| Reaction Details |
|---|
 | Report a problem with these data |
| Target | Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase |
|---|
| Ligand | BDBM50579526 |
|---|
| Substrate/Competitor | n/a |
|---|
| Meas. Tech. | ChEMBL_2135455 (CHEMBL4845065) |
|---|
| IC50 | >820±n/a nM |
|---|
| Citation |  Turdi, H; Chao, H; Hangeland, JJ; Ahmad, S; Meng, W; Brigance, R; Zhao, G; Wang, W; Moore, F; Ye, XY; Mathur, A; Hou, X; Kempson, J; Wu, DR; Li, YX; Azzara, AV; Ma, Z; Chu, CH; Chen, L; Cullen, MJ; Rooney, S; Harvey, S; Kopcho, L; Panemangelor, R; Abell, L; O'Malley, K; Keim, WJ; Dierks, E; Chang, S; Foster, K; Apedo, A; Harden, D; Dabros, M; Gao, Q; Pelleymounter, MA; Whaley, JM; Robl, JA; Cheng, D; Lawrence, RM; Devasthale, P Screening Hit to Clinical Candidate: Discovery of BMS-963272, a Potent, Selective MGAT2 Inhibitor for the Treatment of Metabolic Disorders. J Med Chem64:14773-14792 (2021) [PubMed] Article Turdi, H; Chao, H; Hangeland, JJ; Ahmad, S; Meng, W; Brigance, R; Zhao, G; Wang, W; Moore, F; Ye, XY; Mathur, A; Hou, X; Kempson, J; Wu, DR; Li, YX; Azzara, AV; Ma, Z; Chu, CH; Chen, L; Cullen, MJ; Rooney, S; Harvey, S; Kopcho, L; Panemangelor, R; Abell, L; O'Malley, K; Keim, WJ; Dierks, E; Chang, S; Foster, K; Apedo, A; Harden, D; Dabros, M; Gao, Q; Pelleymounter, MA; Whaley, JM; Robl, JA; Cheng, D; Lawrence, RM; Devasthale, P Screening Hit to Clinical Candidate: Discovery of BMS-963272, a Potent, Selective MGAT2 Inhibitor for the Treatment of Metabolic Disorders. J Med Chem64:14773-14792 (2021) [PubMed] Article |
|---|
| More Info.: | Get all data from this article, Assay Method |
|---|
| |
| Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase |
|---|
| Name: | Beta-1,4-mannosyl-glycoprotein 4-beta-N-acetylglucosaminyltransferase |
|---|
| Synonyms: | GGNT3 | GNT-III | GlcNAc-T III | MGAT3 | MGAT3_HUMAN | N-acetylglucosaminyltransferase III | N-glycosyl-oligosaccharide-glycoprotein N-acetylglucosaminyltransferase III |
|---|
| Type: | PROTEIN |
|---|
| Mol. Mass.: | 61324.81 |
|---|
| Organism: | Homo sapiens (Human) |
|---|
| Description: | ChEMBL_107586 |
|---|
| Residue: | 533 |
|---|
| Sequence: | MKMRRYKLFLMFCMAGLCLISFLHFFKTLSYVTFPRELASLSPNLVSSFFWNNAPVTPQA
