trg + Lig Links | Ki nM | ΔG° kJ/mole | IC50 nM | Kd nM | EC50/IC50 nM | koff s-1 | kon M-1s-1 | pH | Temp °C |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313357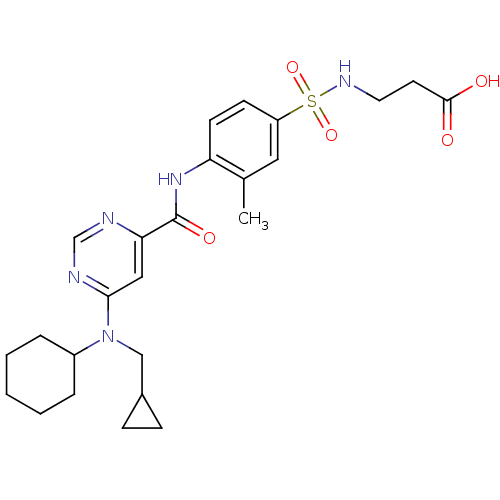 (3-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 2 | -46.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313351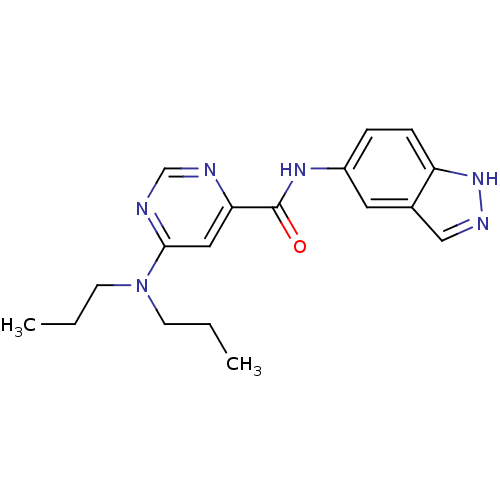 (6-(dipropylamino)-N-(1H-indazol-5-yl)pyrimidine-4-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 3 | -45.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183667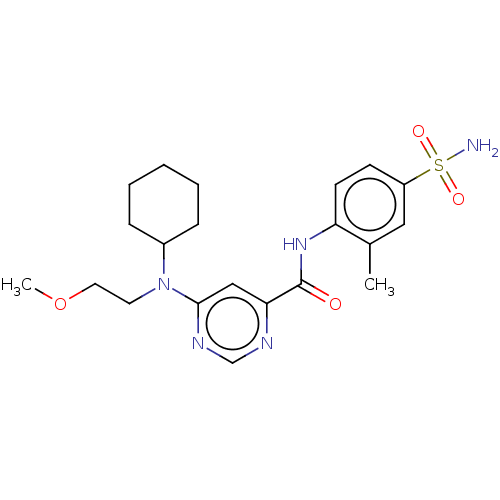 (US9150519, 1-55) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.20 | -45.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313358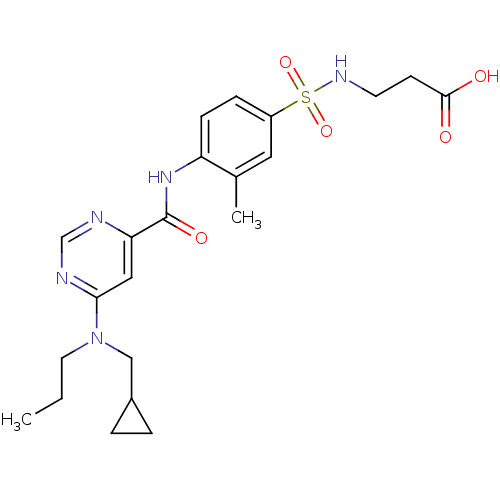 (3-(4-(6-((cyclopropylmethyl)(propyl)amino)pyrimidi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 6 | -43.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183668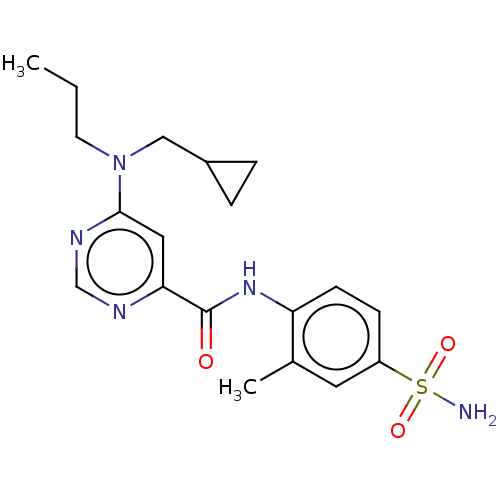 (US9150519, 1-56) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183686 (US9150519, 1-84) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 7 | -43.