| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Cytochrome P450 2B6 |
|---|
| Ligand | BDBM372346 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2206496 (CHEMBL5119204) |
|---|
| IC50 | >13300±n/a nM |
|---|
| Citation |  Regueiro-Ren, A; Sit, SY; Chen, Y; Chen, J; Swidorski, JJ; Liu, Z; Venables, BL; Sin, N; Hartz, RA; Protack, T; Lin, Z; Zhang, S; Li, Z; Wu, DR; Li, P; Kempson, J; Hou, X; Gupta, A; Rampulla, R; Mathur, A; Park, H; Sarjeant, A; Benitex, Y; Rahematpura, S; Parker, D; Phillips, T; Haskell, R; Jenkins, S; Santone, KS; Cockett, M; Hanumegowda, U; Dicker, I; Meanwell, NA; Krystal, M The Discovery of GSK3640254, a Next-Generation Inhibitor of HIV-1 Maturation. J Med Chem65:11927-11948 (2022) [PubMed] Article Regueiro-Ren, A; Sit, SY; Chen, Y; Chen, J; Swidorski, JJ; Liu, Z; Venables, BL; Sin, N; Hartz, RA; Protack, T; Lin, Z; Zhang, S; Li, Z; Wu, DR; Li, P; Kempson, J; Hou, X; Gupta, A; Rampulla, R; Mathur, A; Park, H; Sarjeant, A; Benitex, Y; Rahematpura, S; Parker, D; Phillips, T; Haskell, R; Jenkins, S; Santone, KS; Cockett, M; Hanumegowda, U; Dicker, I; Meanwell, NA; Krystal, M The Discovery of GSK3640254, a Next-Generation Inhibitor of HIV-1 Maturation. J Med Chem65:11927-11948 (2022) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Cytochrome P450 2B6 |
|---|
| Name: | Cytochrome P450 2B6 |
|---|
| Synonyms: | CP2B6_HUMAN | CYP2B6 | Cytochrome P450 2B6 (CYP2B6) |
|---|
| Type: | Protein |
|---|
| Mol. Mass.: | 56289.75 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | P20813 |
|---|
| Residue: | 491 |
|---|
| Sequence: | MELSVLLFLALLTGLLLLLVQRHPNTHDRLPPGPRPLPLLGNLLQMDRRGLLKSFLRFRE
KYGDVFTVHLGPRPVVMLCGVEAIREALVDKAEAFSGRGKIAMVDPFFRGYGVIFANGNR
WKVLRRFSVTTMRDFGMGKRSVEERIQEEAQCLIEELRKSKGALMDPTFLFQSITANIIC
SIVFGKRFHYQDQEFLKMLNLFYQTFSLISSVFGQLFELFSGFLKYFPGAHRQVYKNLQE
INAYIGHSVEKHRETLDPSAPKDLIDTYLLHMEKEKSNAHSEFSHQNLNLNTLSLFFAGT
ETTSTTLRYGFLLMLKYPHVAERVYREIEQVIGPHRPPELHDRAKMPYTEAVIYEIQRFS
DLLPMGVPHIVTQHTSFRGYIIPKDTEVFLILSTALHDPHYFEKPDAFNPDHFLDANGAL
KKTEAFIPFSLGKRICLGEGIARAELFLFFTTILQNFSMASPVAPEDIDLTPQECGVGKI
PPTYQIRFLPR
|
|
|
|---|
| BDBM372346 |
|---|
| n/a |
|---|
| Name | BDBM372346 |
|---|
| Synonyms: | Method A: (R)-4-((1R,3aS,5aR,5bR,7aR,11aS,11bR,13aR,13bR)-3a-((2-(1,1-dioxidothiomorpholino)ethyl)amino)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)-2,3,3a,4,5,5a,5b,6,7,7a,8,11,11a,11b,12,13,13a,13b-octadecahydro-1H-cyclopenta[a]chrysen-9-yl)-1-(fluoromethyl)cyclohex-3-enecarboxylic acid via chiral (R)-benzyl 1-(fluoromethyl)-4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)cyclohex-3-enecarboxylate | US10245275, Example 2b |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C43H67FN2O4S |
|---|
| Mol. Mass. | 727.066 |
|---|
| SMILES | CC(=C)[C@@H]1CC[C@@]2(CC[C@]3(C)[C@H](CC[C@@H]4[C@@]5(C)CC=C(C6=CC[C@](CF)(CC6)C(O)=O)C(C)(C)[C@@H]5CC[C@@]34C)[C@@H]12)NCCN1CCS(=O)(=O)CC1 |r,t:18,20| |
|---|
| Structure | 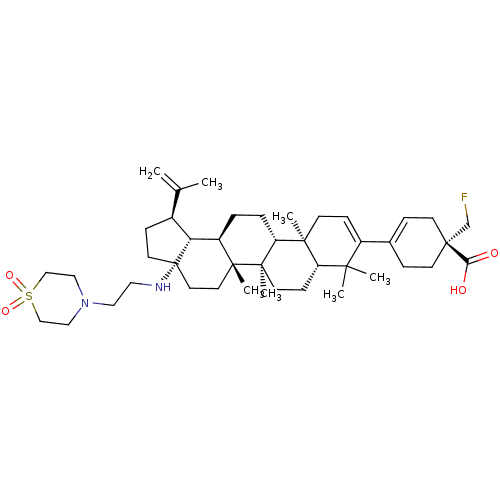
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Regueiro-Ren, A; Sit, SY; Chen, Y; Chen, J; Swidorski, JJ; Liu, Z; Venables, BL; Sin, N; Hartz, RA; Protack, T; Lin, Z; Zhang, S; Li, Z; Wu, DR; Li, P; Kempson, J; Hou, X; Gupta, A; Rampulla, R; Mathur, A; Park, H; Sarjeant, A; Benitex, Y; Rahematpura, S; Parker, D; Phillips, T; Haskell, R; Jenkins, S; Santone, KS; Cockett, M; Hanumegowda, U; Dicker, I; Meanwell, NA; Krystal, M The Discovery of GSK3640254, a Next-Generation Inhibitor of HIV-1 Maturation. J Med Chem65:11927-11948 (2022) [PubMed] Article
Regueiro-Ren, A; Sit, SY; Chen, Y; Chen, J; Swidorski, JJ; Liu, Z; Venables, BL; Sin, N; Hartz, RA; Protack, T; Lin, Z; Zhang, S; Li, Z; Wu, DR; Li, P; Kempson, J; Hou, X; Gupta, A; Rampulla, R; Mathur, A; Park, H; Sarjeant, A; Benitex, Y; Rahematpura, S; Parker, D; Phillips, T; Haskell, R; Jenkins, S; Santone, KS; Cockett, M; Hanumegowda, U; Dicker, I; Meanwell, NA; Krystal, M The Discovery of GSK3640254, a Next-Generation Inhibitor of HIV-1 Maturation. J Med Chem65:11927-11948 (2022) [PubMed] Article