SPEPGGPDLLRTPLYSHSPLLQPLPPSKAAEELHRVDLVLPEDTTEYFVRTKAGGVCFKP
GTKMLERPPPGRPEEKPEGANGSSARRPPRYLLSARERTGGRGARRKWVECVCLPGWHGP
SCGVPTVVQYSNLPTKERLVPREVPRRVINAINVNHEFDLLDVRFHELGDVVDAFVVCES
NFTAYGEPRPLKFREMLTNGTFEYIRHKVLYVFLDHFPPGGRQDGWIADDYLRTFLTQDG
VSRLRNLRPDDVFIIDDADEIPARDGVLFLKLYDGWTEPFAFHMRKSLYGFFWKQPGTLE
VVSGCTVDMLQAVYGLDGIRLRRRQYYTMPNFRQYENRTGHILVQWSLGSPLHFAGWHCS
WCFTPEGIYFKLVSAQNGDFPRWGDYEDKRDLNYIRGLIRTGGWFDGTQQEYPPADPSEH
MYAPKYLLKNYDRFHYLLDNPYQEPRSTAAGGWRHRGPEGRPPARGKLDEAEV
|
|
|
|---|
| BDBM50579526 |
|---|
| n/a |
|---|
| Name | BDBM50579526 |
|---|
| Synonyms: | CHEMBL4878564 |
|---|
| Type | Small organic molecule |
|---|
| Emp. Form. | C26H25F6N5O2 |
|---|
| Mol. Mass. | 553.4994 |
|---|
| SMILES | Cc1ccc(cc1)C1=C(c2nnn[nH]2)C(=O)N[C@@](C1)(c1ccc(OCCCCCC(F)(F)F)cc1)C(F)(F)F |r,c:8| |
|---|
| Structure | 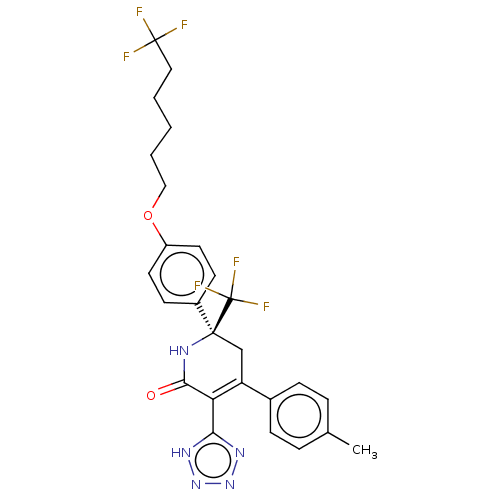
|
|---|


 Search and Browse
Search and Browse
 Download
Download
 Enter Data
Enter Data

 Turdi, H; Chao, H; Hangeland, JJ; Ahmad, S; Meng, W; Brigance, R; Zhao, G; Wang, W; Moore, F; Ye, XY; Mathur, A; Hou, X; Kempson, J; Wu, DR; Li, YX; Azzara, AV; Ma, Z; Chu, CH; Chen, L; Cullen, MJ; Rooney, S; Harvey, S; Kopcho, L; Panemangelor, R; Abell, L; O'Malley, K; Keim, WJ; Dierks, E; Chang, S; Foster, K; Apedo, A; Harden, D; Dabros, M; Gao, Q; Pelleymounter, MA; Whaley, JM; Robl, JA; Cheng, D; Lawrence, RM; Devasthale, P Screening Hit to Clinical Candidate: Discovery of BMS-963272, a Potent, Selective MGAT2 Inhibitor for the Treatment of Metabolic Disorders. J Med Chem64:14773-14792 (2021) [PubMed] Article
Turdi, H; Chao, H; Hangeland, JJ; Ahmad, S; Meng, W; Brigance, R; Zhao, G; Wang, W; Moore, F; Ye, XY; Mathur, A; Hou, X; Kempson, J; Wu, DR; Li, YX; Azzara, AV; Ma, Z; Chu, CH; Chen, L; Cullen, MJ; Rooney, S; Harvey, S; Kopcho, L; Panemangelor, R; Abell, L; O'Malley, K; Keim, WJ; Dierks, E; Chang, S; Foster, K; Apedo, A; Harden, D; Dabros, M; Gao, Q; Pelleymounter, MA; Whaley, JM; Robl, JA; Cheng, D; Lawrence, RM; Devasthale, P Screening Hit to Clinical Candidate: Discovery of BMS-963272, a Potent, Selective MGAT2 Inhibitor for the Treatment of Metabolic Disorders. J Med Chem64:14773-14792 (2021) [PubMed] Article