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183701 (US9150519, 1-100) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 8 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313359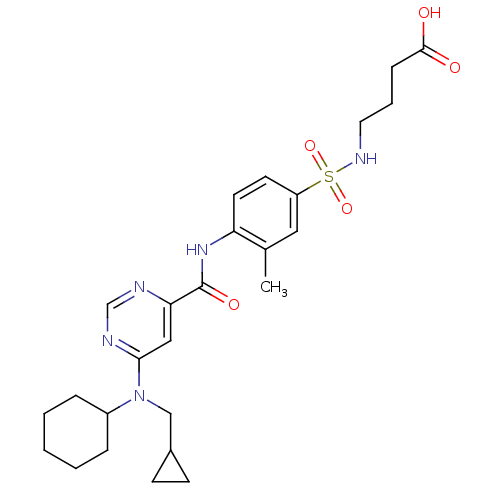 (4-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 8 | -43.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183699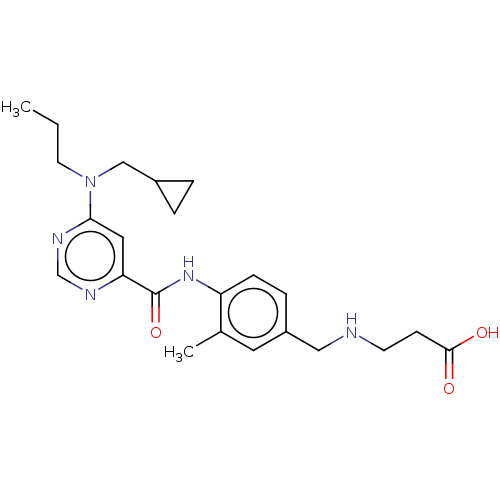 (US9150519, 1-97) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 9 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313363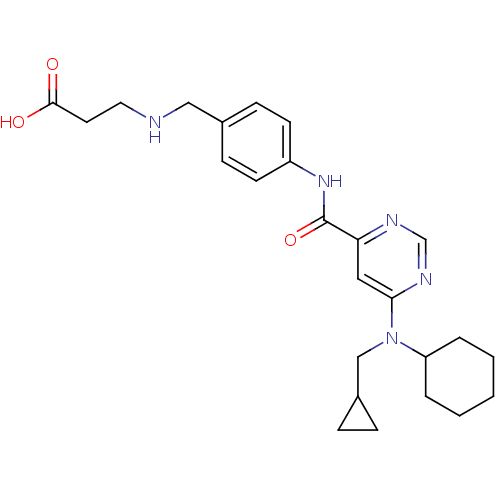 (3-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 9 | -42.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183676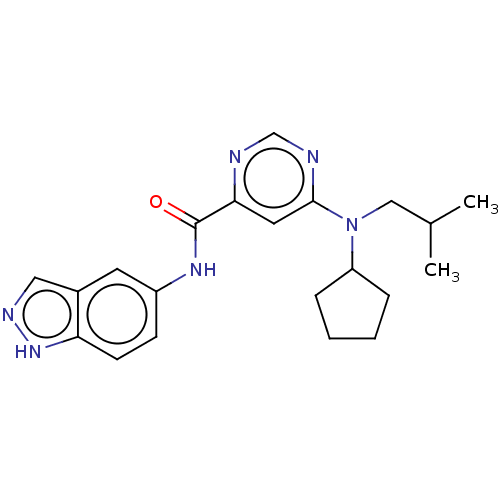 (US9150519, 1-69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 10 | -42.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313361 (2-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 12 | -42.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183692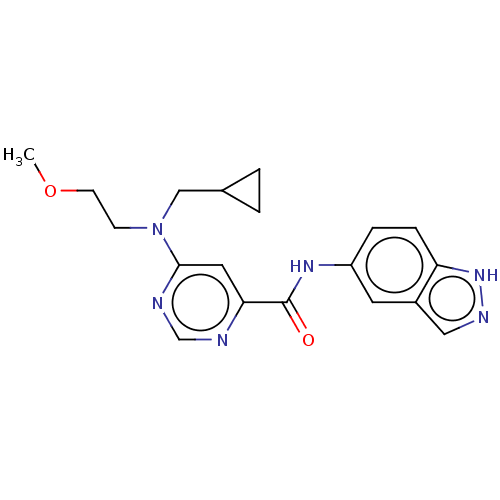 (US9150519, 1-90) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 23 | -40.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183710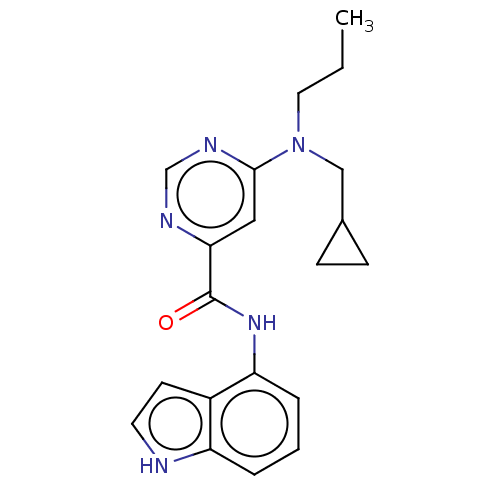 (US9150519, 1-113) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 25 | -40.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313362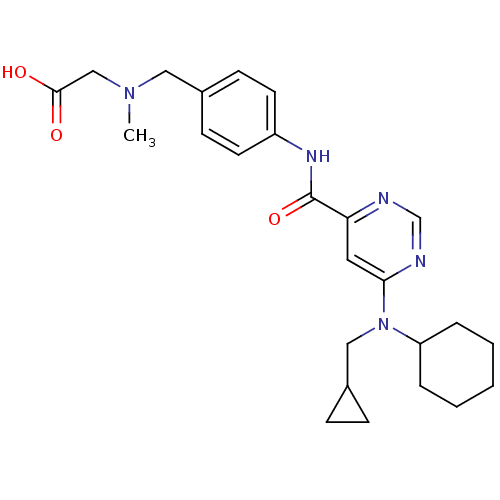 (2-((4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrim...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 29 | -40.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183687 (US9150519, 1-85) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 43 | -39.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313345 (6-(cyclohexyl(cyclopropylmethyl)amino)-N-(4-((dime...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 51 | -38.7 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313333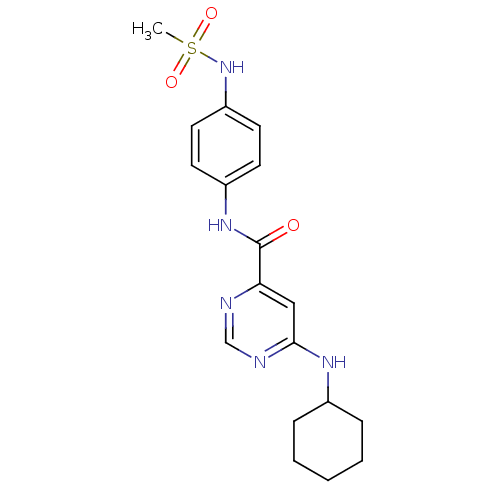 (6-(cyclohexylamino)-N-(4-(methylsulfonamido)phenyl...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 280 | -34.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313326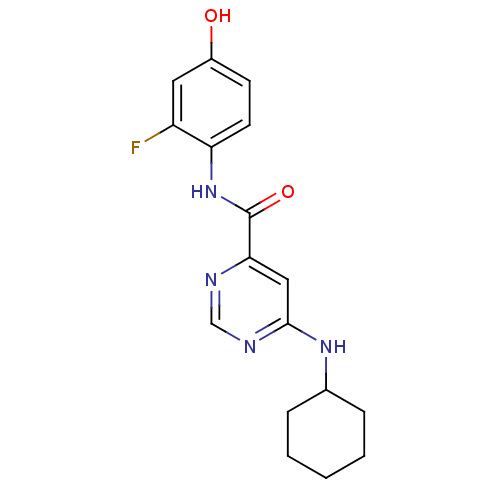 (6-(cyclohexylamino)-N-(2-fluoro-4-hydroxyphenyl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 340 | -34.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313327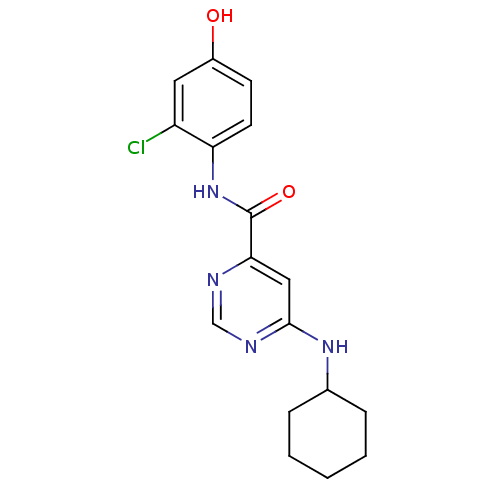 (CHEMBL1076723 | N-(2-chloro-4-hydroxyphenyl)-6-(cy...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 380 | -34.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313346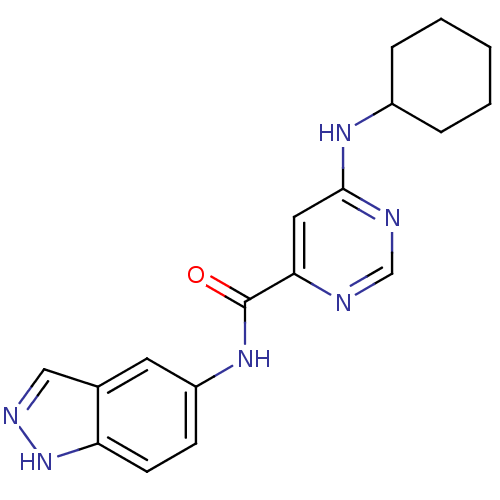 (6-(cyclohexylamino)-N-(1H-indazol-5-yl)pyrimidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 458 | -33.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183644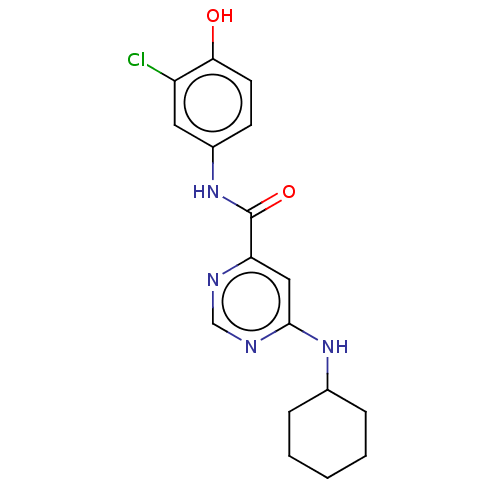 (US9150519, 1-6) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 500 | -33.4 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183643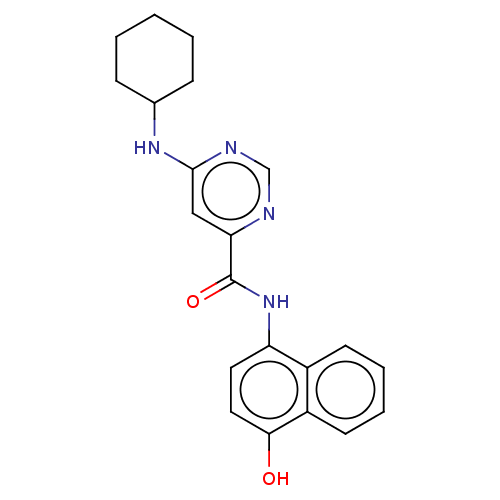 (US9150519, 1-5) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 540 | -33.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313325 (6-(Cyclohexylamino)-N-(4-hydroxy-2-methylphenyl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 550 | -33.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183645 (US9150519, 1-9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 650 | -32.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183646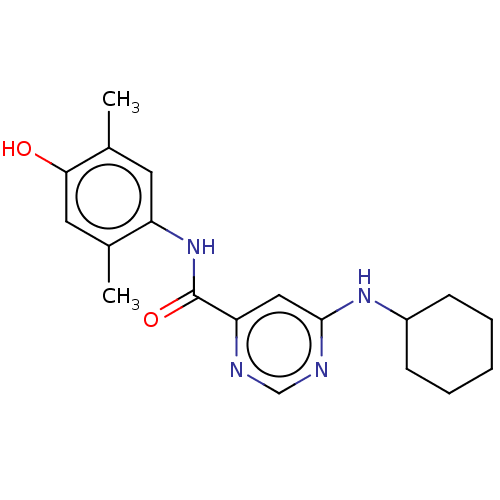 (US9150519, 1-10) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 740 | -32.5 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313320 (6-(cyclohexyl(methyl)amino)-N-(4-hydroxyphenyl)pyr...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 810 | -32.3 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313318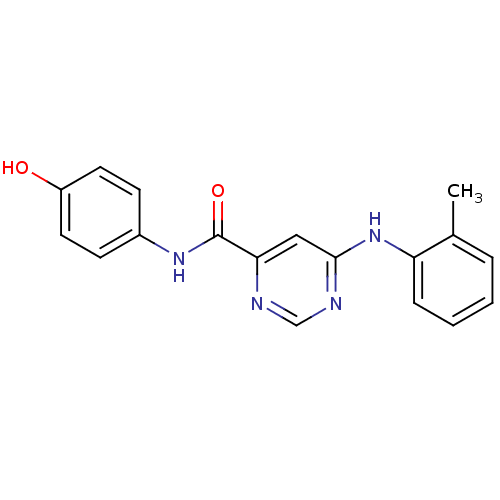 (CHEMBL1081268 | N-(4-hydroxyphenyl)-6-(o-tolylamin...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 840 | -32.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183642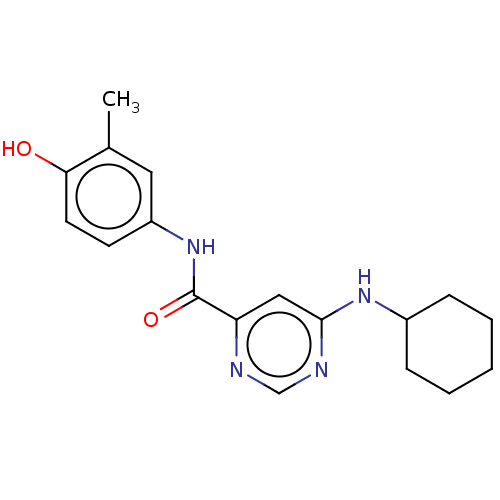 (US9150519, 1-4) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 980 | -31.9 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183672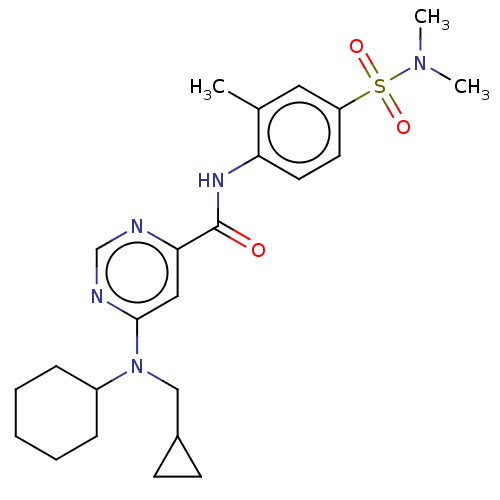 (US9150519, 1-64) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 1.02E+3 | -31.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313313 (6-(cyclohexylamino)-N-(4-hydroxyphenyl)pyrimidine-...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 1.68E+3 | -30.6 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313330 (6-(cyclohexylamino)-N-(4-(2-hydroxyethyl)phenyl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 2.00E+3 | -30.2 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313316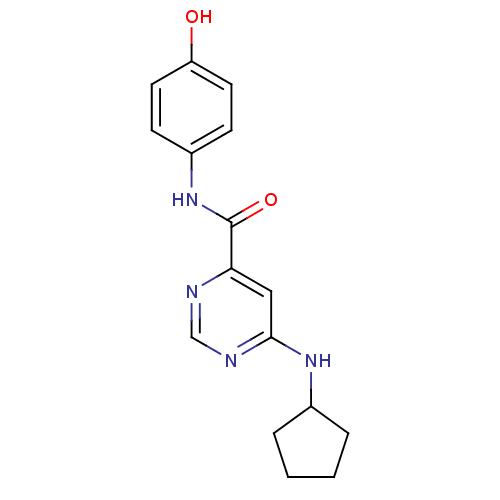 (6-(cyclopentylamino)-N-(4-hydroxyphenyl)pyrimidine...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | 2.13E+3 | -30.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183688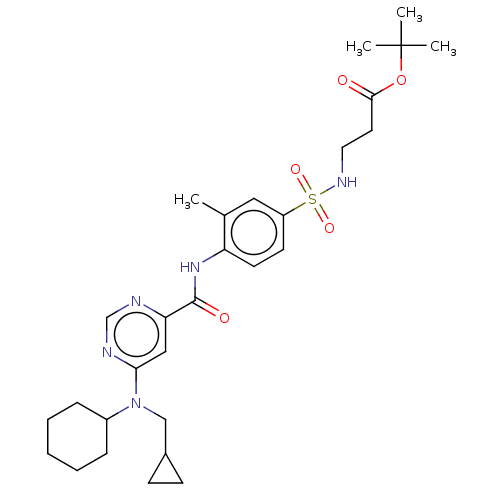 (US9150519, 1-86) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 2.46E+3 | -29.8 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183641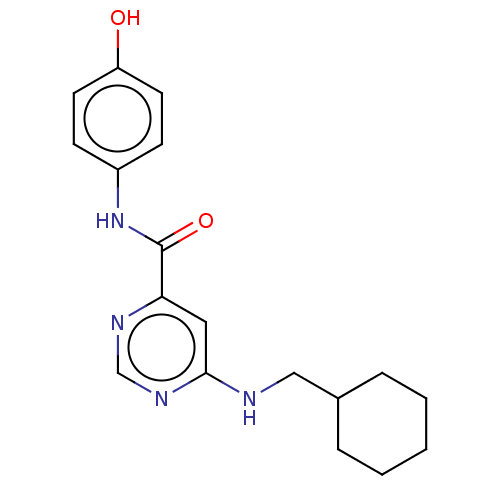 (US9150519, 1-2) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.23E+3 | -29.1 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183648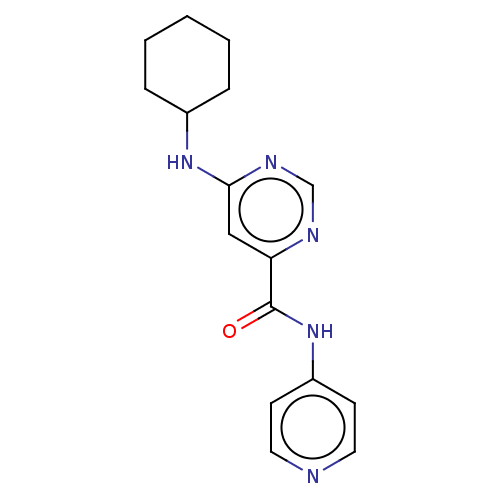 (US9150519, 1-12) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.36E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183647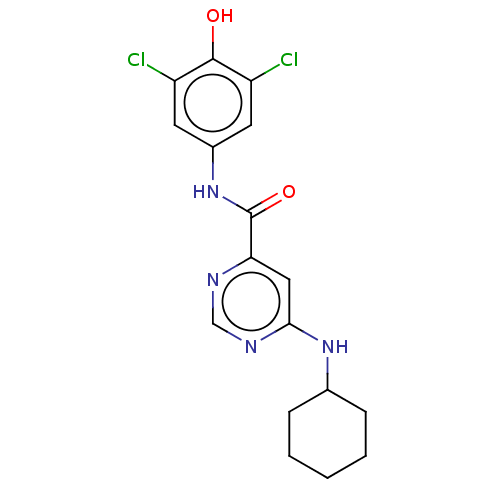 (US9150519, 1-11) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | 3.45E+3 | -29.0 | n/a | n/a | n/a | n/a | n/a | 7.4 | 4 |
MERCK SERONO SA US Patent | Assay Description Membranes were prepared from CHO cells expressing S1P1 or S1P3 for use in ligand and 35S-GTPgammaS binding studies. Cells were suspended in 50 mM TRI... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183645 (US9150519, 1-9) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 1.04E+3 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183646 (US9150519, 1-10) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 1.44E+3 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183653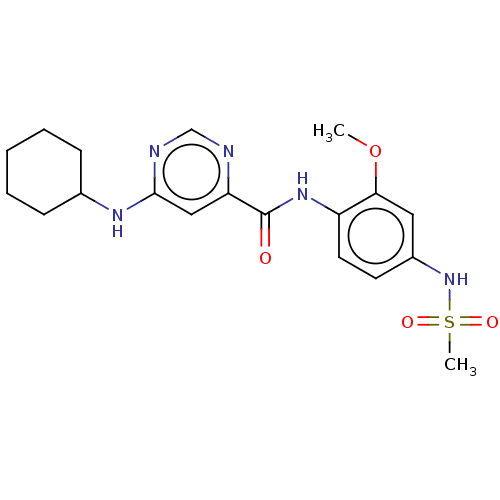 (US9150519, 1-27) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 374 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183662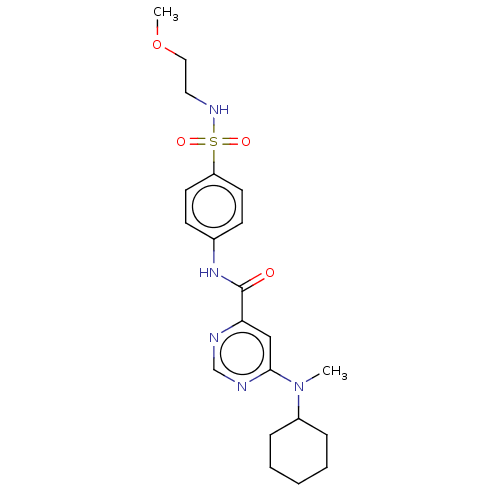 (US9150519, 1-42) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 2.28E+3 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313340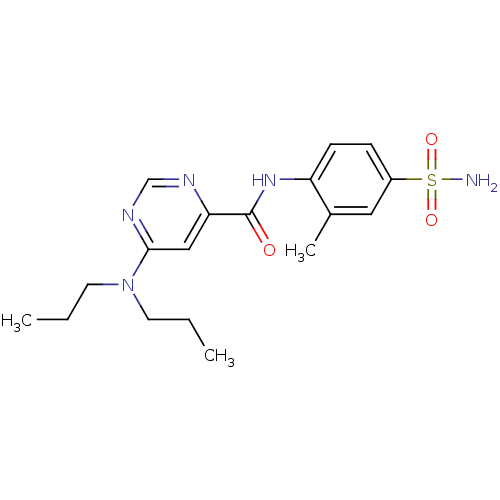 (6-(dipropylamino)-N-(2-methyl-4-sulfamoylphenyl)py...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 36 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183671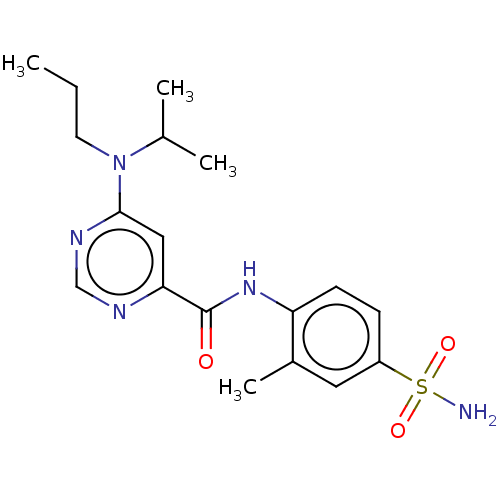 (US9150519, 1-60) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 51 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183673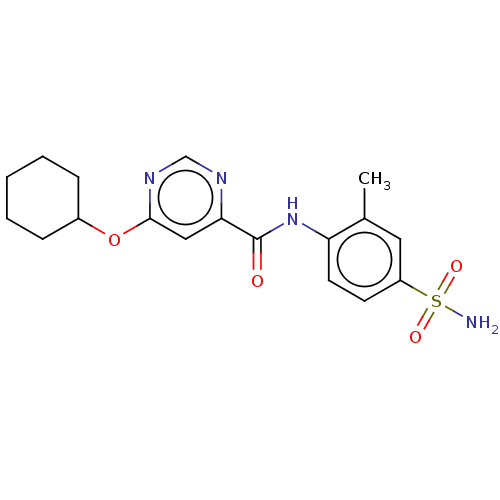 (US9150519, 1-65) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem | US Patent | n/a | n/a | n/a | n/a | 1.33E+4 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183675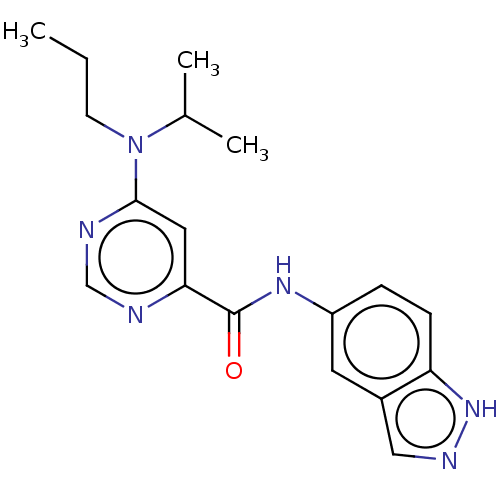 (US9150519, 1-67) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 68 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183676 (US9150519, 1-69) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 19 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183682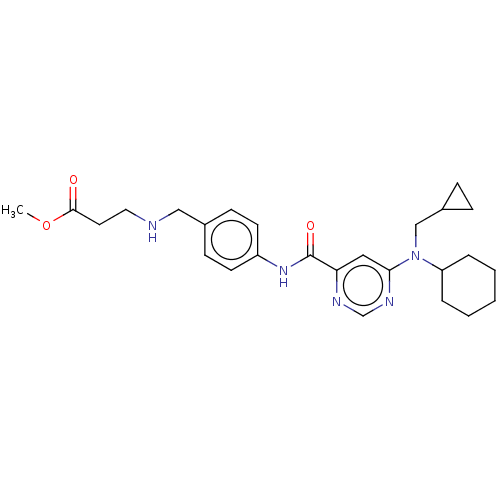 (US9150519, 1-76) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 48 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM50313361 (2-(4-(6-(cyclohexyl(cyclopropylmethyl)amino)pyrimi...) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | CHEMBL PC cid PC sid UniChem Patents Similars | US Patent | n/a | n/a | n/a | n/a | 35 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183698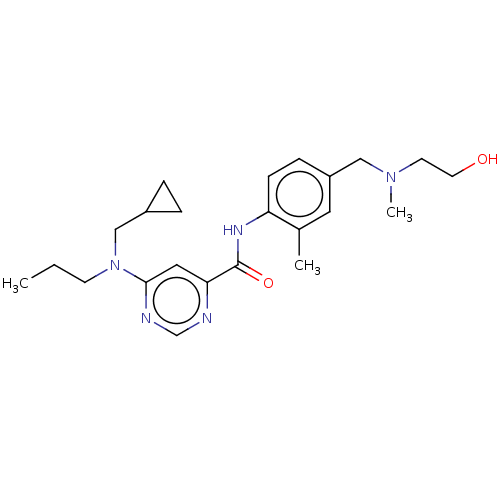 (US9150519, 1-96) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 391 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Sphingosine 1-phosphate receptor 1 (Homo sapiens (Human)) | BDBM183703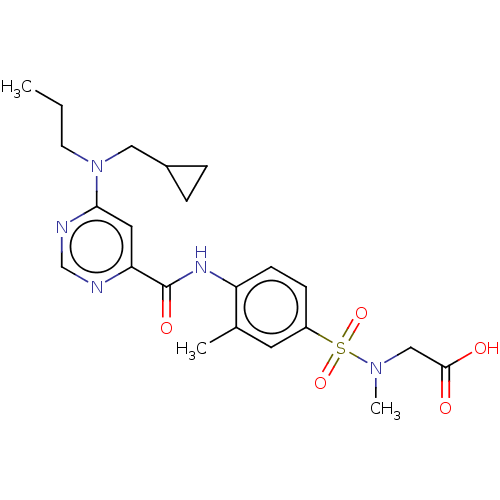 (US9150519, 1-102) | PDB Reactome pathway KEGG UniProtKB/SwissProt B.MOAD antibodypedia GoogleScholar AffyNet | PC cid PC sid UniChem Similars | US Patent | n/a | n/a | n/a | n/a | 1.23E+3 | n/a | n/a | 7.4 | 30 |
MERCK SERONO SA US Patent | Assay Description Measurements of 35S-GTPgammaS Binding: Membranes (1 to 10 µg protein) prepared as described above, were incubated in 96-well Scintiplates (PerkinElm... | US Patent US9150519 (2015) BindingDB Entry DOI: 10.7270/Q2H993ZK | |||||||||||
| More data for this Ligand-Target Pair | |||||||||||||
| Displayed 1 to 50 (of 242 total ) | Next | Last >> |